Abstract
Genetic testing is recommended for all patients with pheochromocytomas and paragangliomas (PPGL) to establish genotype–phenotype associations. We investigated germline mutations in 59 patients with PPGL at six Korean university hospitals using next-generation sequencing (NGS) targeting 38 PPGL-associated genes, including those recommended by the Korean PPGL Task Force. Germline mutations were identified in 13 patients (22%), and affected four genes: RET, NF1, VHL, and SDHD. Germline mutations were significantly associated with a family history of PPGL, smaller tumor size, and the presence of other types of tumors. Using 95 Korean PPGL cases with germline mutations identified through a literature review and 13 cases from our cohort, we characterized genotype–phenotype correlations. Mutation hotspots were identified in specific codons of RET (codons 631 and 634), VHL (157 and 167), and SDHB (131 and 253). NF1 mutations varied, indicating the absence of common hotspots. These findings highlight the efficacy of the recommended NGS panel for Korean patients with PPGL and the importance of genetic testing in establishing clinical management and personalized therapeutic strategies.
Keywords: Genotype, Germline, Korean, Paraganglioma, Phenotype, Pheochromocytoma
Pheochromocytomas (PCCs) and paragangliomas (PGLs) (PPGL) are neuroendocrine tumors derived from chromaffin cells that demonstrate notable clinical heterogeneity [1]. While solid tumors have a low incidence of germline mutations [2], PPGL exhibits a high prevalence, with germline mutations detected in up to 40% of cases and somatic mutations in 20%–30% [3-5]. The variability in the mutation prevalence is influenced by variability in factors such as mutation complexity, geographic location, and the patient selection method used [6]. Additionally, genotype–phenotype correlations appear to be race- and genetic background-dependent [7], highlighting the significance of germline mutation analysis in Korean patients with PPGL. Identifying germline mutations in patients with PPGL is crucial for prognosis prediction and genetic counseling, as germline mutations increase the risk of multiple tumors, metastasis, and recurrence [8-10]. While studies on the Korean population have explored these associations, the limited case numbers call for further research to generalize the findings to a broader population [11-13].
This study was approved by the Institutional Review Board of the Catholic University, Seoul, Korea (IRB approval number: XC19TEDI0046), and written informed consent was obtained from all participants. We enrolled 60 unrelated Korean patients diagnosed with PPGL between January 1994 and June 2021 across six university hospitals (Supplemental Data Fig. S1). We assessed clinical characteristics, tumor features, and outcomes, including recurrence and metastasis, by reviewing medical records. Recurrence was defined as the reappearance of PPGL post-surgery, with an elevated catecholamine level [12], whereas metastasis was identified in non-chromaffin organs [13]. One patient was excluded because of an incomplete medical record. Genomic DNA was extracted from peripheral blood and subjected to next-generation sequencing (NGS) using a custom panel (Thermo Fisher Scientific, Waltham, MA, USA) targeting 38 susceptibility genes, including all 10 basic genes and five genes recommended by the Korean PPGL Task Force [7] (Supplemental Data Fig. S2). Genomic mutations in PPGL, categorized into pseudohypoxia and kinase signaling clusters according to The Cancer Genome Atlas (TCGA) [14], alongside various candidate genes, highlight the utility of the NGS panel for efficient genetic analysis.
A total of 59 unrelated patients with PPGL, predominantly women (67.8%), had a mean age of 52.5 yrs at diagnosis. The characteristics of the patients with PPGL are summarized in Supplemental Data Table S1. Germline mutations were identified in 13 patients (22%): four in RET and four in NF1, both associated with kinase signaling; four in VHL, a pseudohypoxic VHL/EPAS1-related gene; and one in SDHD, a pseudohypoxic tricarboxylic acid (TCA) cycle-related gene (Table 1). We found no actual deletions/duplications at the exon level while checking copy number variation using Integrative Genomics Viewer and Detection of Exon Copy Number (version 2). A genotype–phenotype analysis was performed to explore the clinical characteristics of the 13 Korean patients harboring PPGL germline mutations [mutation (+)] compared with those who lacked germline mutations [mutation (–), N=46; Table 2]. The mutation (+) group had a higher prevalence of a family history of PPGL and the concomitant presence of other tumor types (P<0.001, each), but their tumors had a significantly smaller diameter (P<0.001). We found no statistically significant differences between the groups in terms of sex, tumor type, recurrence rate, or biochemical status. Although not significant, the mutation (+) group tended to have been diagnosed at a younger age and had higher proportions of bilateral PCCs and multiple PPGL tumors.
Table 1. Germline mutations and clinical characteristics of patients with PPGL at six university hospitals.
| Cluster | Gene | No. case | Sex | Age at diagnosis (yrs) | Family history | Clinical diagnosis | Tumorlocation | Tumor size(max, cm) | Multiple tumors | Metastasis | Recurrence | Biochemical status | Othertumors | cDNA change | Amino acid change | ACMG interpretation | Consequence | ClinVar accession |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1. Pseudohypoxia (TCA cycle-related) | SDHD | 1 | F | 44 | Yes | PCC/PGL | Rt adrenal, carotid body | 2.0 | Mu | No | Yes | Nor | NM_003002.3(SDHD):c.119delT | p.Ile40ThrfsTer46 | P | Frameshift | Not reported | |
| Cluster 1. Pseudohypoxia (VHL/EPAS-1-related) | VHL | 2 | M | 16 | No | PCC | Both adrenal | 4.0 | Mu | No | No | Nor | NM_000551.3(VHL):c.208G>A | p.Glu70Lys | P | Missense | VCV000043598.16 | |
| VHL | 3 | F | 49 | No | PGL | Retroperitoneum | 3.5 | S | No | No | Nor | Lymphoma | NM_000551.3(VHL):c.242C>T | p.Pro81Leu | LP | Missense | VCV000036899.24 | |
| VHL | 4 | F | 55 | No | PCC | Lt adrenal | 7.5 | S | No | No | Nor | NM_000551.3(VHL):c.499C>T | p.Arg167Trp | P | Missense | VCV000002218.31 | ||
| VHL | 5 | M | 70 | Yes | PCC | Lt adrenal | 2.6 | Mu | Yes (bladder) | No | Nor | NM_000551.3(VHL):c.640T>A | p.*214Argext*14 | LP | Stop lost | VCV000801933.6 | ||
| Cluster 2. Kinase signaling | NF1 | 6 | M | 35 | No | PCC | Rt adrenal | 4.5 | S | No | No | Nor | NF1 | NM_001042492.2(NF1):c.6596del | p.Leu2199Ter | P | Nonsense | Not reported |
| NF1 | 7 | F | 56 | No | PCC | Lt adrenal | 1.6 | S | No | No | Nor | NF1 | NM_001042492.2(NF1):c.1748A>G | p.Lys583Arg | P | Missense | VCV000068306.21 | |
| NF1 | 8 | M | 66 | No | PCC | Rt adrenal | 4.3 | S | No | No | Adr/Nor | NF1 | NM_001042492.2(NF1):c.4800dup | p.Ala1601SerfsTer21 | P | Frameshift | Not reported | |
| NF1 | 9 | M | 40 | No | PCC | Lt adrenal | 6.0 | S | No | Yes | Adr/Nor | NF1 | NM_001042492.2(NF1):c.7869+1G>A | p? | P | Splice donor | VCV000480091.8 | |
| RET | 10 | F | 64 | Yes | PCC | Both adrenal | 4.6 | Mu | No | No | Adr/Nor | NM_020975.4(RET):c.1891G>T | p.Asp631Tyr | P | Missense | VCV000024914.9 | ||
| RET | 11 | F | 41 | No | PCC | Lt adrenal | 6.0 | S | No | Yes | Adr/Nor | MTC | NM_020975.4(RET):c.1902C>G | p.Cys634Trp | P | Missense | VCV000013918.19 | |
| RET | 12 | F | 52 | No | PCC | Lt adrenal | 6.5 | S | No | No | Adr/Nor | MTC | NM_020975.4(RET):c.1902C>G | p.Cys634Trp | P | Missense | VCV000013918.19 | |
| RET | 13 | F | 54 | Yes | PCC | Rt adrenal | 2.8 | S | No | No | Adr/Nor | MTC | NM_020975.4(RET):c.1902C>G | p.Cys634Trp | P | Missense | VCV000013918.19 |
Abbreviations: PPGL, pheochromocytoma and paraganglioma; F, female; M, male; PCC, pheochromocytoma; PGL, paraganglioma; Rt, right; Lt, left; S, single; Mu, multiple; Nor, noradrenergic; Adr, adrenergic; NF1, neurofibromatosis type 1; MTC, medullary thyroid carcinoma; P, pathogenic; LP, likely pathogenic
Table 2. Characteristics of patients according to the mutation status.
| Characteristic | Mutation-negative (N=46) | Mutation-positive (N=13) | P |
|---|---|---|---|
| Female sex, N (%) | 32 (69.6) | 5 (62.5)* | 0.588 |
| Age at diagnosis, year, median (IQR) | 54.5 (44–63) | 52 (41–60) | 0.146 |
| Family history | 0 (0) | 4 (30.8) | <0.001 |
| Type | 0.160 | ||
| PCC, N (%) | 41 (89.1) | 11 (84.6) | |
| PGL, N (%) | 5 (10.2) | 1 (7.7) | |
| PCC and PGL, N (%) | 0 (0) | 1 (7.7) | |
| Location | 0.058 | ||
| Adrenal, unilateral, N (%) | 40 (87.0) | 9 (69.2) | |
| Adrenal, bilateral, N (%) | 1 (2.3) | 2 (15.4) | |
| Adrenal, head, and neck, N (%) | 0 (0) | 1 (7.7) | |
| Head and neck, N (%) | 0 (0) | 1 (7.7) | |
| Other sites, N (%) | 5 (10.9) | 0 (0) | |
| Multiple tumors, N (%) | 5 (10.9) | 4 (30.8) | 0.081 |
| Tumor diameter, cm, median (IQR) | 4.3 (2.5–6.1) | 4.0 (2.7–6.0) | <0.001 |
| Metastasis, N (%) | 3 (6.5) | 1 (7.7) | 0.883 |
| Recurrence, N (%) | 4 (9.1) | 2 (15.4) | 0.315 |
| Biochemical status | 0.861 | ||
| Adrenergic, N (%) | 3 (6.5) | 0 (0) | |
| Noradrenergic, N (%) | 21 (45.7) | 7 (53.8) | |
| Adrenergic/noradrenergic, N (%) | 18 (39.1) | 6 (46.2) | |
| Silent, N (%) | 4 (8.7) | 0 (0) | |
| Presence of other tumors, N (%) | 3 (6.5) | 8 (61.5) | <0.001 |
*The proportion was based on eight parent samples with available gender information.
For all characteristics, clinical data were collected from available cases only.
Abbreviations: PPGL, pheochromocytoma and paraganglioma; PCC, pheochromocytoma; PGL, paraganglioma; IQR, interquartile range
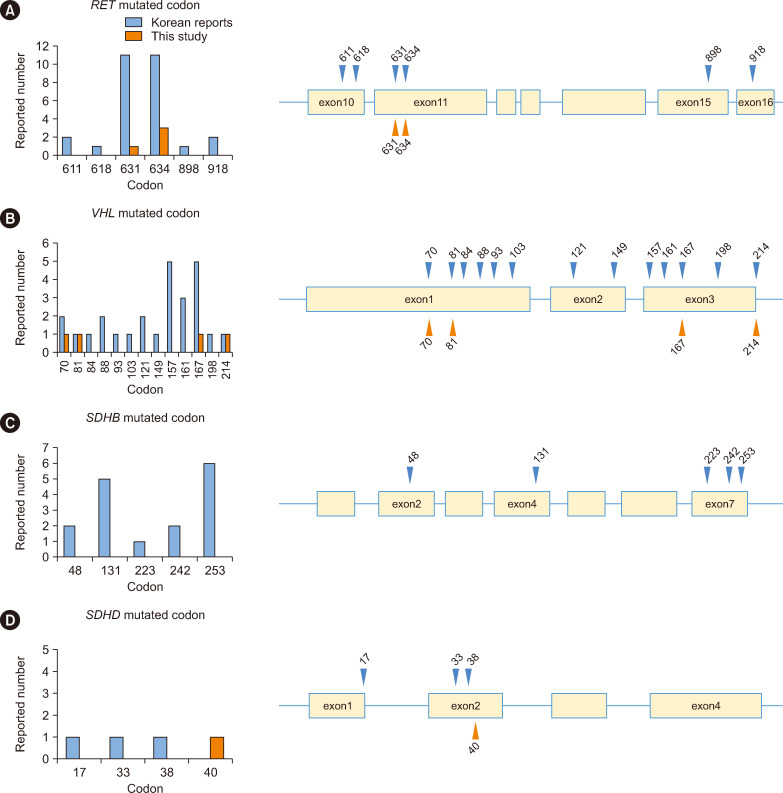
Next, we reviewed previous reports on Korean PPGL cases, and mutations of pathogenicity were reclassified according to the ACMG guidelines [15]. We combined potentially overlapping cases from each study’s institution into a single case to minimize the possibility of duplication bias in the statistics of mutation prevalence and associated clinical characteristics. In total, 95 Korean PPGL cases with germline mutations were collected, including 82 cases from 12 previous reports, as summarized in Supplemental Data Table S2, and 13 cases from the present study cohort (Supplemental Data Fig. S3). We compared the genotypic and phenotypic characteristics of the following four genes that were common in Korean patients with PPGL according to molecular clustering based on TCGA: SDHB (N=16), VHL (N=30), NF1 (N=8), and RET (N=31). Tumor type, tumor location, tumor diameter, metastasis, biochemical status, the presence of other tumors, and the consequences of the germline mutations significantly differed among the four groups (all, P<0.05) (Supplemental Data Table S3). Particularly, RET was the most frequently mutated at codons 631 and 634 of exon 11 (Fig. 1). VHL mutations were distributed widely across all exons; however, mutations typically occurred after codon 70. SDHB was frequently mutated at codons 131 and 253. The frequency of germline mutations in our cohort was 22%, which was significantly lower than the 40% previously reported. A review of Korean and international literature revealed a lower mutation positivity rate in Korean studies (5.6%–32.6%) [6, 11, 12], suggesting that the 40% frequency may be overestimated. Our finding of a 22% positivity rate aligns with those in global studies [16-18], challenging the accuracy of the previously reported 40% rate [3, 4]. Pathogenic mutations were found only in the genes recommended for Korean PPGL screening, aligning with the recommendations of the Korean PPGL Task Force [7]. Therefore, these findings support the suitability of the genes recommended by the Korean PPGL Task Force for genetic testing [8].
Fig. 1. Locations and frequencies of major causative gene mutations in Korean patients with PPGL) (N=95). Mutated codons and mutation frequencies in RET (A), VHL (B), SDHB (C), and SDHD (D). Blue triangles indicate sites of mutations reported previously, and orange triangles indicate sites of mutations detected in patients at six university hospitals in this study.
Abbreviation: PPGL, pheochromocytoma and paraganglioma.
In our cohort, mutation (+) patients had a family history of PPGL, smaller tumors at diagnosis, and a higher incidence of concurrent tumors. However, metastasis and recurrence rates were not significantly different from those of mutation (–) patients, potentially because of the limited sample size. PPGL germline mutation (+) cases were diagnosed earlier and often had a family history of PPGL and higher incidences of bilateral tumors, multiple tumors, metastases, and non-PPGL tumors, as reported in previous Korean and European studies [9, 12, 16, 17]. Previous studies on the Korean population [11, 12] indicated notable clinical differences=among TCGA-based molecular clusters [14]. Furthermore, based on the literature review, we focused on germline mutations in genes with high mutation frequencies, including RET, VHL, SDHB, and NF1, and their phenotypic associations. We found high PGL and metastasis rates in SDHB mutation (+) cases and significant family history rates in RET, VHL, and SDHB mutation (+) cases. Comparing Korean PPGL cases with SDHB, VHL, RET, and NF1 mutations with those in international studies revealed parallels and distinctions. Korean SDHB mutation (+) cases had a higher PGL frequency, mirroring trends in Asian Indians [18]. VHL and RET mutations in Koreans were associated with a higher incidence of bilateral PCCs, aligning with findings in previous Korean studies [11, 12]. However, SDHB and VHL mutations in Koreans exhibited a higher metastasis rate, differing from findings in Europeans [19] and Japanese [8]. Biochemically, pseudohypoxic cases with SDHB and VHL mutations were mainly noradrenergic or silent, whereas kinase signaling types with NF1 and RET mutations were predominantly adrenergic/noradrenergic. These results highlight mutations among molecular clusters and detailed gene-specific characteristics, indicating that even when genes within a molecular cluster exhibit similar characteristics, the features of individual genes may differ. Therefore, our findings highlight the importance of comparing molecular clusters when analyzing causative variants associated with PPGL, as well as the necessity for a detailed comparison at the level of individual genes.
Our findings regarding the mutation types and sites in patients with PPGL were largely consistent with the mutation sites previously identified in Korean studies [11, 12]. SDHB mutations predominantly involved frameshifts, whereas VHL mutations were mainly missense. RET mutations were exclusively missense, and NF1 mutations varied without specific hotspots. Notably, the occurrence of specific SDHB mutations (e.g., c.137G>A, c.470delT) was lower in Korean patients than in Japanese patients, suggesting racial differences. VHL missense mutations, particularly in exons 1 and 3, were the most frequent across Japanese, Asian Indian, and Korean studies [8, 11, 12, 18]. Korean RET mutations primarily occurred in exon 11, consistent with global findings. Our research on mutation sites at the exon level in Koreans offers a foundation for future genotype–phenotype analyses, highlighting the complexity and diversity of PPGL mutations within this population.
The limitations of this study include the reliance on targeted NGS, which does not identify large deletions or duplications despite their reported prevalence in SDHB-, SDHD-, and VHL-related cases. Future research may benefit from whole-exome or whole-genome sequencing for a more comprehensive genetic analysis. Despite being a multicenter effort using a 38-gene panel, the case number was limited, thereby highlighting the need for a broad review of Korean cases to establish genotype–phenotype correlations. To avoid missing or duplicate cases, potentially overlapping cases from each author’s institution were combined into one case to minimize the possibility of duplicate bias in the statistics.
In Korea, PPGL is rare, but its clinical features differ depending on the germline mutations involved. Therefore, genetic testing is imperative for patients with PPGL. The genotype–phenotype associations and frequently mutated sites in primary causative genes identified in this study will be useful for understanding the characteristics of PPGL in Korea.
ACKNOWLEDGEMENTS
We thank Seung Jae Lee of the Medical Library, The Catholic University of Korea, Seoul, Korea, for excellent support.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.3343/alm.2023.0376
Footnotes
AUTHOR CONTRIBUTIONS
Study conception or design: Lee S, Moon SD; data acquisition, analysis, or interpretation: Jo KH, Kim ES, Han JH, Jang YS, Yun JS, Son JW, Yoo SJ, Lee SH, Kwon HS, Moon SD, Lee S, Lee J, Yoo J, Kim HS, Kim M; drafting or revising the manuscript: Jo KH, Lee J, Lee S, Moon SD; final approval of the manuscript: Lee S, Moon SD. All authors have read and approved the final manuscript.
CONFLICTS OF INTEREST
None declared.
RESEARCH FUNDING
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT; 2019R1F1A1058741). The authors acknowledge the financial support of the Catholic Medical Center Research Foundation in the program year 2019.
References
- 1.Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–27. doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 2.Qing T, Mohsen H, Marczyk M, Ye Y, O'Meara T, Zhao H, et al. Germline variant burden in cancer genes correlates with age at diagnosis and somatic mutation burden. Nat Commun. 2020;11:2438. doi: 10.1038/s41467-020-16293-7.6465db3a292f40d4a8d14f8e7096e431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buffet A, Burnichon N, Amar L, Gimenez-Roqueplo AP. Pheochromocytoma: when to search a germline defect? Presse Med. 2018;47:e109–18. doi: 10.1016/j.lpm.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108–19. doi: 10.1038/nrc3648. [DOI] [PubMed] [Google Scholar]
- 5.Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, et al. NGS in PPGL (NGSnPPGL) Study Group, author. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13:233–47. doi: 10.1038/nrendo.2016.185. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Seong MW, Lee KE, Choi HJ, Ku EJ, Bae JH, et al. Germline mutations and genotype-phenotype correlations in patients with apparently sporadic pheochromocytoma/paraganglioma in Korea. Clin Genet. 2014;86:482–6. doi: 10.1111/cge.12304. [DOI] [PubMed] [Google Scholar]
- 7.Ku EJ, Kim KJ, Kim JH, Kim MK, Ahn CH, Lee KA, et al. Diagnosis for pheochromocytoma and paraganglioma: a joint position statement of the Korean pheochromocytoma and paraganglioma task force. Endocrinol Metab (Seoul) 2021;36:322–38. doi: 10.3803/EnM.2020.908.18a53aff05e747e7999e95b118af67b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonamine M, Wasano K, Aita Y, Sugasawa T, Takahashi K, Kawakami Y, et al. Prevalence of germline Variants in a Large Cohort of Japanese Patients with pheochromocytoma and/or paraganglioma. Cancers. 2021;13 doi: 10.3390/cancers13164014.666a21de9804443d9d93ce3b0d8cb293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–21. doi: 10.3410/f.1015808.198505. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Li M, Guan X, Yu A, Xiao Q, Wang C, et al. Clinical syndromes and genetic screening strategies of pheochromocytoma and paraganglioma. J Kidney Cancer VHL. 2018;5:14–22. doi: 10.15586/jkcvhl.2018.113.1567afa14d64456bb716dfe11f11d167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Kim MJ, Kong SH, Kim SJ, Kang H, Shin CS, et al. Characteristics of germline mutations in Korean patients with pheochromocytoma/paraganglioma. J Med Genet. 2022;59:56–64. doi: 10.1136/jmedgenet-2020-107102. [DOI] [PubMed] [Google Scholar]
- 12.Choi H, Kim KJ, Hong N, Shin S, Choi JR, Kang SW, et al. Genetic analysis and clinical characteristics of hereditary pheochromocytoma and paraganglioma syndrome in Korean population. Endocrinol Metab (Seoul) 2020;35:858–72. doi: 10.3803/EnM.2020.683.491bb741d3db4e64963bb492c13519d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo SH, Kim JH, Kim MJ, Cho SI, Kim SJ, Kang H, et al. Whole exome sequencing identifies novel genetic alterations in patients with pheochromocytoma/paraganglioma. Endocrinol Metab (Seoul) 2020;35:909–17. doi: 10.3803/EnM.2020.756.81ee7b51c73c40798a778e121f695152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38:489–515. doi: 10.1210/er.2017-00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 17.Cascón A, Pita G, Burnichon N, Landa I, López-Jiménez E, Montero-Conde C, et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab. 2009;94:1701–5. doi: 10.1210/jc.2008-2756. [DOI] [PubMed] [Google Scholar]
- 18.Pandit R, Khadilkar K, Sarathi V, Kasaliwal R, Goroshi M, Khare S, et al. Germline mutations and genotype-phenotype correlation in Asian Indian patients with pheochromocytoma and paraganglioma. Eur J Endocrinol. 2016;175:311–23. doi: 10.1530/EJE-16-0126. [DOI] [PubMed] [Google Scholar]
- 19.Currás-Freixes M, Piñeiro-Yañez E, Montero-Conde C, Apellániz-Ruiz M, Calsina B, Mancikova V, et al. PheoSeq: A targeted next-generation sequencing assay for pheochromocytoma and paraganglioma diagnostics. J Mol Diagn. 2017;19:575–88. doi: 10.1016/j.jmoldx.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.