Abstract
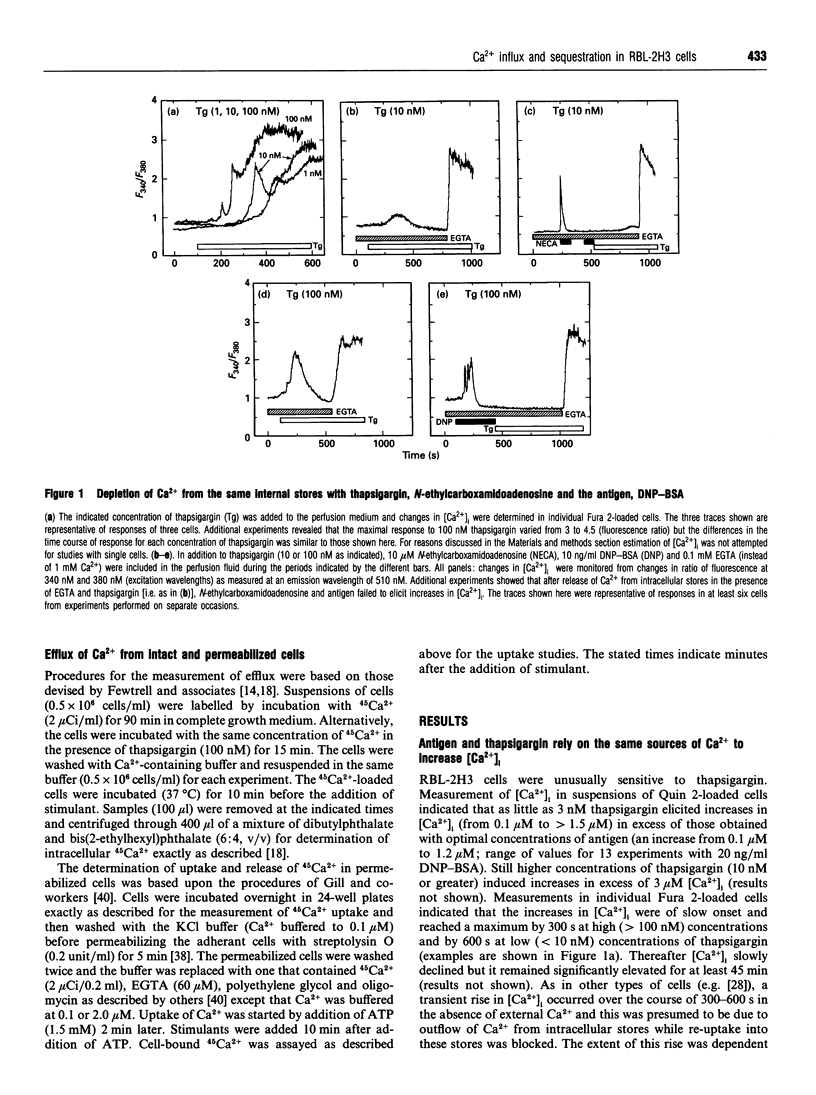
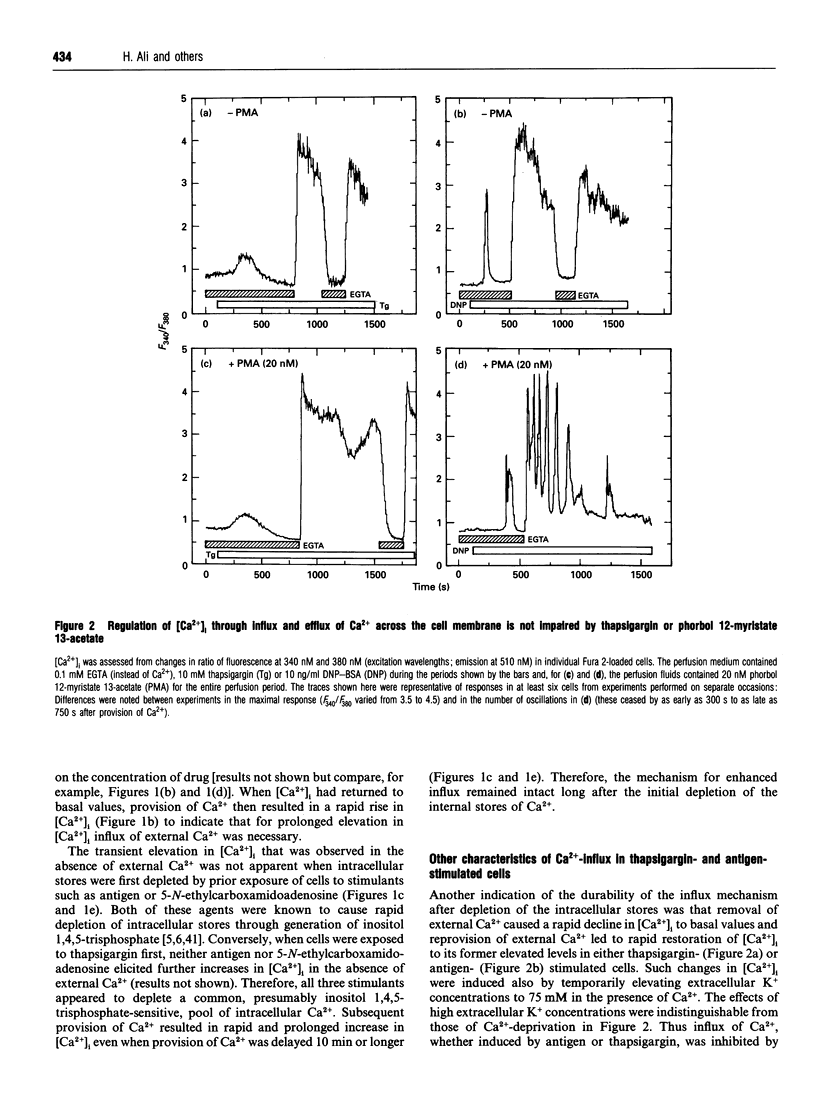
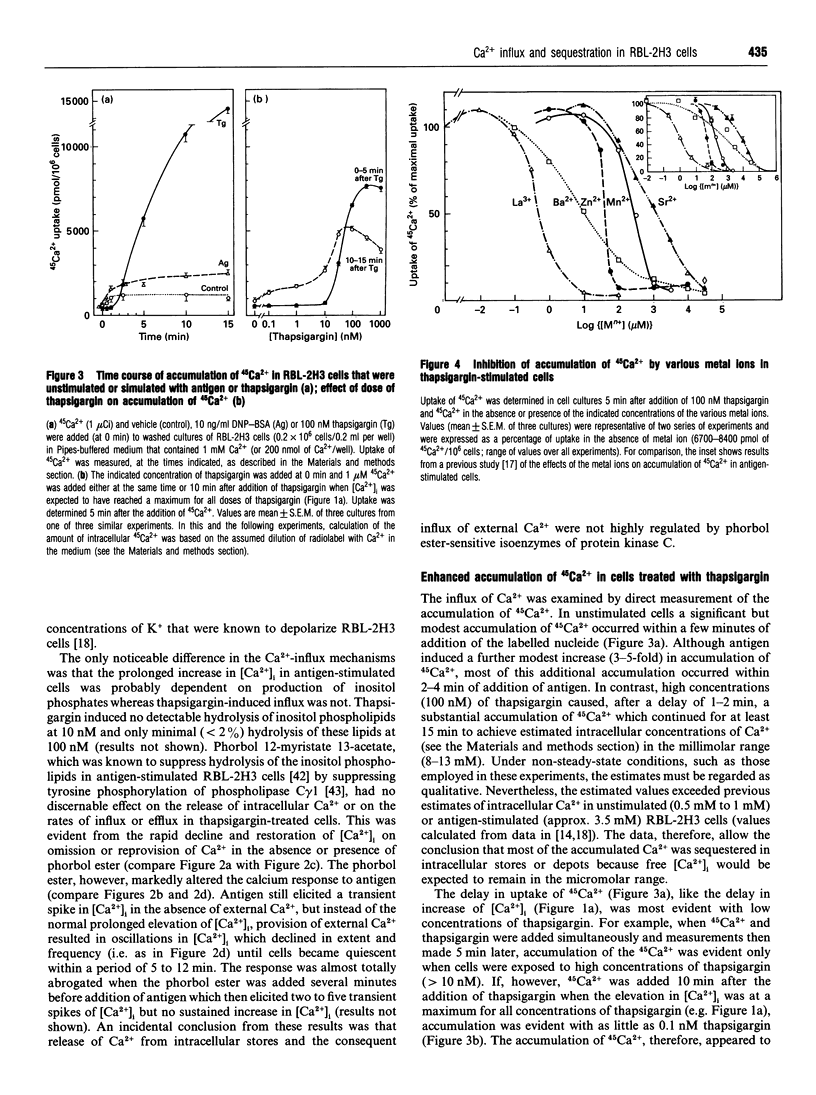
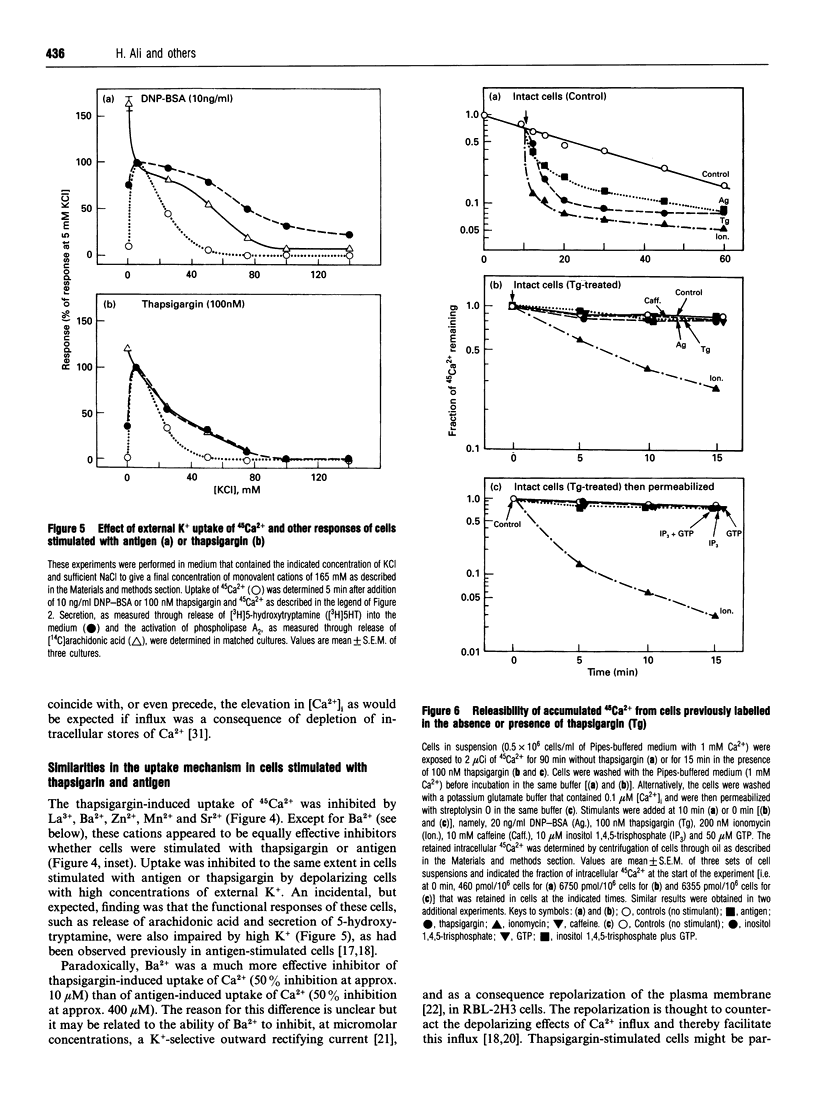
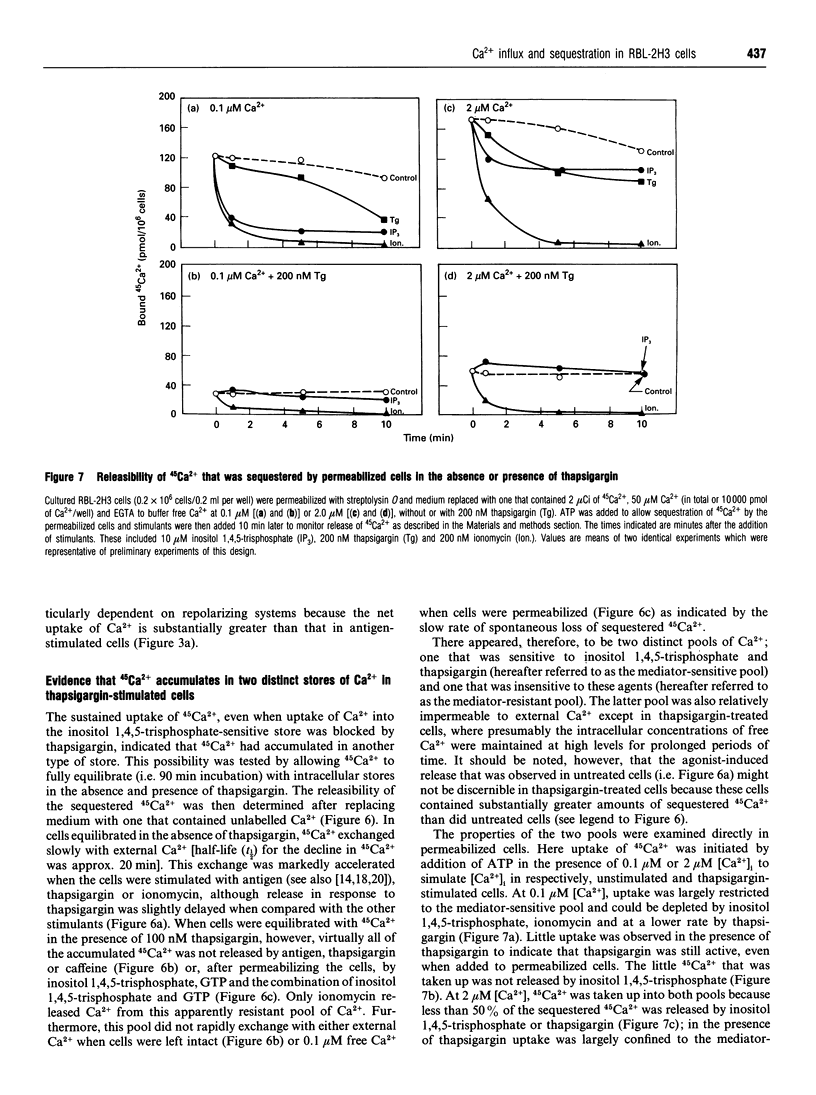
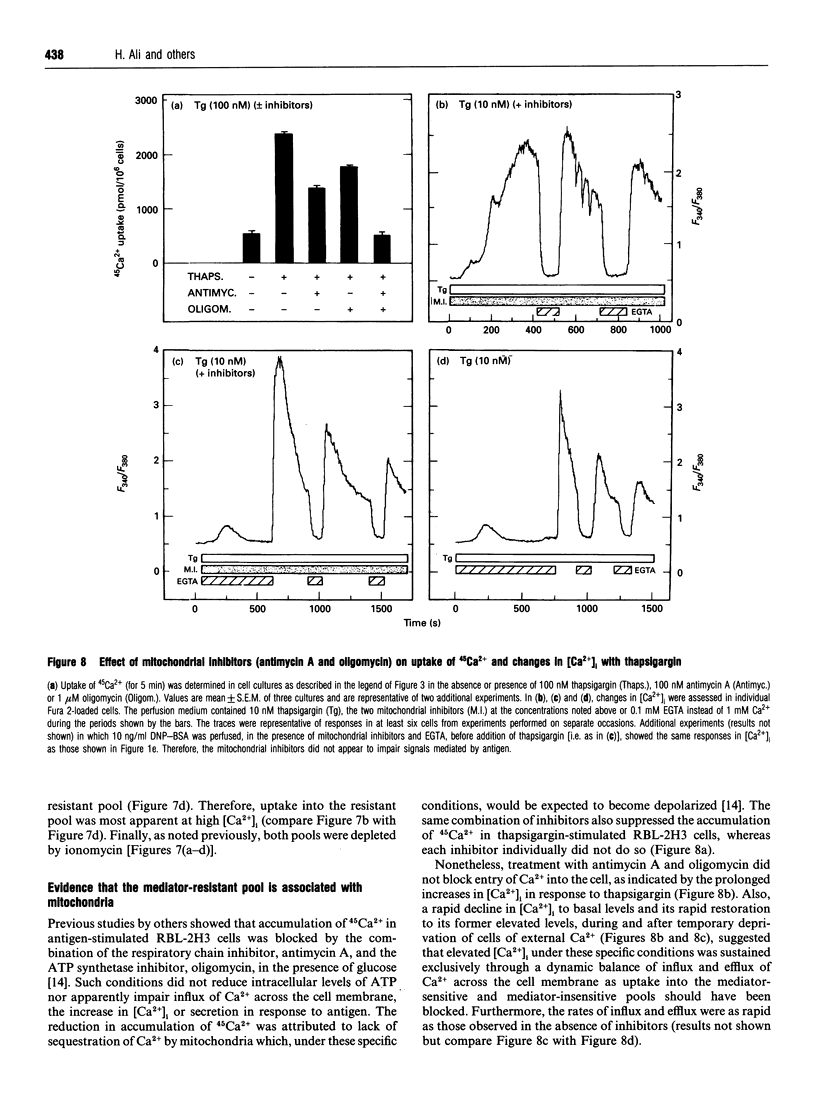
In single, Fura 2-loaded RBL-2H3 cells, antigen and thapsigargin depleted the same intracellular pool of Ca2+ in the absence of external Ca2+; provision of external Ca2+ induced immediate increases in levels of free Ca2+ ([Ca2+]i). These increases were dependent on the presence of external Ca2+ and, presumably, on influx of Ca2+ across the cell membrane. Both stimulants enhanced intracellular accumulation of 45Ca2+ through ostensibly similar mechanisms because accumulation was blocked to similar extents by various multivalent cations or by depolarization with K+. Because thapsigargin blocked reuptake of Ca2+ into inositol 1,4,5-trisphosphate sensitive stores, uptake occurred independently of the refilling of these stores. Uptake was dependent instead on sequestration of 45Ca2+ in a pool of high capacity that was insensitive to thapsigargin, caffeine, GTP and inositol 1,4,5-trisphosphate but sensitive to ionomycin and mitochondrial inhibitors. The existence of an inositol 1,4,5-trisphosphate-insensitive pool was also apparent in permeabilized cells; at 0.1 microM [Ca2+]i, uptake of 45Ca2+ was largely confined (> 80%) to the inositol 1,4,5-trisphosphate-sensitive pool, but at 2 microM [Ca2+]i uptake was largely (> 60%) into the inositol 1,4,5-trisphosphate-insensitive pool. Provision of mitochondrial inhibitors along with thapsigargin to block uptake into both pools, did not impair the thapsigargin-induced increase in [Ca2+]i or influx of Ca2+, as indicated by changes in Fura 2 fluorescence, but did block the intracellular accumulation of 45Ca2+. The studies illustrate the utility of simultaneous measurements of [Ca2+]i and 45Ca2+ uptake for a full accounting of Ca2+ homoeostasis as exemplified by the ability to distinguish between influx and mitochondrial uptake of Ca2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Beaven M. A. Receptor-mediated release of inositol 1,4,5-trisphosphate and inositol 1,4-bisphosphate in rat basophilic leukemia RBL-2H3 cells permeabilized with streptolysin O. Biochim Biophys Acta. 1989 Jan 17;1010(1):88–99. doi: 10.1016/0167-4889(89)90188-2. [DOI] [PubMed] [Google Scholar]
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Beaven M. A., Guthrie D. F., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Synergistic signals in the mechanism of antigen-induced exocytosis in 2H3 cells: evidence for an unidentified signal required for histamine release. J Cell Biol. 1987 Sep;105(3):1129–1136. doi: 10.1083/jcb.105.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993 May;14(5):222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Rogers J., Moore J. P., Hesketh T. R., Smith G. A., Metcalfe J. C. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7129–7136. [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Bird G. S., Putney J. W., Jr Inhibition of thapsigargin-induced calcium entry by microinjected guanine nucleotide analogues. Evidence for the involvement of a small G-protein in capacitative calcium entry. J Biol Chem. 1993 Oct 15;268(29):21486–21488. [PubMed] [Google Scholar]
- Brown G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992 May 15;284(Pt 1):1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Chandra S., Fewtrell C., Millard P. J., Sandison D. R., Webb W. W., Morrison G. H. Imaging of total intracellular calcium and calcium influx and efflux in individual resting and stimulated tumor mast cells using ion microscopy. J Biol Chem. 1994 May 27;269(21):15186–15194. [PubMed] [Google Scholar]
- Choi O. H., Lee J. H., Kassessinoff T., Cunha-Melo J. R., Jones S. V., Beaven M. A. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses ml muscarinic receptors. J Immunol. 1993 Nov 15;151(10):5586–5595. [PubMed] [Google Scholar]
- Cleveland P. L., Millard P. J., Showell H. J., Fewtrell C. M. Tenidap: a novel inhibitor of calcium influx in a mast cell line. Cell Calcium. 1993 Jan;14(1):1–16. doi: 10.1016/0143-4160(93)90013-v. [DOI] [PubMed] [Google Scholar]
- Cunha-Melo J. R., Dean N. M., Moyer J. D., Maeyama K., Beaven M. A. The kinetics of phosphoinositide hydrolysis in rat basophilic leukemia (RBL-2H3) cells varies with the type of IgE receptor cross-linking agent used. J Biol Chem. 1987 Aug 25;262(24):11455–11463. [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993 Oct 5;268(28):20737–20740. [PubMed] [Google Scholar]
- Fewtrell C., Sherman E. IgE receptor-activated calcium permeability pathway in rat basophilic leukemia cells: measurement of the unidirectional influx of calcium using quin2-buffered cells. Biochemistry. 1987 Nov 3;26(22):6995–7003. doi: 10.1021/bi00396a021. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ghosh T. K., Mullaney J. M. Calcium signalling mechanisms in endoplasmic reticulum activated by inositol 1,4,5-trisphosphate and GTP. Cell Calcium. 1989 Jul;10(5):363–374. doi: 10.1016/0143-4160(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Dehydrogenase activation by Ca2+ in cells and tissues. J Bioenerg Biomembr. 1991 Dec;23(6):823–854. doi: 10.1007/BF00786004. [DOI] [PubMed] [Google Scholar]
- Hide M., Beaven M. A. Calcium influx in a rat mast cell (RBL-2H3) line. Use of multivalent metal ions to define its characteristics and role in exocytosis. J Biol Chem. 1991 Aug 15;266(23):15221–15229. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jones S. V., Choi O. H., Beaven M. A. Carbachol induces secretion in a mast cell line (RBL-2H3) transfected with the ml muscarinic receptor gene. FEBS Lett. 1991 Sep 2;289(1):47–50. doi: 10.1016/0014-5793(91)80905-i. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Metzger H. Initial characterization of the calcium channel activated by the cross-linking of the receptors for immunoglobulin E. J Biol Chem. 1984 Aug 25;259(16):10188–10193. [PubMed] [Google Scholar]
- Kuno M., Okada T., Shibata T. A patch-clamp study: secretagogue-induced currents in rat peritoneal mast cells. Am J Physiol. 1989 Mar;256(3 Pt 1):C560–C568. doi: 10.1152/ajpcell.1989.256.3.C560. [DOI] [PubMed] [Google Scholar]
- Labrecque G. F., Holowka D., Baird B. Antigen-triggered membrane potential changes in IgE-sensitized rat basophilic leukemia cells: evidence for a repolarizing response that is important in the stimulation of cellular degranulation. J Immunol. 1989 Jan 1;142(1):236–243. [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M. A., Qian Y. X. Selective expression of potassium channels during mast cell differentiation. J Biol Chem. 1994 May 20;269(20):14813–14819. [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr, Drzewiecki G., Triggle D. Effects of smooth muscle calcium antagonists on human basophil histamine release. Biochem Pharmacol. 1981 Oct;30(20):2867–2869. doi: 10.1016/0006-2952(81)90428-7. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Gross D., Webb W. W., Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard P. J., Ryan T. A., Webb W. W., Fewtrell C. Immunoglobulin E receptor cross-linking induces oscillations in intracellular free ionized calcium in individual tumor mast cells. J Biol Chem. 1989 Nov 25;264(33):19730–19739. [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. The effect of mitochondrial inhibitors on calcium homeostasis in tumor mast cells. Am J Physiol. 1990 Feb;258(2 Pt 1):C217–C226. doi: 10.1152/ajpcell.1990.258.2.C217. [DOI] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. The relative contributions of extracellular and intracellular calcium to secretion from tumor mast cells. Multiple effects of the proton ionophore carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1987 Aug 5;262(22):10638–10643. [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Yamada K., Kazanietz M. G., Blumberg P. M., Beaven M. A. Different isozymes of protein kinase C mediate feedback inhibition of phospholipase C and stimulatory signals for exocytosis in rat RBL-2H3 cells. J Biol Chem. 1993 Feb 5;268(4):2280–2283. [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Pribluda V. S., Metzger H. Calcium-independent phosphoinositide breakdown in rat basophilic leukemia cells. Evidence for an early rise in inositol 1,4,5-trisphosphate which precedes the rise in other inositol phosphates and in cytoplasmic calcium. J Biol Chem. 1987 Aug 25;262(24):11449–11454. [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Excitement about calcium signaling in inexcitable cells. Science. 1993 Oct 29;262(5134):676–678. doi: 10.1126/science.8235587. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L., Beaven M. A., Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993 Aug 15;268(23):16887–16890. [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rink T. J. Receptor-mediated calcium entry. FEBS Lett. 1990 Aug 1;268(2):381–385. doi: 10.1016/0014-5793(90)81290-5. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Romanin C., Reinsprecht M., Pecht I., Schindler H. Immunologically activated chloride channels involved in degranulation of rat mucosal mast cells. EMBO J. 1991 Dec;10(12):3603–3608. doi: 10.1002/j.1460-2075.1991.tb04926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian R. P., McGivney A., Barsumian E. L., Crews F. T., Hirata F., Axelrod J. Variants of the rat basophilic leukemia cell line for the study of histamine release. Fed Proc. 1982 Jan;41(1):30–34. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Yamada K., Jelsema C. L., Beaven M. A. Certain inhibitors of protein serine/threonine kinases also inhibit tyrosine phosphorylation of phospholipase C gamma 1 and other proteins and reveal distinct roles for tyrosine kinase(s) and protein kinase C in stimulated, rat basophilic RBL-2H3 cells. J Immunol. 1992 Aug 1;149(3):1031–1037. [PubMed] [Google Scholar]


