Abstract
Organic dyestuff are mostly toxic compounds that pose serious dangers to the environment. Adsorption using low-cost adsorbents is the most favorable method for its economic aspects. Recently, geopolymers have been introduced as an effective adsorbent for dyes and heavy metals. In this investigation, the synthesis of geopolymers from fired brick waste (Homra) was studied with full characterization using X-ray Diffraction, Fourier Transform Infrared Spectroscopy, Brunauer–Emmett–Teller, Energy dispersive X-ray, Scanning electron microscope tests and Transmission electron microscopy. The synthesized nano-Homra geopolymer (NHGP) was then subjected to the removal of one of the most used basic dyes, Methylene Blue (MB). Adsorption optimization was applied using Response surface methodology to study dye adsorption by the synthesized nano-geopolymer. The independent variables studied were: temperature, contact time, and concentration of dye in the elimination process, which were varied in the range of (25–60 ℃), (10–180 min), and (20–300 mg/L) respectively. The results obtained from ANOVA indicated that the maximum removal efficiency of 95% and adsorption capacity of 80.65 mg/g at a temperature of 59 ℃, contact time of 163 min, and an initial concentration of 254 mg/L. The results showed that the data obtained from the adsorption of MB onto NHGP was compatible with the Pseudo second order (R2 = 0.9838) and Langmuir isotherm model (R2 = 0.9882).
Keywords: Box-Behnken design, RSM, Adsorption, Nanogeopolymer, Methylene blue, Optimization
Subject terms: Chemical engineering, Surface chemistry, Sustainability, Pollution remediation
Introduction
One of the most important issues facing humanity today is water pollution, which is getting worse every day as a result of massive industrial expansion taking place all over the world1. The main sources of toxins for the environment in general and water in particular are mostly the organic dyestuffs used in various industries, such as plastics, cosmetics, painting, varnish, and textiles2. Many of the operations in these manufacturing sectors include the use of water, and a sizable portion of the tainted water is released into the environment as wastewater3. Because organic dyes are highly persistent, poisonous, and nonbiodegradable, they pose a threat to living organisms in water and have the power to alter the water's properties even at low concentrations4. Artificial dyes can alter the color of water and block off sunlight, which interferes with aquatic plants' ability to photosynthesize. Furthermore, artificial coloring raises the water's BOD, COD, and particles in suspension5–8. Nowadays, more than 700,000 tons of synthetic dyes (about 10,000 commercial varieties) are produced each year9. Not only are most dyes toxic and carcinogenic but decontaminating dye-containing effluents can also be a useful means of reusing clean water resources from the treatment of industrial wastewater, which assists in addressing one of humanity's most pressing concerns: water scarcity. Consequently, the advancement of wastewater treatment technology holds significant importance since it has the potential to augment the world's water supply. The flocculation precipitation method, electrolysis, membrane filtration, oxidation, biological degradation, and adsorption, among others, are the techniques that are frequently used in the treatment of industrial wastewater 10–15. Because of adsorption's many benefits, including its high efficiency, affordability, and simplicity, it has gained continual attention in the domains of dye removal. Many materials, including activated carbon, coal ash, resins, zeolite, metal oxides, kaolin, magnetic material adsorbents, polypyrrole-functionalized adsorbents, and molecular sieves, have been used as adsorbents in the present day with positive results 16–23.
Inorganic polymers known as geopolymers are prepared through the dissolution, polycondensation, and structural reorganization of low-cost aluminosilicate minerals, such as metakaolin, fly ash, and slag. At the moment, geopolymer materials have been thoroughly studied and used in a number of industrial applications because of their favorable chemical and physical characteristics, including excellent mechanical qualities, high durability, great chemical stability, potent immobilization, and strong heat resistance24. Due to the fact that industrial wastes like fly ash, silica fume, slag, glass slag, and biomass ash are abundant, readily available, and less likely to deplete the benchmark raw material, metakaolin, as well as their notable environmental benefits, the synthesis of geopolymers from these materials is thought to be a cost-effective option25–27. In terms of structure and functionality, geopolymer can be thought of as a three-dimensional aluminosilicate structure with an amorphous or semi-crystalline microstructure, similar to zeolite. It is possible for geopolymerization to occur at temperatures below 100 ◦C, which simplifies and uses less energy and water than zeolites in the production of geopolymer. Low porosity, on the other hand, hinders the industrial applicability of geopolymers. Consequently, to enhance the ability of geopolymers to remove colors from wastewater, it is imperative that their chemical and physical properties be updated on a regular basis. According to Barbosa et al., soybean oil was added to the reaction system of 1SiO2:0.25Al2O3:5.2H2O:0.5 K2O to direct the mesostructured. To create mesoporous geopolymer, which has a higher adsorption capacity than those made without oil because of its optimized pore characteristics, a friable solid monolith was first created hydrothermally. It was then crushed and cleaned with hexane to remove the oil from the material28.
In recent years, there has been increasing emphasis on the removal of dyes from these materials2,29–31. Geopolymer's chemical structure is an aluminosilicate framework that is negatively charged and well-adjusted by some cations e.g. (Na+, K+, or Cs+), which can be swapped out for cations in a solution. As a result, this characteristic serves as the foundation for the adsorption of cationic dyes32,33. Even though the use of geopolymer as an adsorbent in treating wastewater is still in its beginnings, some important variables have been thoroughly studied, including temperature, pH, the amount of absorbent, and the initial concentration of pollutants. Numerous initiatives have been put in place to boost the capability of adsorption. Ash-based geopolymer materials spheres were manufactured by Novais et al. using a suspension-solidification technique. Because of its superior diffusion behavior and high surface area, methylene blue has an adsorption capacity of 79.7 mg/g34. Jin et al. also fabricated a metakaolin-based geopolymer (MG) that served as a quick removal for nickel ions and methylene blue (MB) to aid in the separation and recycling of adsorbents35. Li et al. created reusable, porous geopolymer adsorbents with a high adsorption efficiency of 97.8% for the treatment of dye wastewater36. According to Hua et al., procion red can be effectively removed from synthetic wastewater by using magnetic geopolymer as an adsorbent for water decolorization37. Ali et al. studied employing Fe3O4 magnetic nanoparticle adsorbents in a batch method to remove the anionic azo dye from wastewater. The impact of trembling speed, time, and adsorbent dose on the removal effectiveness of the dye has been investigated38. The sepals of waste tomato plants were used to develop a new green nanocatalyst (GNC) using a thermal approach. The effectiveness of this GNC in reducing the chemical oxygen demand (COD) during a photocatalytic procedure with simulated petroleum refinery liquid waste was studied by Khader et. al.39. Also, Khader et al. assessed the efficacy of the adsorption treatment approach in eliminating phenol and acetone in wastewater from industries40.
In this work, a novel nano geopolymer was developed for the treatment of methylene blue MB in wastewater, based on the high value-added usage of burnt clay brick waste (Homra). XRD, FTIR, SEM, and BET were used to examine the microstructural characteristics. The RSM method was used to optimize the most effective adsorption parameters at the optimum pH value, and repeated experiments were used to confirm the correctness of the optimized prediction model. According to the findings, nano Homra -geopolymer (NHGP) is a powerful adsorbent that has great potential for eliminating MB from wastewater. The effectiveness of geopolymers' adsorption in eliminating organic contaminants is contingent upon ionic interactions. The positively charged part of methylene blue, one of the most well-known cationic dyes, the cationic part of the dye may trade sites with the geopolymer structure's Na+ charge-balancing cations41. In addition to standard isotherm and kinetic modeling, the suggested adsorbent underwent concurrent comprehensive characterization, regeneration, reusability, and actual wastewater treatment studies. This is in line with the ideas of sustainable and green development, which emphasize using solid waste to create high-value products and using waste to treat poisoning.
Materials and methodology
Raw materials
Fired clay brick waste (Homra) was used as a good substitute for the calcined kaolin which is less expensive. The waste was sieved using mesh size 200, the undersize was used in the preparation of the nano geopolymer. Sodium hydroxide and sodium silicate were used as an alkaline activator in the preparation of geopolymer. They were purchased from El Shark El-Awsat company for chemicals, in Cairo, Egypt. The chemical composition and the mineralogical analysis of Homra were determined using the X-ray fluorescence technique (Axios, panalytical 2005, wavelength dispersive sequential spectrometer machine) and X-ray diffraction Brukur D8 advanced computerized apparatus respectively.
Methylene blue
Methylene Blue, Methylthioninium chloride, (MB), is a formal derivative of phenothiazine. It is a dark green powder that yields a blue solution in water. The hydrated form has 3 molecules of water per unit of methylene blue.
The MB was obtained from RFCL LIMITED with 99% purity, its chemical formula is C16H18ClN3S and its structure is presented in Fig. 1.
Figure 1.
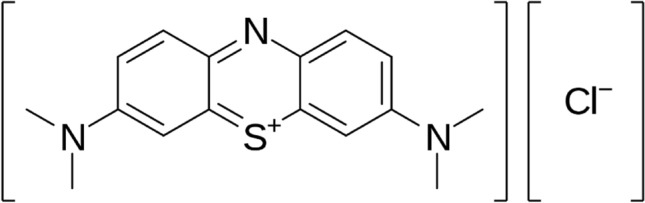
Methylene Blue Structure.
Alkaline activator preparation
The pellets of sodium hydroxide (99%) were dissolved in water to prepare 12 M of an aqueous solution of NaOH. The prepared solution was cooled at room temperature and then mixed with sodium silicate solution (16.7% Na2O, 30.3% SiO2, and 53% H2O) to produce the alkaline activator. The ratio of Na2SiO3/ NaOH is 2.5, this mixture was prepared before the synthesis of the geopolymer adsorbent about 24 h9.
Preparation of the geopolymer adsorbent
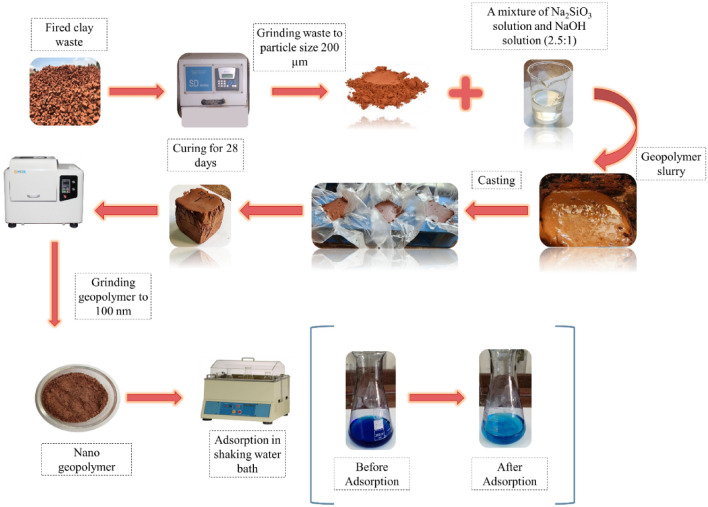
The aluminum silicates that were present in the Homra waste were mixed with the alkaline activator solution with a solid–liquid (S/L) mass ratio (3/1) using a mechanical mixer to obtain a homogenous mixture. The geopolymer slurry was placed in a cubic iron mold with dimensions (5cm x 5cm). The cubic mold was vibrated using an electric mechanical vibrator to remove any air voids. The sample was kept in the mould for 24 h then de-molded to be cured at room temperature for 28 days. The synthetic geopolymer was crushed and ground to particle size less than100 nm.
Characterization of the adsorbent
The mineralogical analysis was performed on the synthetic geopolymer with X-ray diffraction to determine the crystalline phases (XRD) using BRUKER apparatus for XRD, Axs, D8- ADVANCE (Germany 2001). Brunauer- Emmett- Teller (BET) analysis was used to determine the surface area presence in the synthetic geopolymer and pore size distribution42. BET was performed in the presence of nitrogen adsorption–desorption isotherm analysis at a temperature of 77 K using a 3 Flex 3500 microscopic model analyzer. Scanning electron microscope (SEM) analysis was accomplished to determine the prepared adsorbent morphology43 using JEOL5410 apparatus. The shapes and sizes of the prepared nano-geopolymer were determined using Transmission electron microscopy (TEM) with micro-analyzer electron technique (JEOL JX 1230), while energy dispersive X-ray analysis (EDX) was determined using JEOL JX 2840 apparatus. The main functional group presence in the synthetic geopolymer was determined using Fourier-transform infrared spectroscopy (FT-IR). FT-IR spectrums were recorded on a Jasco FTIR- 6100 system, using a pellet made with dehydrated KBr and an instrument in the reflectance mode. The FTIR spectrums were determined between 400 to 4000 cm-1.
Adsorption experiments
A stock solution of 1g/L was prepared by dissolving (1g) of MB in one liter of double distilled water. The working solutions were prepared by diluting the stock solution with double distilled water to give the required concentration of the working solutions. The hydrogen ion concentration (pH) of the solutions was adjusted using either 0.1N HCl or 0.1N NaOH solutions. The dye concentration was detected by Spectrophotometer (HACH DR 2800) at the maximum wavelength of the dye, λmax = 667 nm. The hydrogen ion concentrations were determined using XS Instrument pH meter. The optimum pH was firstly determined for pH ranging from 2 to 12.
Adsorption can be affected by various factors such as pH, initial concentration of adsorbates, contact time, adsorbent dosage, adsorbent size, and temperature, in addition to agitation speed. The optimization of the above-mentioned factors is essential to maximize outcomes of adsorption, percentage removal, or the adsorption capacity. The adsorption capacity is the predominant. The optimum pH was firstly determined for pH ranging from 2 to 12. The effect of initial conc, time, and temperature were examined according to the principle of Box-Behnken Design.
Experimental design of adsorption of MB on nano-geopolymer
Box-Behnken design (BBD) is used to design and optimize the main parameters affecting the removal efficiency of the MB solution using Design-Expert version 13 software. Three variables were used in the design of the experiments: adsorption time (10–180 min), adsorption temperature (25–60 ℃), and initial concentration of the MB (20–300 mg/L) while keeping the adsorbent dose fixed during the experiments (0.1 g). The removal efficiency of the MB solution was used as a response value of the experimental design.
0.1 g of the synthetic nano-Homra-geopolymer (NHGP) was immersed in 100 mg/L of MB solution at room temperature followed by adjusting pH to 12 then placed in a water bath shaker with a speeding rate of 130 rpm. The experiment procedure is illustrated in Fig. 2. The effect of the initial concentration, time, and temperature were studied. Table 1 shows the conditions of the experimental design. The removal efficiency, R, and the adsorption capacity, q, were determined using the following Eqs. (1 and 2)44.
| 1 |
| 2 |
where is the initial concentration of MB solution in (mg/L), is the concentration at final time in (mg/L), is the volume of the solution sample (L), is the amount of the adsorbent in (g).
Figure 2.
Experimental Procedure of MB Adsorption Using NHGP.
Table 1.
Experimental Condition of Removal Efficiency of MB Using Design Expert.
| Run | A: Time min | B: Temperature ℃ | C: Initial Conc mg/L |
|---|---|---|---|
| 1 | 95 | 60 | 300 |
| 2 | 10 | 42.5 | 300 |
| 3 | 95 | 60 | 20 |
| 4 | 10 | 25 | 160 |
| 5 | 95 | 42.5 | 160 |
| 6 | 95 | 42.5 | 160 |
| 7 | 180 | 42.5 | 300 |
| 8 | 10 | 42.5 | 20 |
| 9 | 95 | 42.5 | 160 |
| 10 | 180 | 60 | 160 |
| 11 | 95 | 42.5 | 160 |
| 12 | 180 | 42.5 | 20 |
| 13 | 180 | 25 | 160 |
| 14 | 95 | 25 | 20 |
| 15 | 95 | 25 | 300 |
| 16 | 95 | 42.5 | 160 |
| 17 | 10 | 60 | 160 |
Adsorption isotherm, kinetics and thermodynamics of MB
The adsorption isotherm of the MB solution was determined at the optimum conditions using 0.1 g of nano geopolymer adsorbent at different initial concentrations ranging from 20 to 300 mg/L which mainly affects the adsorption capacity. Different adsorption models45,46 (Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich) were used to identify the most fitted adsorption isotherm model. Langmuir adsorption isotherm assumes the absence of interactions between the adsorbed components, equal energy of sorption, and also homogenous binding sites47. Freundlich adsorption isotherm can describe the adsorption for both mono and multilayer48. Temkin adsorption isotherm assumes a linear equation and ignores the low and high concentrations. The uniform distribution of bounding energy was also assumed45. Dubinin–Radushkevich (Dub-Rad) isotherm model is used to determine the apparent free energy of the adsorption process and also the characteristic porosity49–51.
The adsorption isotherm model can be determined according to the following Eqs. (3) to (7):
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
where is the concentration of MB solution at equilibrium in (mg.l-1); is the adsorption capacity at equilibrium in (mg.g-1); is the constant of Langmuir (l.mg-1); is the maximum theoretical adsorption capacity of the adsorbent in (mg.g-1); is the constant of Freundlich in (mg (1-n) .Ln.g-1); finally is the adsorption strength constant for Freundlich. B is a Temkin constant (J.mol-1) related to the heat of adsorption and is equal to (RT/b), where T is the absolute temperature in (K) and R is a gas constant (8.314 J.mol-1.k-1) while Temkin isotherm constant is expressed by A (L.g-1). β is the Dub-Rad constant in (mol2/J2), while ε is the Polanyi potential that represents the theoretical adsorption capacity in (J/mol)52.
The kinetics of the adsorption process of MB at the optimum conditions using the Pseudo-first order, the Pseudo second order, the Intraparticle, Interparticle diffusion, and Elovich models. Equations (8) to (12) are used to investigate the kinetic model of the adsorption in the linear form35,53–56.
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
where qt and qe are the adsorption capacity at any time and equilibrium respectively in (mg/g), t is the time (min) and c is constant (mg.g-1). The rate constants for Pseudo-first order, Pseudo-second order, and Intraparticle diffusion are k1 (min-1), k2 (g. mg-1. min-1) and kp (mg.g-1.min-0.5) respectively. kMD represents the mass transfer diffusion rate constant. α represents the initial absorption rate in (mg.g-1.min-1), while β is the Elovich constant.
Adsorption thermodynamics was used to identify the energy changes accompanied by the adsorption process. Different parameters were used to check the spontaneous of the adsorption process such as the change of Gibbs free energy (ΔG, kJ/mol), entropy change (ΔS, kJ/K), and enthalpy change (ΔH, kJ/mol)24. These parameters were calculated using the following equations from (13) to (15)57,58.
| 13 |
| 14 |
| 15 |
where Ce is the concentration of the liquid phase at equilibrium in (mg/L), qe is the adsorption capacity at equilibrium, R is the universal gas constant, Kd is the coefficient of distribution and T is the absolute temperature in Kelvin.
Reusability test of nanogeopolymer
After obtaining the optimal removal efficiency of the adsorbent, a regeneration experiment utilizing a nano-geopolymer adsorbent was conducted. The ideal nano-geopolymer adsorbent, as determined by the experiment, was submerged in a certain pH solution at the optimum conditions. This allowed for three cycles of adsorption tests to ascertain the adsorption stability. After being filtered out, the nano-geopolymer adsorbent was washed five times with methanol (99%) before being dried one last time to remove any last traces of methanol. For every experiment, the MB dye's adsorption capacity and removal efficiency were computed.
Results and discussion
Characterization of adsorbent
Mineralogical analysis of nano-geopolymer adsorbent
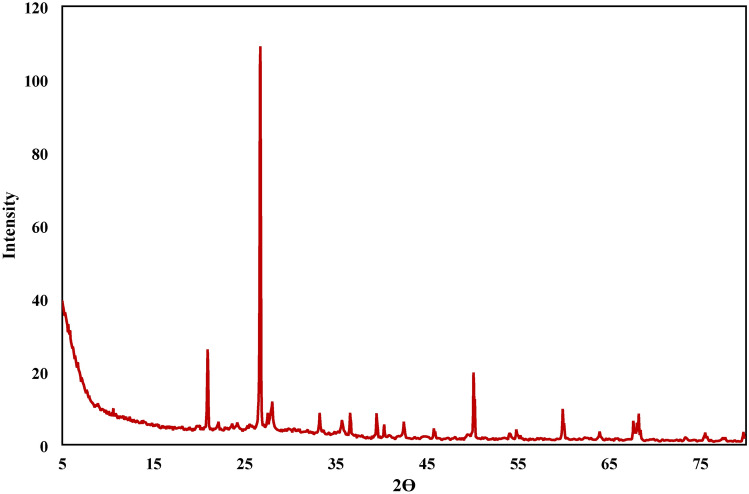
X-ray Diffraction (XRD) is a technique used for determining the atomic and molecular structure of a crystalline material, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions.
XRD has revealed that the main phase present in clay brick waste (Homra) is quartz at 2 ϴ equal to 20.936 and 26.7 as shown in Fig. 3. The firing of the bricks was done below 900 ℃, so the main constituents are quartz and amorphous meta-kaolin.
Figure 3.
XRD of Adsorbent.
SEM of NHGP
To inspect the NHGP surface, SEM analysis was investigated. The SEM image with 12,000 × magnification depicted in Fig. 4 shows the formation of the heterogeneous matrix after the polycondensation of raw material. The geopolymer surface was consistent and coarse, with irregular aggregates, as seen in Figure. There were several tiny cavities and holes found with different shapes and sizes in the range of (12–44 nm). This suggests that the geopolymer may operate as an excellent adsorbent.
Figure 4.

SEM of Homra Nano-geopolymer (NHGP).
The surface structure of the NHGP is valuable because it gives superior vacancies for adsorbate molecules.
TEM analysis of NHGP
Figure 5 shows an image of the prepared geopolymer matrix with Na2SiO3/ NaOH ratio 2.5 after 28 days of aging. There are many small particles with sizes ranging from 11.61 to 53.52 nm. These small particles are crystalline material as illustrated in Sect. (3.1.1).
Figure 5.
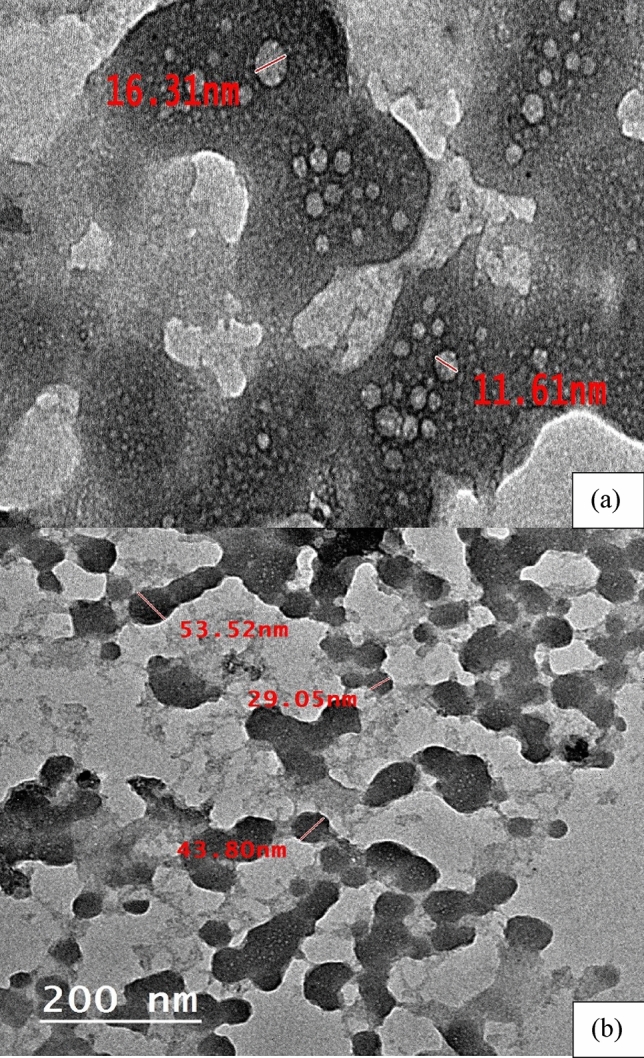
TEM of Homra Nano-geopolymer (NHGP): (a) 100 nm Magnification, (b) 200 nm Magnification.
It also indicates the presence of two different phases which are light and dark grains. The image of the dark grains is related to the presence of SiO2 in quartz form while the light-colored grains are related to Al2O3. The remaining different gray shade zones are related to aluminosilicates and a mixture of the most common phases. Aluminosilicates partially dissolve in the alkaline medium, producing an amorphous geopolymer gel along with undissolved particles of crystalline material during the reaction of geopolymerizatio59.
Analysis of specific area (BET)
The specific surface area of GP and NHGP were analyzed using BET and the results are illustrated in Table 2. It can be observed that the NHGP has a surface area of 18.36 m2/g more than that of GP 11.82 m2/g. The high specific surface area of NHGP makes it a good candidate to be used as an adsorbent medium. Generally, the adsorbent’s pores are classified into micropores, mesopores, and macropores with diameters less than 2 nm, ranging from 2 to 50 nm and more than 50 nm respectively according to IUPAC regulations60. The obtained results show that the produced adsorbent is mesopores type (as discussed in section "TEM analysis of NHGP") which is the most accessible pores to adsorb MB. The prepared geopolymer is an ideal adsorbent that is eco-friendly, non-soluble, mostly efficient, and easy preparation materials. Table 2 shows the BET analysis of the produced adsorbent and different adsorbent from previous studies.
Table 2.
BET Analysis.
| Average pore reduction (nm) | Surface area BET (m2/g) | Pore volume (cm3/g) | Total pore volume (cm3/g) | References | |
|---|---|---|---|---|---|
| Nano geopolymer | 7.0738 | 18.3658 | 0.061697 | 6.496 × 10–2 | Present study |
| Geopolymer | 7.545 | 11.82 | 0.058 | 6.12 × 10–2 | Present study |
| Kaolin, Metakaolin, Fly ash Geopolymer | 9.68428 | 9.5076 | 0.02387 | – | 9 |
| Gangue waste geopolymer | 30.08 | 18.54 | 0.024 | – | 61 |
| Amino-bagasse/metakaolin geopolymer | 7.7604 | 49.16 | 0.1227 | – | 62 |
| Steel slag, Fly ash, Metakaolin geopolymer | 9.73 | – | 0.027 | – | 63 |
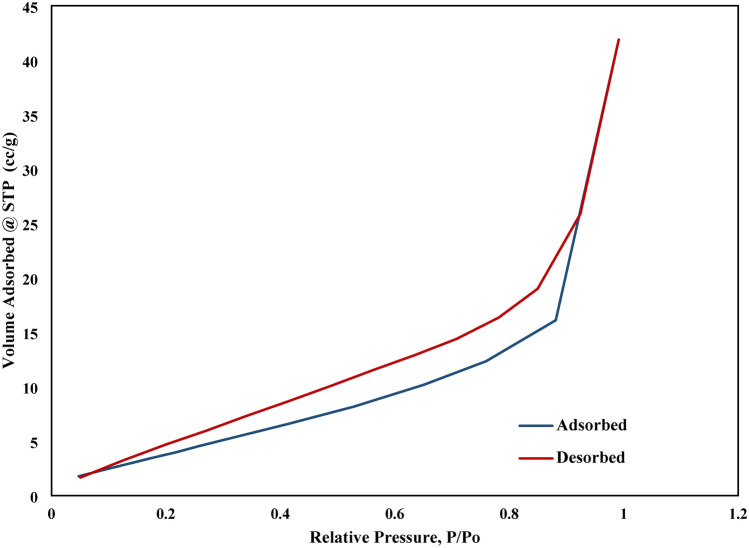
From Fig. 6, it was observed that lower pressure regions show the formation of a monolayer followed by a formation of multilayers. This model is Type IV isotherms with hysteresis loop at a relative pressure till 0.9. BET surface area characterization of NHGP is mesoporous materials, with pore diameters ranging between 2—50 nm giving this type of isotherm.
Figure 6.
N2 Adsorption–Desorption Isotherms of N2 onto NHGP.
EDX and FTIR analyses
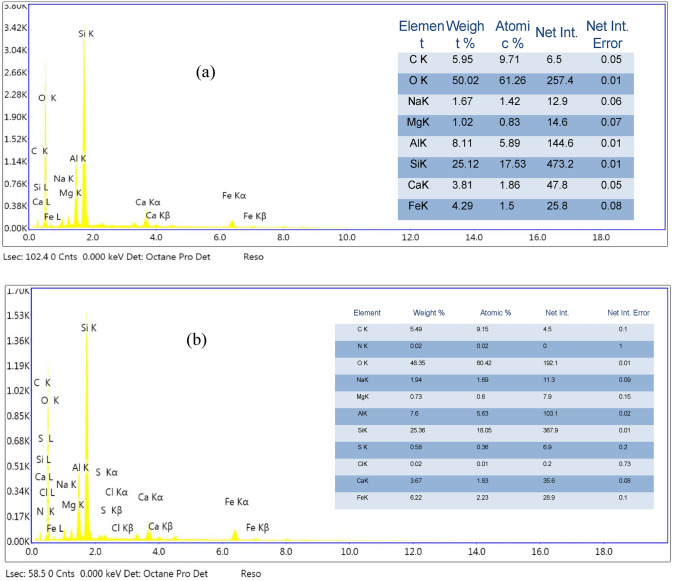
The chemical analysis of NHGP (EDX analysis) depicted in Fig. 7a contains 25.12 wt% Si, 8.11 wt% Al, and 50.02 wt% O, and there is no sulfur, Cl, or N content in the analysis of NHGP as native adsorbent. It illustrates the composition ratio of Si and Al to be 3.1.
Figure 7.
EDX Analysis (a) before adsorption, (b) after adsorption of MB.
Compared with NHGP after the adsorption of Methylene blue dye chemical analysis, Fig. 7b, which contains mainly 25.36 wt % Si, 7.6 wt% Al, 48.35 wt% O, with the presence of S, Cl, and N which are compatible with the adsorption of MB.
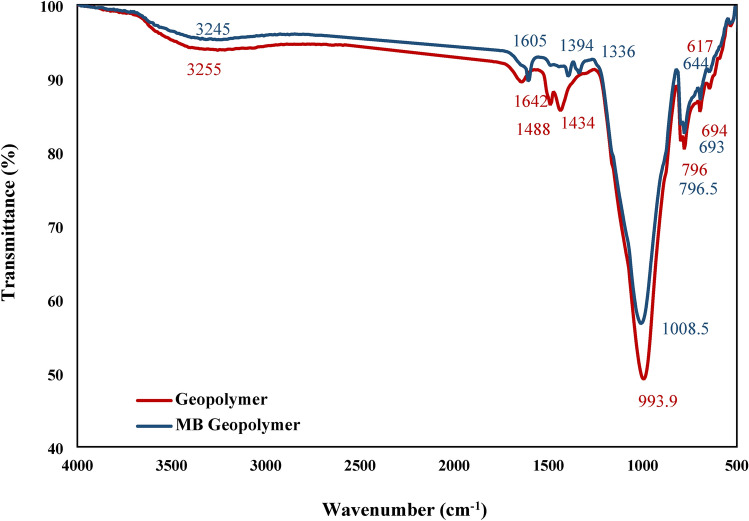
FTIR was used to identify the functional groups involved in the adsorption of MB before and after the adsorption process onto NHGP as shown in Fig. 8. A strong vibration peak centered at 993 cm-1 typical for aluminosilicates can be seen. This peak shifts to 1008 cm-1 with the adsorption of MB. Al–O–Si vibrations correspond to the absorption bands 600–800 cm-1. The absorption peak of 796 cm-1 was an indication of the presence of quartz64. The absorption band around 1443 cm-1 is attributed to stretching vibrations of CO32- ions confirming the existence of carbonate species65. The broad band observed at 3255 cm-1 and for geopolymer, is due to the OH and the adsorbed water this peak shifted to 3245 on the adsorption of MB. The stretching bands at 1008.5 cm-1 are for (-NH2) and 1390 cm-1 for (-CH3) that are present in MB that adsorbed onto geopolymer. The peak at 1605 cm-1 is due to asymmetric stretching vibrations of C = O and the peak observed at 1394 cm-1 can be assigned to the aromatic compound group66.
Figure 8.
FTIR Analysis of Geopolymer before and after Adsorption.
Effect of pH on MB adsorption
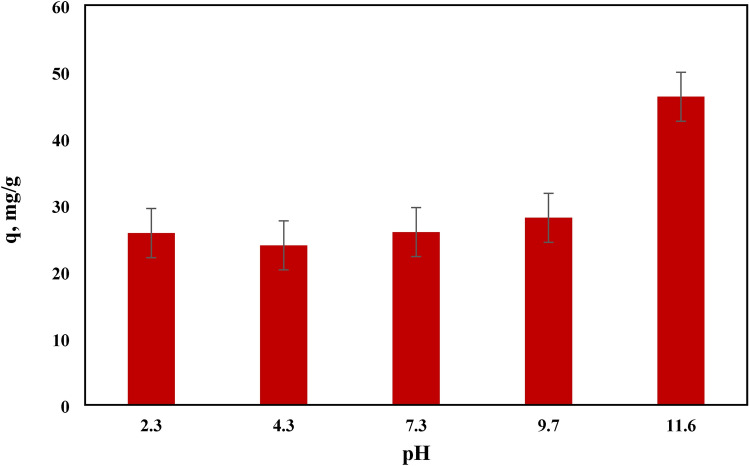
The adsorption of methylene blue (MB) using NHGP at different pH values is presented in Fig. 9. As demonstrated from the figure, the quantities of MB adsorbed onto the resin were largely raised with the increase in pH, designating that the alkaline environment was more advantageous for MB adsorption. Bearing in mind the cationic nature of MB, electrostatic interactions may occur between MB and the adsorbent surface67, 68.
Figure 9.
Influence of pH on the Adsorption Capacity of MB Dye onto NHGP.
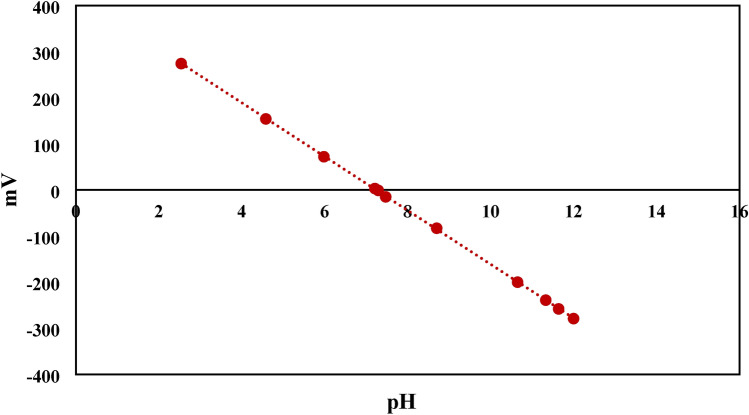
Figure 10 illustrates the zeta potential of the geopolymer at different pH’s. As a consequence of this data, the zeta potential is negative at the alkaline medium, therefore the surface of NHGP was negatively charged. Thus, electrostatic attraction between MB cations and the adsorbent surface was expected higher pH values. The adsorption of MB onto NHGP is not dependent only on the Electrostatic interactions, as there are some adsorptions that occur at low pH values (Fig. 10). Another mechanism as well as hydrophobic adsorption and electron donor–acceptor (EDA) interactions may affect the adsorption process, this proposed interaction can be seen in Fig. 1169–71.
Figure 10.
Zeta Potential of NHGP at Different pH.
Figure 11.
Schematic Diagram for the Proposed Reaction between MB and NHGP.
ANOVA of MB removal
Table 3 lists the actual and the predicted values of the removal efficiency of MB that was performed according to the designed experiments using Design-Expert v13. The relationship between the different parameters and the removal efficiency of MB was described using multivariate analysis. It was found that the quadratic model was the best fitness function that describes this relation as following Eq. (16):
| 16 |
where Y is the removal efficiency of MB, t is the time of the adsorption, T is the temperature and C is the initial concentration. According to the ANOVA analysis in Table 4, the model is significant and the p-value of lack of fit is 0.3895 which is non-significant (more than 0.05).
Table 3.
Experimental Conditions of Actual and Predicted Values of Removal Efficiency of MB from Wastewater.
| Run | A: Time min | B: Temperature ℃ | C: Initial conc mg/L | Actual removal efficiency MB (%) | Predict removal efficiency MB (%) |
|---|---|---|---|---|---|
| 1 | 95 | 60 | 300 | 81.6 | 78.99 |
| 2 | 10 | 42.5 | 300 | 9.6 | 11.21 |
| 3 | 95 | 60 | 20 | 60.15 | 61.42 |
| 4 | 10 | 25 | 160 | 13.9 | 13.56 |
| 5 | 95 | 42.5 | 160 | 52.78 | 52.42 |
| 6 | 95 | 42.5 | 160 | 50.25 | 52.42 |
| 7 | 180 | 42.5 | 300 | 57.13 | 59.40 |
| 8 | 10 | 42.5 | 20 | 32.76 | 30.49 |
| 9 | 95 | 42.5 | 160 | 52.8 | 52.42 |
| 10 | 180 | 60 | 160 | 91.6 | 91.93 |
| 11 | 95 | 42.5 | 160 | 56.97 | 52.42 |
| 12 | 180 | 42.5 | 20 | 63.8 | 62.19 |
| 13 | 180 | 25 | 160 | 33.13 | 32.13 |
| 14 | 95 | 25 | 20 | 49 | 51.61 |
| 15 | 95 | 25 | 300 | 13.24 | 11.97 |
| 16 | 95 | 42.5 | 160 | 49.3 | 52.42 |
| 17 | 10 | 60 | 160 | 29.6 | 30.60 |
Table 4.
ANOVA Analysis of the Removal Efficiency of MB from Wastewater.
| Source | Sum of squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 8192.33 | 9 | 910.26 | 91.06 | < 0.0001 | Significant |
| A-Time | 3192.01 | 1 | 3192.01 | 319.30 | < 0.0001 | |
| B-Temp | 2952.19 | 1 | 2952.19 | 295.31 | < 0.0001 | |
| C-Initial Conc | 243.54 | 1 | 243.54 | 24.36 | 0.0017 | |
| AB | 457.32 | 1 | 457.32 | 45.75 | 0.0003 | |
| AC | 67.98 | 1 | 67.98 | 6.80 | 0.0350 | |
| BC | 818.25 | 1 | 818.25 | 81.85 | < 0.0001 | |
| A2 | 443.99 | 1 | 443.99 | 44.41 | 0.0003 | |
| B2 | 0.0370 | 1 | 0.0370 | 0.0037 | 0.9532 | |
| C2 | 7.43 | 1 | 7.43 | 0.7436 | 0.4171 | |
| Residual | 69.98 | 7 | 10.00 | |||
| Lack of Fit | 34.56 | 3 | 11.52 | 1.30 | 0.3895 | not significant |
| Pure Error | 35.42 | 4 | 8.85 | |||
| Cor Total | 8262.31 | 16 |
The values of R2 and R2adj are proportional rises with the significance of the model. Table 5 shows the coefficient of determination of R2 and R2adj equal 0.9915 and 0.9805 respectively for the removal efficiency of MB, while the predicted value of R2 is 0.9264. Those values show a good agreement between the actual and predicted values presented by the model.
Table 5.
Statistics fit model.
| Std. dev | 3.16 | R2 | 0.9915 |
| Mean | 46.92 | Adjusted R2 | 0.9806 |
| C.V. % | 6.74 | Predicted R2 | 0.9264 |
| Adeq Precision | 33.2902 |
Table 6 shows that all adsorption parameters affect the adsorption performance and removal efficiency of MB. The positive signs for parameters such as time, temperature, and the interaction between time and temperature have a great effect on the adsorption performance, while the negative signs for parameters such as initial concentration and the time square imply that increasing those parameters causes decreasing the performance of the adsorption.
Table 6.
Values of VIF.
| Factor | Coefficient estimate | df | Standard error | 95% CI Low | 95% CI high | VIF |
|---|---|---|---|---|---|---|
| Intercept | 52.42 | 1 | 1.41 | 49.08 | 55.76 | |
| t | 19.98 | 1 | 1.12 | 17.33 | 22.62 | 1.0000 |
| T | 19.21 | 1 | 1.12 | 16.57 | 21.85 | 1.0000 |
| C | − 5.52 | 1 | 1.12 | − 8.16 | − 2.87 | 1.0000 |
| t T | 10.69 | 1 | 1.58 | 6.95 | 14.43 | 1.0000 |
| t C | 4.12 | 1 | 1.58 | 0.3843 | 7.86 | 1.0000 |
| TC | 14.30 | 1 | 1.58 | 10.56 | 18.04 | 1.0000 |
| t2 | − 10.27 | 1 | 1.54 | − 13.91 | − 6.63 | 1.01 |
| T2 | − 0.0938 | 1 | 1.54 | − 3.74 | 3.55 | 1.01 |
| C2 | − 1.33 | 1 | 1.54 | − 4.97 | 2.31 | 1.01 |
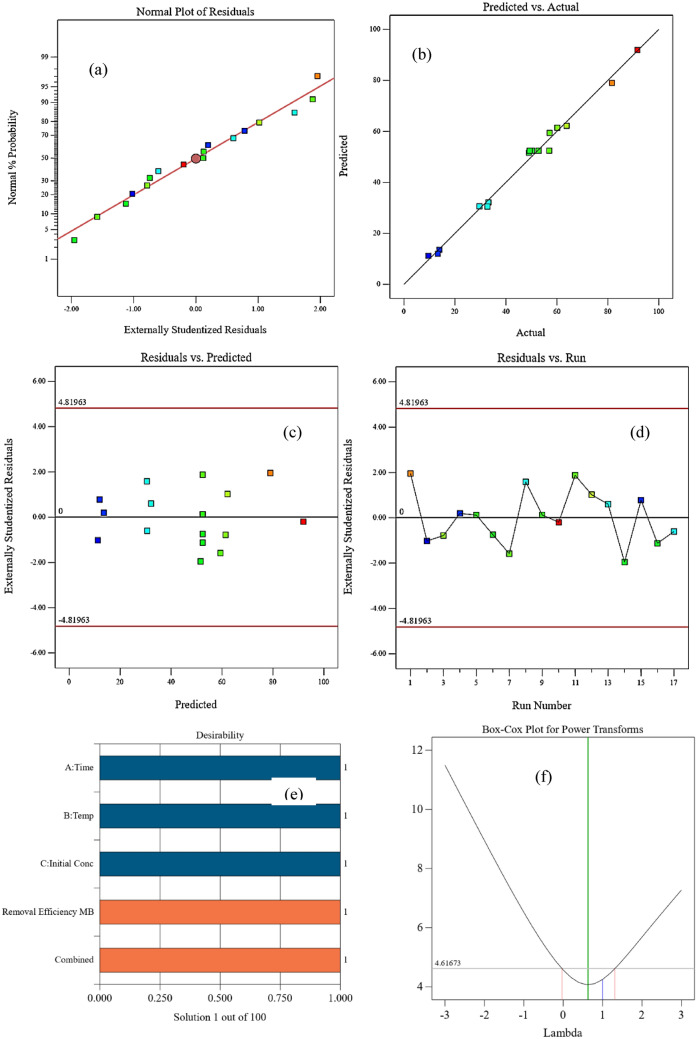
The main diagnostic plots were observed in Fig. 12. The residual of the function model shows a good distribution probability that confirms the assumption accuracy and the independence of the residuals as shown in Fig. 12a. A linear correlation was observed between the predicted and actual values of the experimental data as shown in Fig. 12b while Fig. 12c shows a random distribution of the data points obtained from the experiments with the error line, it was indicated a good prediction and model accuracy. It was observed that the different data of the residuals are in the interval ranges of (4.81963) and (-4.81963) indicating a good prediction of the model as illustrated in Fig. 11d. Figure 12e shows the Pareto graph for the adsorption process that indicates the satisfaction of each variable to the criteria, their values near 1 represent the maximum adsorption reached. The best lambda for the adsorption process was found at 0.63 as illustrated in Fig. 12f the Box-Cox diagram which indicates that the data was suitable and didn’t need any improvement44, 50.
Figure 12.
Diagnostic Plots for the Adsorption Removal Efficiency of MB: (a) Normal Probability, (b) Predicted and Actual Values, (c) Externally Studentized Residuals versus Predicted Values, (d) Externally Studentized Residuals versus Run Number, (e) Pareto Diagram, (f) Box-Cox Diagram.
Effect of interactive different parameters on adsorption removal efficiency of MB
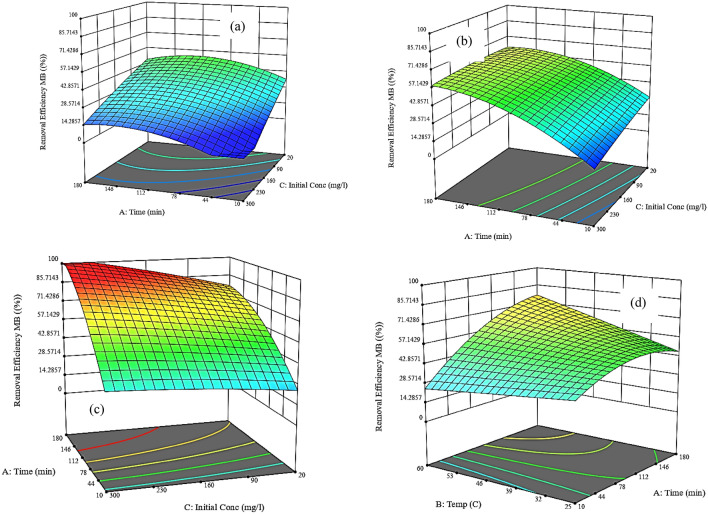
Figure 13a–c show the relationship between the time, initial concentrations of MB at different adsorption temperatures (25 ℃, 42.5 ℃, and 60 ℃), and the adsorption removal efficiency of MB. It can be observed that increasing the temperature causes increasing in the removal efficiency of MB at different initial concentrations and times. Temperature affects the adsorption process based on the type of bonds formed between adsorbate sites and adsorbents. This behavior is due to the rapid diffusion of the MB dye molecules from the synthetic wastewater to reach the active sites present in the NHGP72 which indicates that a higher temperature is preferred for the adsorption process.
Figure 13.
3D Response Surface Plots of for the Effect of Different Parameters on the Adsorption Removal Efficiency of MB.
Figure 13d–f show the effect of contact time and adsorption temperatures at initial concentrations ranging from 20 to 300 ppm on the removal efficiency of MB. It can be understood that the effect of the adsorption time is more dominant than the effect of the adsorption temperature. It can be observed that the maximum removal efficiency of MB at a temperature of 60 ℃, a time of 180 min ranging from 80 to 100% at initial concentrations of 20 ppm and 300 ppm respectively. The reason for this behavior is that the high initial concentration of MB causes a high concentration gradient leading to the high driving force for the transfer of the molecules quickly to the active sites73. It was observed that increasing the initial concentration of MB causes increasing the loading capacity of NHGP adsorbent that complies with the results obtained from the adsorption of MB using green olive stones66.
Finally, Fig. 13g–i show the relationship between the adsorption temperature and initial concentrations at different contact times ranging from 10 to 180 min on the removal efficiency. It can be observed that the effect of the adsorption temperature is more dominant than the initial concentration and also increasing the contact time between the adsorbent and the MB solution causes increasing in the removal efficiency due to allowing a sufficient time to adsorb MB on the NHGP. Adsorption capacity increases as the contact time increases to a point, after which further increase in the contact time does not increase adsorbate uptake on adsorbent due to saturation and there will be no significant change in the removal efficiency of MB74.
Optimization of MB removal efficiency
The optimum removal efficiency of MB was obtained from the program software using the optimization tab. The constraints of each parameter were set as illustrated in Table 7. The optimal parameters were found to be at a temperature of 59 ℃, initial concentration of MB of 254 ppm, and adsorption time of 163.2 min to obtain a predicted value for removal efficiency of MB of 95% with the desirability of 1 as illustrated in Table 8. To validate these results the suggested optimum parameters were performed five times at the optimum conditions to calculate the mean removal efficiency of MB of 94.4% with a standard deviation of 1.4% with an error percentage of 0.63 which proves the validity of the model.
Table 7.
Constraints Optimization.
| Name | Goal | Lower limit | Upper limit | Lower weight | Upper weight | Importance |
|---|---|---|---|---|---|---|
| A: Time | is in range | 10 | 180 | 1 | 1 | 3 |
| B: Temp | is in range | 25 | 60 | 1 | 1 | 3 |
| C: Initial Conc | is in range | 20 | 300 | 1 | 1 | 3 |
| Removal Efficiency MB | maximize | 9.6 | 91.6 | 1 | 1 | 5 |
Table 8.
Results of the Optimum Solutions.
| Number | Time | Temp | Initial conc | Removal efficiency MB | Desirability | |
|---|---|---|---|---|---|---|
| 1 | 163.23 | 59.081 | 254.168 | 95.116 | 1 | Selected |
| 2 | 175.299 | 58.074 | 237.562 | 93.882 | 1 | |
| 3 | 167.275 | 58.437 | 223.843 | 92.423 | 1 | |
| 4 | 158.502 | 58.587 | 230.888 | 91.617 | 1 | |
| 5 | 179.406 | 57.34 | 222.832 | 91.893 | 1 | |
| 6 | 135.101 | 59.820 | 297.520 | 92.584 | 1 | |
| 7 | 154.257 | 59.565 | 229.569 | 92.556 | 1 |
Adsorption isotherm, kinetics and thermodynamics studies
Isotherm models of the adsorption of MB onto NHGP
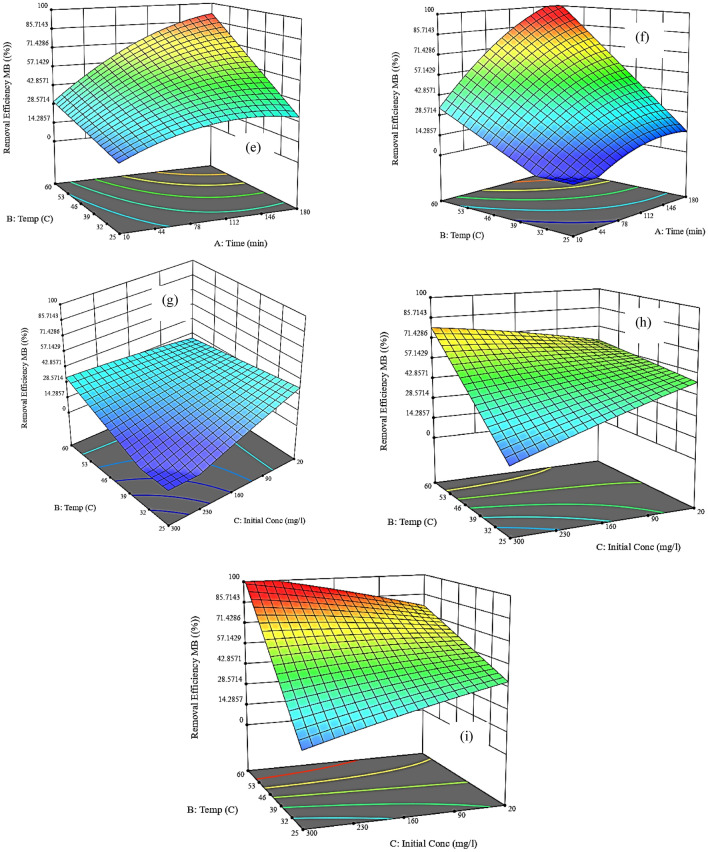
Studying the Langmuir isotherm model, it has been stated that the adsorbent surface is homogeneously distributed having identical adsorbent sites and there is no lateral interaction forming a monolayer. From Fig. 14a it was found that the correlation coefficient of the model (R2) is equal to 0.988 which indicates that the adsorption of MB with NHGP is well fitted with this model with a maximum capacity of 80.65 mg/g.
Figure 14.
Adsorption Isotherm for MB onto NHGP; (a) Langmuir, (b) Freundlich, (c) Temkin.
Figure 14b represents the fitting of the isotherm data with the linear form of the Freundlish model. The Freundlich adsorption isotherm model is used to describe heterogeneous systems. From the fitting data and the correlation factor (R2 = 0.9418), we deduced that this system is moderately fitted with the Freundlish model.
The 1/n value obtained from the Freundlich isotherm plot is 0.6036 which falls between 0 and 1 indicating the adsorption is linear and uniform throughout the surface. According to the 1/n value (0 < 1/n < 1), this isotherm is a favorable physical process.
The Temkin model in Fig. 14c clarifies the variation in the heat of adsorption and the interactions of adsorbate–adsorbate molecules during the progression of the adsorbed layer. The correlation coefficient of this model is 0.8846 which indicates poor fitting for the adsorption of MB with NHGP. Figure 14d shows the Dub-Rad model that is suitable for moderate concentration of the adsorbate and assumes multilayer properties as Vander Waals forces are involved and the physical adsorption process can be applied75. Table 9 shows the fitting parameter of the four adsorption isotherm models.
Table 9.
Isotherm Models Parameters of the Removal of MB by NHGP.
| Model | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg/g) | 80.65 |
| kL (L/mg) | 0.9077 | |
| R2 | 0.9882 | |
| Ep | 0.05–0.0037 | |
| Freundlich | Kf, (mg/g) (mg/L)(1/n) | 2.589 |
| n-1 | 0.6036 | |
| R2 | 0.9418 | |
| Temkin | A (L/g) | 0.151 |
| B (J/mol) | 16.003 | |
| R2 | 0.8846 | |
| Dub–Rad | qm (mg/g) | 45.388 |
| β (mol2/J2) | 5.01153E-05 | |
| R2 | 0.9383 |
Kinetics of the adsorption of MB onto NHGP
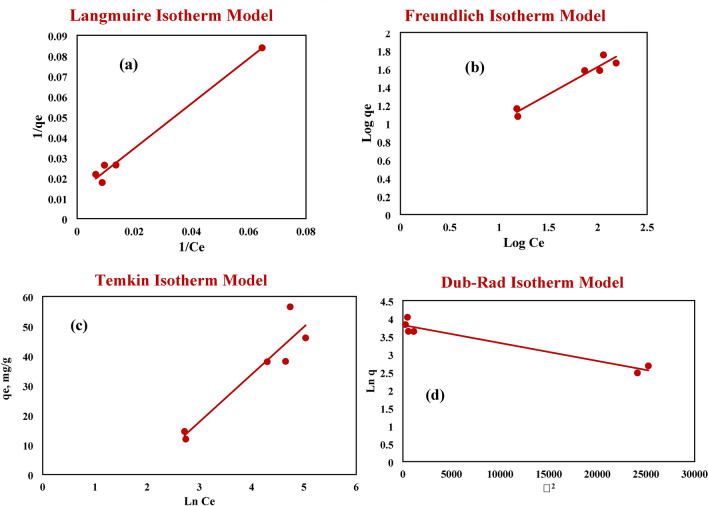
Figure 15a illustrates the adsorption capacity and the rate of the adsorption of MB onto the NHGP. It can be observed there is rapid adsorption takes place at a time ranging from 0 to 45 min to increase the adsorption capacity from 0 mg/g to 31.57 mg/g respectively. The removal efficiency of MB is enhanced as the contact time increases till reaches 45 min, then at times ranging from 45 to 120 min the adsorption capacity was nearly unchanged ranging from 31.57 mg/g to 28.44 mg/g due to reaching equilibrium.
Figure 15.
Kinetics of MB Adsorption; (a) Adsorption Capacity of MB versus Contact Time, (b) Pesudo First Order, (c) Pesudo Second Order, (d) Intraparticle Diffusion, (e) Interparticle Diffusion, (f) Elovich Model.
Kinetics of MB adsorption on NHGP were determined using Pseudo first order, Pseudo second order, Intraparticle diffusion, Interparticle diffusion, and Elovich models as illustrated in Fig. 15b–f to identify the best-fitted model and analyze the MB adsorption process. Table 10 shows the fitting parameters for the five kinetic adsorption models, which designated that the Pseudo Second Order kinetic model is the most applicable model for this process. It has a correlation coefficient of (R2 = 0.9838) which is higher than the four other models. According to this model, it was found that the equilibrium adsorption capacity and adsorption rate constant were 28.41 mg. g-1 and 9.14 × 10–3 g. mg-1.min-1 respectively. Choosing of Pseudo Second Order model is considered the most suitable model for the adsorption process. It is also indicated that the adsorption is a chemisorption type and the adsorption rate doesn’t depend on the adsorbate concentration but depends on the adsorption capacity76 confirmed by the results obtained from the Design-Expert software.
Table 10.
Kinetics Parameters of MB Adsorption onto NHGP.
| Kinetics model | Parameters | NHGP Adsorbent |
|---|---|---|
| Pesudo First Order | k1 (min-1) | 1.41 × 10–2 |
| qe (mg/g) | 31.57 | |
| R2 | 0.57075 | |
| Pesudo Second Order | k2 (g. mg-1.min-1) | 9.15 × 10–3 |
| qe (mg/g) | 28.41 | |
| R2 | 0.9838 | |
| Intraparticle Diffusion | kp (mg.g-1.min-0.5) | 2.1499 |
| C (mg.g-1) | 9.529 | |
| R2 | 0.6301 | |
| Interparticle Diffusion | kMD | 0.009 |
| R2 | 0.041 | |
| Elovich | β | 0.313 |
| α | 270.106 | |
| R2 | 0.4647 |
Adsorption thermodynamics
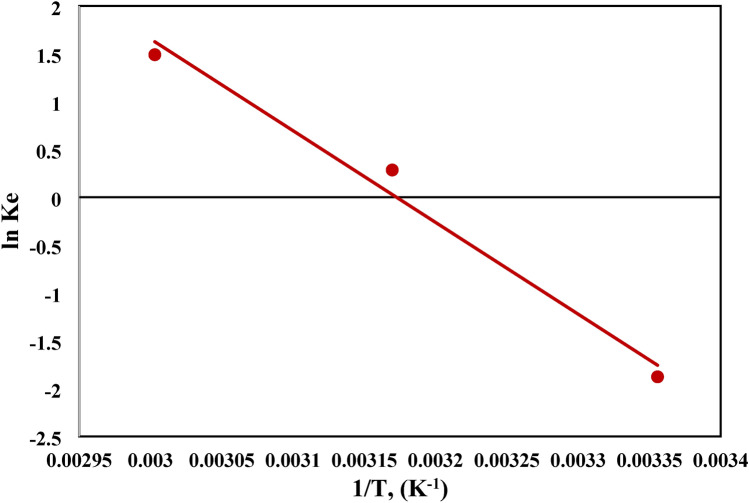
Thermodynamic parameters were assessed for the removal of MB dye by using NHGP synthesized adsorbent in the temperature range of 25 to 60 ℃. Figure 16 illustrates the calculations of the thermodynamics variables ΔS° and ΔH°. The Kd values were obtained from the adsorption data at different temperatures. The ΔG° values shown in Table 11 confirm that the adsorption process was spontaneous at the selected temperature range (25–60 ℃), which is in concurrence with the ΔS° value (ΔS > 0 for the spontaneous process). The calculated value of ΔH° (+ ve 79.9 kJ/mol) is implicit in the endothermic characteristics of the process.
Figure 16.
Linear Relationship of Van’t Hoff Equation at Different Adsorption Temperatures.
Table 11.
Thermodynamic Parameters of the removal of MB Dye by using NHGP.
| Temperature (K) | Kd | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) |
|---|---|---|---|---|
| 298 | 0.15 | 4651.18892 | 79.726 | 252.94 |
| 315.5 | 1.33 | − 748.0434725 | ||
| 333 | 4.44 | − 4126.969062 |
Moreover, the adsorption process may be classified as a chemical process or, a physical process, or may be operated by the two mechanisms simultaneously77. The ΔH value for van Edward force, hydrogen bond, ligand exchange, dipole interaction, and chemical bond is 4–10, 2– 40, ≈ 40, 2–29, and > 60 kJ⋅mol-1, respectively78. The ΔH value of the adsorption of MB onto synthesized NHGP was 79.7 kJ.mol-1, indicating the adsorption by a chemical bond.
The adsorption capacity of NHGP synthesized in the current study for methylene blue is compared with the adsorption capacity of different composite geopolymers as shown in Table 12. According to the data below, it can be indicated that the NHGP produced from fired brick waste (Homra) is a suitable sorbent for MB removal from economic and environmental perspectives.
Table 12.
Comparison between Different Geopolymer Adsorbents for MB Adsorption Uptake.
| Dye | Type of geopolymer composite | Adsorption capacity, mg/g | Refs. |
|---|---|---|---|
| Methylene Blue | CTAB-Cu2O/TiO2 Geopolymer composite | 20.11 | 79 |
| Fly ash geopolymer (FAG) | 37.04 | 80 | |
| Fly ash geopolymer | 15.4 | 81 | |
| Volcanic ash-metakaolin based geopolymer (GP0 and GP10 and GP30) | 13.9, 14 and 14.1 | 82 | |
| Metakaolin-based geopolymer (MKG) | 43.48 | 24 | |
| New graphitic carbon nitride/geopolymer | 170.9 | 41 | |
| Fly ash–based geopolymer spheres | 30.1 | 34 | |
| Magnetite /geopolymer composite MGP | 76.34 | 83 | |
| Pyrophyllite clay porous geopolymer | 64.1 | 84 | |
| Coal fly ash geopolymer | 50.7 | 85 | |
| Present study | 80.65 |
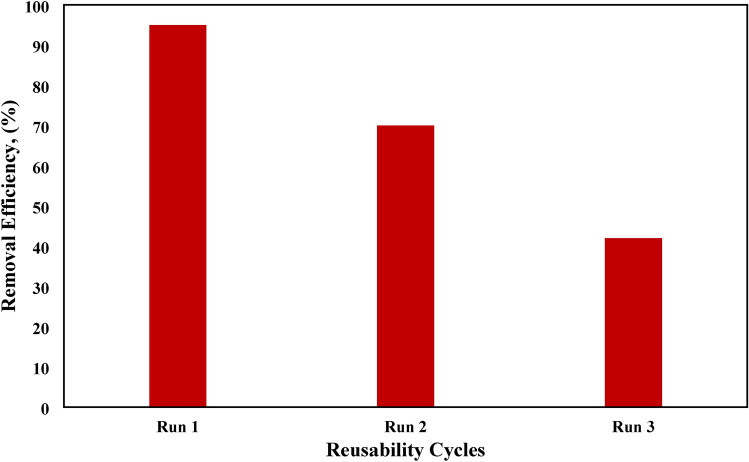
Desorption and reusability for MB adsorption
The commonly employed alcohol-washing method was first used for the regeneration of NHGP. During alcohol washing, the color of the solution turned from transparent to blue, indicating the desorption of MB from the spent adsorbent. After three times washing, the regenerated NHGP was used for the next adsorption run. As shown in Fig. 17, qe of MB decreased sharply in the next adsorption runs. The decreased amount of adsorption was attributed to residual MB on the surface of NHGP. As discussed in the “Effect of pH” section, although electrostatic repulsion existed between MB and the adsorbent surface at lower pH values, hydrophobic adsorption, and electron donor–acceptor (EDA) interaction could still lead to the adsorption of MB. In other words, the adsorbed MB could not completely desorb in the regeneration process, and also 20% of the adsorbent weight was lost during the regeneration. In addition, although repeated washing could enhance the desorption of MB, more spent washing alcohol and water would be produced, which needs further treatment. Thus, the regeneration method was not favorable from a practical viewpoint that decreased the removal efficiency from 95% at the first use to 70% at the second use and finally to 42%.
Figure 17.
Reusability of NHGP.
Conclusion
This work effectively synthesized a nano-geopolymer from waste-fired brick (Homra) as a novel solid particle adsorbent for wastewater purification, offering an unanticipated opportunity to control the geopolymer materials' structure for high-efficiency dye adsorption. The nano geopolymer was prepared using a 12M NaOH solution as an activator, with a 3:1 Na2SiO3/NaOH ratio. The nano-Homra geopolymer (NHGP) ideal SBET-specific surface area was 18.3658 m2/g, which offered enough channels for the mass transfer and adsorption processes. At the optimal conditions of 59 ℃ temperature, 254 mg/L initial concentration, and 163 min experiment duration, the novel nano-geopolymer adsorbent particles demonstrated high adsorption efficiency for MB dye, with a maximum adsorption capacity reaching 80.65 mg/g with a removal efficiency of 95.11%. Additionally, the test findings also demonstrated that the main external constraints affecting the entire adsorption process were the adsorbent concentration and experiment time. Additionally, the adsorbent's regeneration removal effectiveness was tested without causing a significant mass loss. The modified matrix structure of the nano-geopolymer was associated with the enhanced porosity and adsorption characteristics of the adsorbent. Because of their straightforward construction and practical treatment method, the resulting adsorbents were the predominant substitutes for more expensive or powder adsorbents. The novel nano-geopolymers have the potential to be used as adsorbent or carrier materials in various industrial applications.
Acknowledgements
The authors express their gratitude for the funding provided by the Science, Technology, and Innovation Funding Authority (STDF) in partnership with the Egyptian Knowledge Bank (EKB).
Author contributions
The idea for the study was contributed to by all authors. E.M. Abdel Hamid, H.M. Aly, and K. A.M. El Naggar handled the material preparation, data gathering, and analysis. E.M. Abdel Hamid and K.A.M. El Naggar wrote the first draft of the manuscript, and all authors provided feedback on earlier drafts. The final manuscript was read and approved by all authors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Açışlı, Ö., Acar, İ & Khataee, A. Preparation of a surface modified fly ash-based geopolymer for removal of an anionic dye: Parameters and adsorption mechanism. Chemosphere1(295), 133870. 10.1016/j.chemosphere.2022.133870 (2022). 10.1016/j.chemosphere.2022.133870 [DOI] [PubMed] [Google Scholar]
- 2.El Alouani, M. et al. Application of geopolymers for treatment of water contaminated with organic and inorganic pollutants: State-of-the-art review. J. Environ. Chem. Eng.9(2), 105095. 10.1016/j.jece.2021.105095 (2021). 10.1016/j.jece.2021.105095 [DOI] [Google Scholar]
- 3.Altaleb, H. A. et al. Efficient electrospun terpolymer nanofibers for the removal of cationic dyes from polluted waters: A non-linear isotherm and kinetic study. J. Environ. Chem. Eng.9(4), 105361. 10.1016/j.jece.2021.105361 (2021). 10.1016/j.jece.2021.105361 [DOI] [Google Scholar]
- 4.Guo, R. T. et al. Recent advances and perspectives of g–C3N4–based materials for photocatalytic dyes degradation. Chemosphere1(295), 133834. 10.1016/j.chemosphere.2022.133834 (2022). 10.1016/j.chemosphere.2022.133834 [DOI] [PubMed] [Google Scholar]
- 5.Peighambardoust, S. J., Boffito, D. C., Foroutan, R. & Ramavandi, B. Sono-photocatalytic activity of sea sediment@400/ZnO catalyst to remove cationic dyes from wastewater. J. Mol. Liq.367, 120478. 10.1016/J.MOLLIQ.2022.120478 (2022). 10.1016/J.MOLLIQ.2022.120478 [DOI] [Google Scholar]
- 6.Pooladi, H., Foroutan, R. & Esmaeili, H. Synthesis of wheat bran sawdust/Fe3O4 composite for the removal of methylene blue and methyl violet. Environ. Monitor. Assess.193, 1–7. 10.1007/s10661-021-09051-9 (2021). 10.1007/s10661-021-09051-9 [DOI] [PubMed] [Google Scholar]
- 7.Foroutan, R. et al. Development of a magnetic orange seed/Fe3O4 composite for the removal of methylene blue and crystal violet from aqueous media. Biomass Convers. Biorefinery10.1007/s13399-023-04692-x (2023). 10.1007/s13399-023-04692-x [DOI] [Google Scholar]
- 8.Khatooni, H., Peighambardoust, S. J., Foroutan, R., Mohammadi, R. & Ramavandi, B. Adsorption of methylene blue using sodium carboxymethyl cellulose-g-poly (acrylamide-co-methacrylic acid)/Cloisite 30B nanocomposite hydrogel. J. Polym. Environ.31(1), 297–311. 10.1007/s10924-022-02623-x (2023). 10.1007/s10924-022-02623-x [DOI] [Google Scholar]
- 9.Aouan, B. et al. Development and optimization of geopolymer adsorbent for water treatment: Application of mixture design approach. J. Environ. Manage.338, 117853. 10.1016/J.JENVMAN.2023.117853 (2023). 10.1016/J.JENVMAN.2023.117853 [DOI] [PubMed] [Google Scholar]
- 10.Shi, B., Li, G., Wang, D., Feng, C. & Tang, H. Removal of direct dyes by coagulation: The performance of preformed polymeric aluminum species. J. Hazard. Mater.143(1–2), 567–574. 10.1016/j.jhazmat.2006.09.076 (2007). 10.1016/j.jhazmat.2006.09.076 [DOI] [PubMed] [Google Scholar]
- 11.Sirés, I. & Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int.1(40), 212–229. 10.1016/j.envint.2011.07.012 (2012). 10.1016/j.envint.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad, R., Guo, J. & Kim, J. Structural characteristics of hazardous organic dyes and relationship between membrane fouling and organic removal efficiency in fluidized ceramic membrane reactor. J. Cleaner Product.20(232), 608–616. 10.1016/j.jclepro.2019.05.244 (2019). 10.1016/j.jclepro.2019.05.244 [DOI] [Google Scholar]
- 13.Maniyam, M. N., Yaacob, N. S., Azman, H. H., Ab Ghaffar, N. A. & Abdullah, H. Immobilized cells of Rhodococcus strain UCC 0004 as source of green biocatalyst for decolourization and biodegradation of methyl orange. Biocatal. Agricult. Biotechnol.1(16), 569–578. 10.1016/J.BCAB.2018.10.008 (2018). 10.1016/J.BCAB.2018.10.008 [DOI] [Google Scholar]
- 14.El Alouani, M., Alehyen, S., Achouri, M. E. & Taibi, M. Potential use of moroccan fly ash as low cost adsorbent for the removal of two anionic dyes (indigo carmine and acid orange). J. Mater. Environ. Sci.8, 3397–3409 (2017). [Google Scholar]
- 15.Bensalah, H., Younssi, S. A., Ouammou, M., Gurlo, A. & Bekheet, M. F. Azo dye adsorption on an industrial waste-transformed hydroxyapatite adsorbent: Kinetics, isotherms, mechanism and regeneration studies. J. Environ. Chem. Eng.8(3), 103807. 10.1016/j.jece.2020.103807 (2020). 10.1016/j.jece.2020.103807 [DOI] [Google Scholar]
- 16.Park, J. H., Hwang, S. W., Lee, S. L., Lee, J. H. & Seo, D. C. Sorption behavior of phosphate by fly ash discharged from biomass thermal power plant. Appl. Biol. Chem.64(1), 43. 10.1186/s13765-021-00614-5 (2021). 10.1186/s13765-021-00614-5 [DOI] [Google Scholar]
- 17.Chai, Y. et al. Hydroxyapatite reinforced inorganic-organic hybrid nanocomposite as high-performance adsorbents for bilirubin removal in vitro and in pig models. Bioact. Mater.6(12), 4772–4785. 10.1016/j.bioactmat.2021.05.017 (2021). 10.1016/j.bioactmat.2021.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, S. et al. Computation-guided descriptor for efficient zeolite catalysts screening in C4 alkylation process. Chem. Eng. Sci.21(241), 116726. 10.1016/j.ces.2021.116726 (2021). 10.1016/j.ces.2021.116726 [DOI] [Google Scholar]
- 19.Zhang, Z. Q., Yang, Y. X., Li, J. A., Zeng, R. C. & Guan, S. K. Advances in coatings on magnesium alloys for cardiovascular stents–a review. Bioact. Mater.6(12), 4729–4757. 10.1016/j.bioactmat.2021.04.044 (2021). 10.1016/j.bioactmat.2021.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Q. et al. Mussel inspired preparation of amine-functionalized Kaolin for effective removal of heavy metal ions. Mater. Chem. Phys.15(181), 116–125. 10.1016/j.matchemphys.2016.06.041 (2016). 10.1016/j.matchemphys.2016.06.041 [DOI] [Google Scholar]
- 21.Lin, Z. et al. Removal of Hg2+ with polypyrrole-functionalized Fe3O4/kaolin: synthesis, performance and optimization with response surface methodology. Nanomaterials10(7), 1370. 10.3390/nano10071370 (2020). 10.3390/nano10071370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roto, R., Yusran, Y. & Kuncaka, A. Magnetic adsorbent of Fe3O4@SiO2 core-shell nanoparticles modified with thiol group for chloroauric ion adsorption. Appl. Surf. Sci.377, 30–36. 10.1016/J.APSUSC.2016.03.099 (2016). 10.1016/J.APSUSC.2016.03.099 [DOI] [Google Scholar]
- 23.Ren, C. et al. Preparation of amino-functionalized CoFe2O4@ SiO2 magnetic nanocomposites for potential application in absorbing heavy metal ions. RSC Adv.6(76), 72479–72486. 10.1039/c6ra13304e (2016). 10.1039/c6ra13304e [DOI] [Google Scholar]
- 24.El Alouani, M., Alehyen, S., El Achouri, M. & Taibi, M. Preparation, characterization, and application of metakaolin-based geopolymer for removal of methylene blue from aqueous solution. J. Chem.10.1155/2019/4212901 (2019). 10.1155/2019/4212901 [DOI] [Google Scholar]
- 25.Minelli, M. et al. Characterization of novel geopolymer – Zeolite composites as solid adsorbents for CO2 capture. Chem. Eng. J.10.1016/j.cej.2018.02.050 (2018). 10.1016/j.cej.2018.02.050 [DOI] [Google Scholar]
- 26.Kaewmee, P., Song, M., Iwanami, M., Tsutsumi, H. & Takahashi, F. Porous and reusable potassium-activated geopolymer adsorbent with high compressive strength fabricated from coal fly ash wastes. J. Clean. Prod.10.1016/j.jclepro.2020.122617 (2020). 10.1016/j.jclepro.2020.122617 [DOI] [Google Scholar]
- 27.Siyal, A. A., Shamsuddin, M. R. & Low, A. Fly ash based geopolymer for the adsorption of cationic and nonionic surfactants from aqueous solution – A feasibility study. Mater. Lett.10.1016/j.matlet.2020.128758 (2021). 10.1016/j.matlet.2020.128758 [DOI] [Google Scholar]
- 28.Barbosa, T. R., Foletto, E. L., Dotto, G. L. & Jahn, S. L. Preparation of mesoporous geopolymer using metakaolin and rice husk ash as synthesis precursors and its use as potential adsorbent to remove organic dye from aqueous solutions. Ceram. Int.10.1016/j.ceramint.2017.09.193 (2018). 10.1016/j.ceramint.2017.09.193 [DOI] [Google Scholar]
- 29.Thakur, N. & Armstrong, D. W. Arsenic sequestration by iron oxide coated geopolymer microspheres. J. Clean. Prod.10.1016/j.jclepro.2021.125931 (2021).33349739 10.1016/j.jclepro.2021.125931 [DOI] [Google Scholar]
- 30.Ji, Z., Su, L. & Pei, Y. Characterization and adsorption performance of waste-based porous open-cell geopolymer with one-pot preparation. Ceram. Int.10.1016/j.ceramint.2021.01.062 (2021). 10.1016/j.ceramint.2021.01.062 [DOI] [Google Scholar]
- 31.Bouna, L. et al. Synthesis and characterization of mesoporous geopolymer based on Moroccan kaolinite rich clay. Appl. Clay Sci.10.1016/j.clay.2020.105764 (2020). 10.1016/j.clay.2020.105764 [DOI] [Google Scholar]
- 32.Luukkonen, T. et al. Simultaneous removal of Ni(II), As(III), and Sb(III) from spiked mine effluent with metakaolin and blast-furnace-slag geopolymers. J. Environ. Manage.10.1016/j.jenvman.2015.11.007 (2016). 10.1016/j.jenvman.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Al-Zboon, K., Al-Harahsheh, M. S. & Hani, F. B. Fly ash-based geopolymer for Pb removal from aqueous solution. J. Hazard. Mater.10.1016/j.jhazmat.2011.01.133 (2011). 10.1016/j.jhazmat.2011.01.133 [DOI] [PubMed] [Google Scholar]
- 34.Novais, R. M. et al. Synthesis of porous biomass fly ash-based geopolymer spheres for efficient removal of methylene blue from wastewaters. J. Clean. Prod.10.1016/j.jclepro.2018.09.265 (2019). 10.1016/j.jclepro.2018.09.265 [DOI] [Google Scholar]
- 35.Jin, H., Zhang, Y., Wang, Q., Chang, Q. & Li, C. Rapid removal of methylene blue and nickel ions and adsorption/desorption mechanism based on geopolymer adsorbent. Colloid Interface Sci. Commun.45, 100551. 10.1016/J.COLCOM.2021.100551 (2021). 10.1016/J.COLCOM.2021.100551 [DOI] [Google Scholar]
- 36.Li, C. J., Zhang, Y. J., Chen, H., He, P. Y. & Meng, Q. Development of porous and reusable geopolymer adsorbents for dye wastewater treatment. J. Clean. Prod.10.1016/j.jclepro.2022.131278 (2022).36160313 10.1016/j.jclepro.2022.131278 [DOI] [Google Scholar]
- 37.Hua, P. et al. Adsorption of acid green and procion red on a magnetic geopolymer based adsorbent: Experiments, characterization and theoretical treatment. Chem. Eng. J.10.1016/j.cej.2019.123113 (2020). 10.1016/j.cej.2019.123113 [DOI] [Google Scholar]
- 38.Ali, N. S., Khader, E. H., Abdulrahman, M. A., Salih, I. K. & Albayati, T. M. Removal of anionic azo dye from wastewater using Fe3O4 magnetic nanoparticles adsorbents in a batch system. Desalinat. Water Treat.1(317), 100033. 10.1016/J.DWT.2024.100033 (2024). 10.1016/J.DWT.2024.100033 [DOI] [Google Scholar]
- 39.Khader, E. H., Mohammed, T. J., Albayati, T. M., Saady, N. M. C. & Zendehboudi, S. Green nanocatalyst for the photocatalytic degradation of organic pollutants in petroleum refinery wastewater: Synthesis, characterization, and optimization. J. Mol. Struct.10.1016/j.molstruc.2024.137688 (2024). 10.1016/j.molstruc.2024.137688 [DOI] [Google Scholar]
- 40.Khader, E. H. et al. Evaluation of adsorption treatment method for removal of phenol and acetone from industrial wastewater. Desalin. Water Treat.10.1016/j.dwt.2024.100091 (2024). 10.1016/j.dwt.2024.100091 [DOI] [Google Scholar]
- 41.Kaya-Özkiper, K., Uzun, A. & Soyer-Uzun, S. Boosting methylene blue adsorption capacity of an industrial waste-based geopolymer by depositing graphitic carbon nitride onto its surface: Towards sustainable materials for wastewater treatment. Chem. Eng. Sci.284, 119398. 10.1016/J.CES.2023.119398 (2024). 10.1016/J.CES.2023.119398 [DOI] [Google Scholar]
- 42.Arokiasamy, P. et al. Metakaolin/sludge based geopolymer adsorbent on high removal efficiency of Cu2+. Case Stud. Constr. Mater.17, e01428. 10.1016/J.CSCM.2022.E01428 (2022). 10.1016/J.CSCM.2022.E01428 [DOI] [Google Scholar]
- 43.Mast, J., Verleysen, E., Hodoroaba, V. D. & Kaegi, R. Characterization of nanomaterials by transmission electron microscopy: Measurement procedures. Charact. Nanopart. Meas. Process. Nanoparticles10.1016/B978-0-12-814182-3.00004-3 (2020). 10.1016/B978-0-12-814182-3.00004-3 [DOI] [Google Scholar]
- 44.Aslani, A., Masoumi, H., Ghanadzadeh Gilani, H. & Ghaemi, A. Improving adsorption performance of l-ascorbic acid from aqueous solution using magnetic rice husk as an adsorbent: experimental and RSM modeling. Sci. Rep.13(1), 10860. 10.1038/s41598-023-38093-x (2023). 10.1038/s41598-023-38093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inyinbor, A. A., Adekola, F. A. & Olatunji, G. A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind.15, 14–27. 10.1016/J.WRI.2016.06.001 (2016). 10.1016/J.WRI.2016.06.001 [DOI] [Google Scholar]
- 46.Akar, S. T., Çolo, H., Sayin, F., Kara, I. & Akar, T. Parametric optimization of Cu (II) removal process by a metakaolin-based geopolymer: Batch and continuous process design. J. Cleaner Product.15(366), 132819. 10.1016/J.JCLEPRO.2022.132819 (2022). 10.1016/J.JCLEPRO.2022.132819 [DOI] [Google Scholar]
- 47.Tripathi, S. et al. Microalgae: An emerging source for mitigation of heavy metals and their potential implications for biodiesel production. Adv. Biofuels Appl. Technol. Environ. Sustain.10.1016/B978-0-08-102791-2.00004-0 (2019). 10.1016/B978-0-08-102791-2.00004-0 [DOI] [Google Scholar]
- 48.Inyinbor, A. A., Adekola, F. A. & Olatunji, G. A. Kinetics and isothermal modeling of liquid phase adsorption of rhodamine B onto urea modified Raphia hookerie epicarp. Appl. Water Sci.10.1007/s13201-016-0471-7 (2017). 10.1007/s13201-016-0471-7 [DOI] [Google Scholar]
- 49.Foroutan, R., Ahmadlouydarab, M., Ramavandi, B. & Mohammadi, R. Studying the physicochemical characteristics and metals adsorptive behavior of CMC-g-HAp/Fe3O4 nanobiocomposite. J. Environ. Chem. Eng.10.1016/j.jece.2018.09.030 (2018). 10.1016/j.jece.2018.09.030 [DOI] [Google Scholar]
- 50.Foroutan, R., Mohammadi, R., Peighambardoust, S. J., Jalali, S. & Ramavandi, B. Application of nano-silica particles generated from offshore white sandstone for cadmium ions elimination from aqueous media. Environ. Technol. Innov.19, 101031. 10.1016/J.ETI.2020.101031 (2020). 10.1016/J.ETI.2020.101031 [DOI] [Google Scholar]
- 51.Chen, Y. et al. Application studies of activated carbon derived from rice husks produced by chemical-thermal process - A review. Adv. Colloid Interface Sci.10.1016/j.cis.2011.01.006 (2011). 10.1016/j.cis.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 52.Foroutan, R., Mohammadi, R., Razeghi, J., Ahmadi, M. & Ramavandi, B. Amendment of Sargassum oligocystum bio-char with MnFe2O4 and lanthanum MOF obtained from PET waste for fluoride removal: A comparative study. Environ. Res.251, 118641. 10.1016/J.ENVRES.2024.118641 (2024). 10.1016/J.ENVRES.2024.118641 [DOI] [PubMed] [Google Scholar]
- 53.Wu, F. C., Tseng, R. L. & Juang, R. S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J.10.1016/j.cej.2009.04.042 (2009). 10.1016/j.cej.2009.04.042 [DOI] [Google Scholar]
- 54.Inda, N. I. et al. A kinetic study of copper (II) extraction using LIX84-I impregnated polymeric particles with different structures. Solvent Extract. Res. Dev.25(1), 23–36. 10.15261/serdj.25.23 (2018). 10.15261/serdj.25.23 [DOI] [Google Scholar]
- 55.Peighambardoust, S. J. et al. Effectiveness of polyacrylamide-g-gelatin/ACL/Mg–Fe LDH composite hydrogel as an eliminator of crystal violet dye. Environ. Res.10.1016/J.ENVRES.2024.119428 (2024). 10.1016/J.ENVRES.2024.119428 [DOI] [PubMed] [Google Scholar]
- 56.Largitte, L. & Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des.109, 495–504. 10.1016/J.CHERD.2016.02.006 (2016). 10.1016/J.CHERD.2016.02.006 [DOI] [Google Scholar]
- 57.Ouaddari, H. et al. Removal of Methylene Blue by adsorption onto natural and purified clays: Kinetic and thermodynamic study. Chem. Phys. Impact1(8), 100405. 10.1016/J.CHPHI.2023.100405 (2024). 10.1016/J.CHPHI.2023.100405 [DOI] [Google Scholar]
- 58.Teimouri, A., Esmaeili, H., Foroutan, R. & Ramavandi, B. Adsorptive performance of calcined Cardita bicolor for attenuating Hg(II) and As(III) from synthetic and real wastewaters. Korean J. Chem. Eng.10.1007/s11814-017-0311-y (2018). 10.1007/s11814-017-0311-y [DOI] [Google Scholar]
- 59.Nenadović, S. S. S. et al. Structural and chemical properties of geopolymer gels incorporated with neodymium and samarium. Gels10.3390/gels7040195 (2021). 10.3390/gels7040195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sing, K. S. W. et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem.10.1351/pac198557040603 (1985). 10.1351/pac198557040603 [DOI] [Google Scholar]
- 61.Dong, C. et al. Optimized preparation of gangue waste-based geopolymer adsorbent based on improved response surface methodology for Cd(II) removal from wastewater. Environ. Res.10.1016/j.envres.2023.115246 (2023). 10.1016/j.envres.2023.115246 [DOI] [PubMed] [Google Scholar]
- 62.Xiong, J., He, Y., Cui, X. & Liu, L. Preparation of amino-bagasse/metakaolin based geopolymer hybrid microsphere as a low-cost and effective adsorbent for Cu(II). J. Environ. Chem. Eng.11(5), 110954. 10.1016/J.JECE.2023.110954 (2023). 10.1016/J.JECE.2023.110954 [DOI] [Google Scholar]
- 63.Lu, M. et al. Potentiality of the porous geopolymer sphere in adsorption of Pb (II) from aqueous solutions: Behaviors and mechanisms. Ceram. Int.10.1016/j.ceramint.2022.09.040 (2023). 10.1016/j.ceramint.2022.09.040 [DOI] [Google Scholar]
- 64.Liu, X., Yan, Z., Wang, H. & Luo, Y. In situ synthesis of NaY Zeolite with coal-based kaolin. J. Natural Gas Chem.12(1), 63–70 (2003). [Google Scholar]
- 65.Fernández-Jiménez, A. & Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater.10.1016/j.micromeso.2005.05.057 (2005). 10.1016/j.micromeso.2005.05.057 [DOI] [Google Scholar]
- 66.Al-Ghouti, M. A. & Al-Absi, R. S. Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Sci. Rep.10.1038/s41598-020-72996-3 (2020). 10.1038/s41598-020-72996-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eltaweil, A. S., Elgarhy, G. S., El-Subruiti, G. M. & Omer, A. M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol.10.1016/j.ijbiomac.2020.03.122 (2020). 10.1016/j.ijbiomac.2020.03.122 [DOI] [PubMed] [Google Scholar]
- 68.Song, G. et al. Sorptive removal of methylene blue from water by magnetic multi-walled carbon nanotube composites. Environ. Sci. Pollut. Res.10.1007/s11356-021-13543-z (2021). 10.1007/s11356-021-13543-z [DOI] [PubMed] [Google Scholar]
- 69.Tao, E., Ma, D., Yang, S. & Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compounds15(832), 154833. 10.1016/j.jallcom.2020.154833 (2020). 10.1016/j.jallcom.2020.154833 [DOI] [Google Scholar]
- 70.Kah, M., Sigmund, G., Xiao, F. & Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res.10.1016/j.watres.2017.07.070 (2017). 10.1016/j.watres.2017.07.070 [DOI] [PubMed] [Google Scholar]
- 71.He, Q. et al. Adsorption of anionic azo dyes using lignite coke by one-step short-time pyrolysis. Fuel10.1016/j.fuel.2020.117140 (2020). 10.1016/j.fuel.2020.117140 [DOI] [Google Scholar]
- 72.Mbachu, C. A. et al. Green synthesis of iron oxide nanoparticles by Taguchi design of experiment method for effective adsorption of methylene blue and methyl orange from textile wastewater. Results Eng.1(19), 101198. 10.1016/j.rineng.2023.101198 (2023). 10.1016/j.rineng.2023.101198 [DOI] [Google Scholar]
- 73.Wang, H. et al. Facile synthesis of magnetic nitrogen-doped porous carbon from bimetallic metal-organic frameworks for efficient norfloxacin removal. Nanomater.10.3390/NANO8090664 (2018). 10.3390/NANO8090664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fito, J. et al. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from Rumex abyssinicus plant. Sci. Rep.10.1038/s41598-023-32341-w (2023). 10.1038/s41598-023-32341-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayawei, N., Ebelegi, A. N. & Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem.10.1155/2017/3039817 (2017). 10.1155/2017/3039817 [DOI] [Google Scholar]
- 76.Sahoo, T. R. & Prelot, B. Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. Nanomater. Detect. Remov. Wastewater Pollut.10.1016/B978-0-12-818489-9.00007-4 (2020). 10.1016/B978-0-12-818489-9.00007-4 [DOI] [Google Scholar]
- 77.J. Fu, Y. Li, C. Ye, and C. Lin, “Study on the adsoption kinetics and thermodynamics of DMF on macroporous adsorbents,” Huanjing Kexue Xuebao/Acta Sci. Circumstantiae, 2012.
- 78.Mokokwe, G. & Letshwenyo, M. W. Investigation of clay brick waste for the removal of copper, nickel and iron from aqueous solution: batch and fixed – bed column studies. Heliyon10.1016/j.heliyon.2022.e09963 (2022). 10.1016/j.heliyon.2022.e09963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falah, M., MacKenzie, K. J. D., Knibbe, R., Page, S. J. & Hanna, J. V. New composites of nanoparticle Cu (I) oxide and titania in a novel inorganic polymer (geopolymer) matrix for destruction of dyes and hazardous organic pollutants. J. Hazard. Mater.10.1016/j.jhazmat.2016.06.016 (2016). 10.1016/j.jhazmat.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 80.Alouani, M. E., Alehyen, S., Achouri, M. E. & Taibi, M. Removal of cationic dye-methylene blue-from aqueous solution by adsorption on fly ash-based geopolymer. J. Mater. Environ. Sci.9(1), 32–46. 10.26872/jmes.2018.9.1.5 (2018). 10.26872/jmes.2018.9.1.5 [DOI] [Google Scholar]
- 81.Novais, R. M., Ascensao, G., Tobaldi, D. M., Seabra, M. P. & Labrincha, J. A. Biomass fly ash geopolymer monoliths for effective methylene blue removal from wastewaters. J. Clean. Product.10(171), 783–794. 10.1016/j.jclepro.2017.10.078 (2018). 10.1016/j.jclepro.2017.10.078 [DOI] [Google Scholar]
- 82.Shikuku, V. O., Tome, S., Hermann, D. T., Tompsett, G. A. & Timko, M. T. Rapid adsorption of cationic methylene blue dye onto volcanic ash-metakaolin based geopolymers. Silicon10.1007/s12633-021-01637-9 (2022). 10.1007/s12633-021-01637-9 [DOI] [Google Scholar]
- 83.Al-husseiny, R. A. & Ebrahim, S. E. Effective removal of methylene blue from wastewater using magnetite/geopolymer composite: synthesis, characterization and column adsorption study. Inorg. Chem. Commun.10.1016/j.inoche.2022.109318 (2022). 10.1016/j.inoche.2022.109318 [DOI] [Google Scholar]
- 84.Ettahiri, Y. et al. Pyrophyllite clay-derived porous geopolymers for removal of methylene blue from aqueous solutions. Mater. Chem. Phys.296, 127281. 10.1016/J.MATCHEMPHYS.2022.127281 (2023). 10.1016/J.MATCHEMPHYS.2022.127281 [DOI] [Google Scholar]
- 85.Liu, Y. et al. A facile method for preparation of floatable and permeable fly ash-based geopolymer block. Mater. Lett.185, 370–373. 10.1016/J.MATLET.2016.09.044 (2016). 10.1016/J.MATLET.2016.09.044 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.