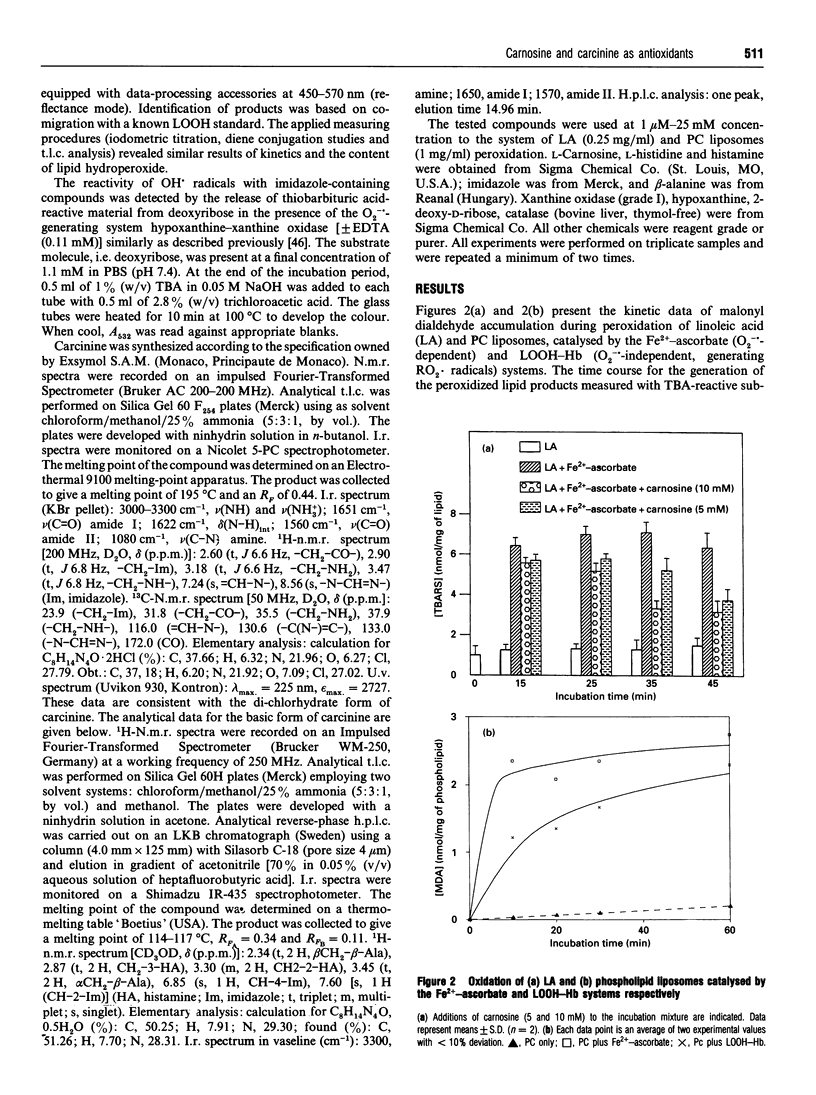
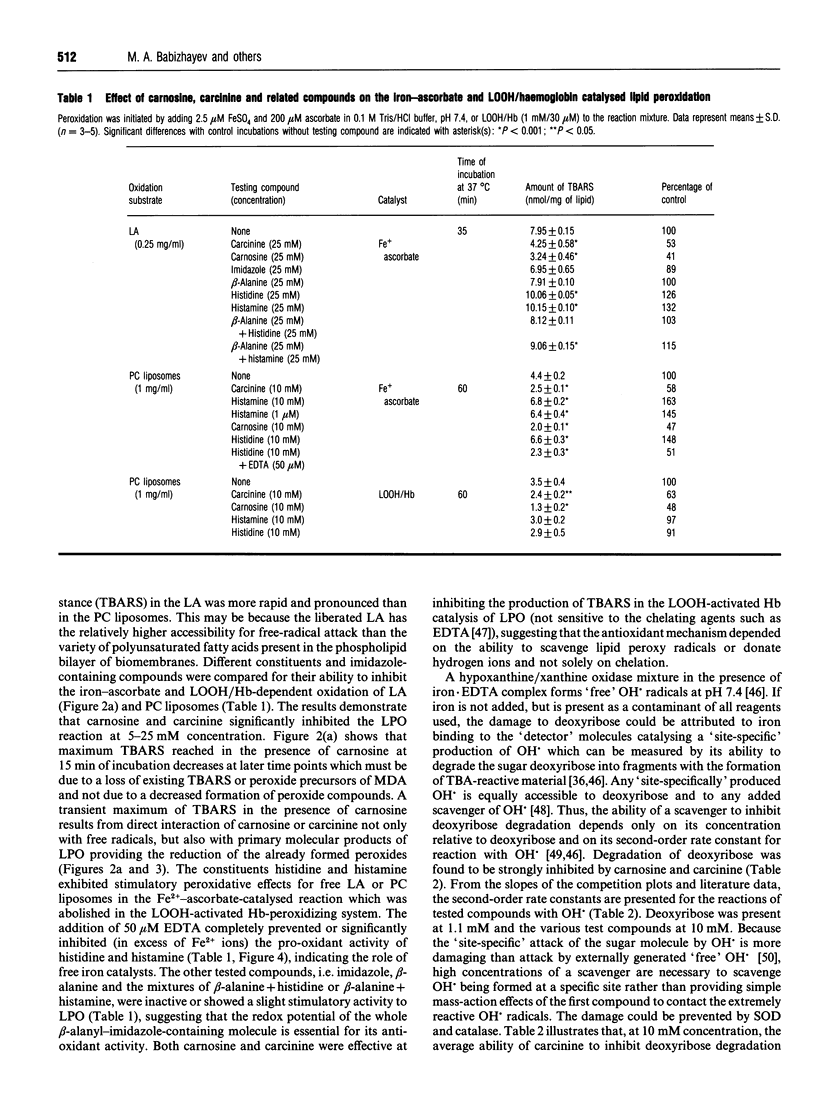
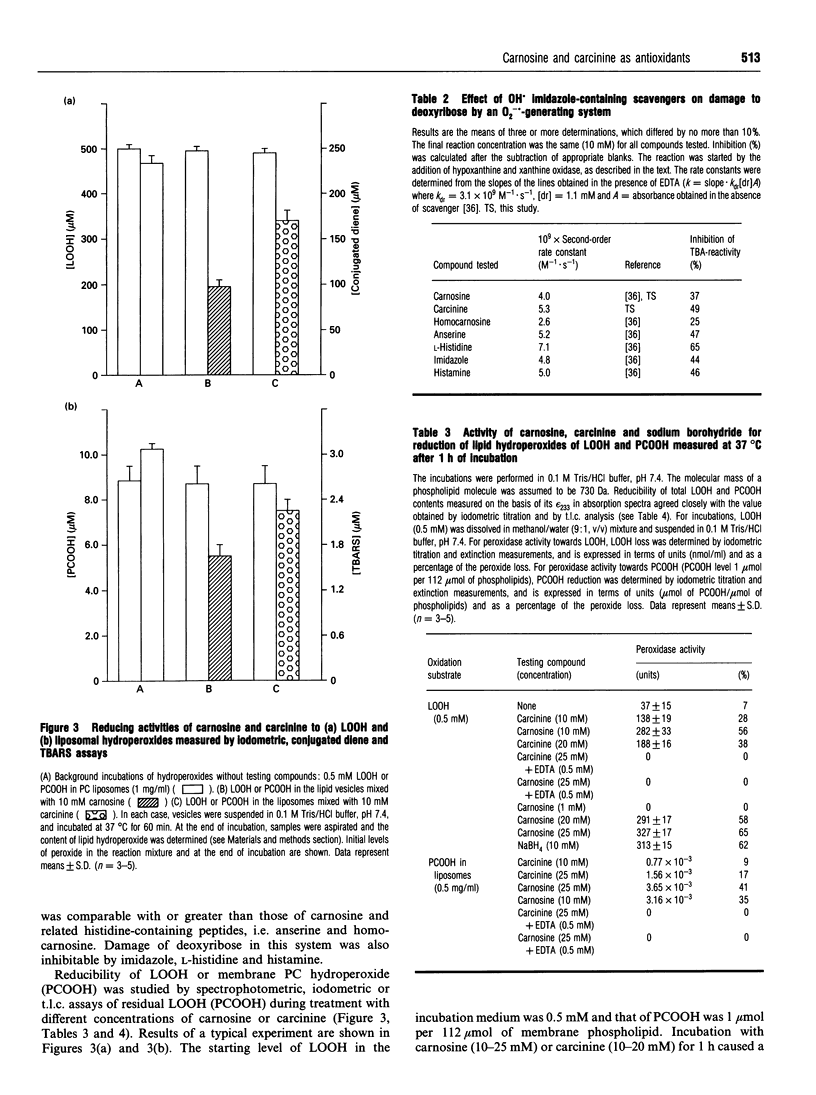
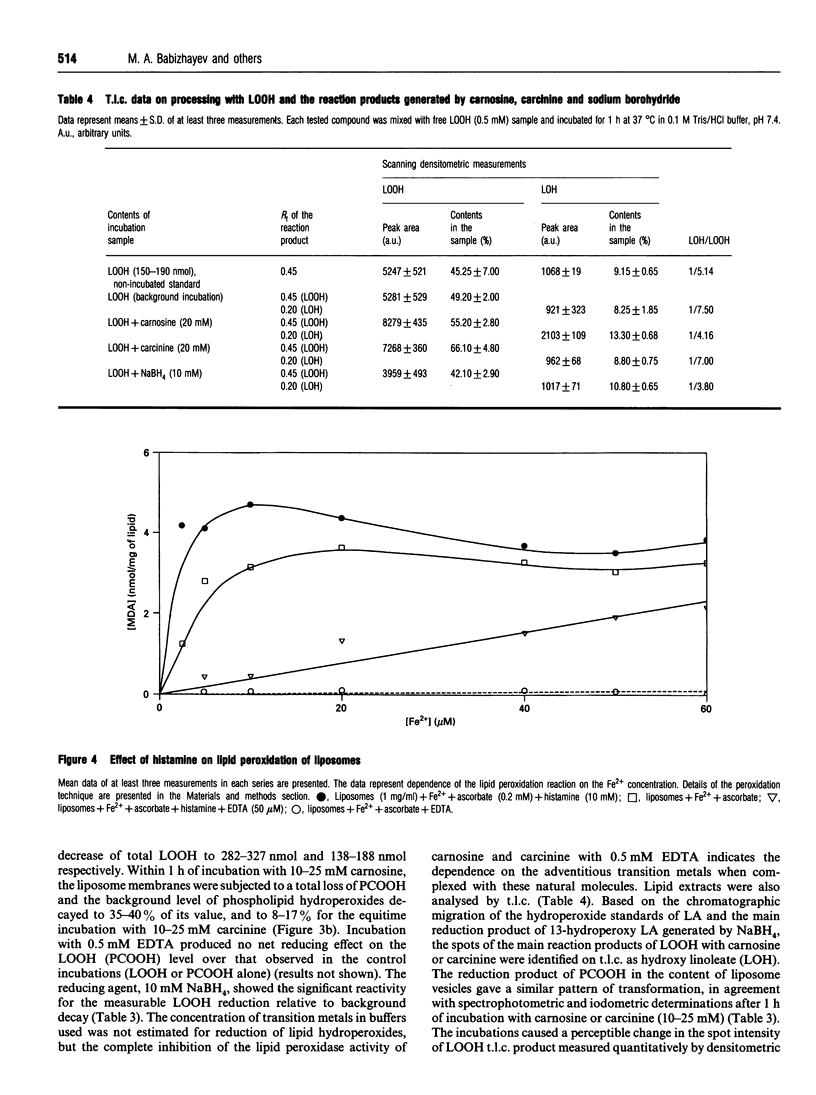
Abstract
Carnosine (beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) are natural imidazole-containing compounds found in the non-protein fraction of mammalian tissues. Carcinine was synthesized by an original procedure and characterized. Both carnosine and carcinine (10-25 mM) are capable of inhibiting the catalysis of linoleic acid and phosphatidylcholine liposomal peroxidation (LPO) by the O2(-.)-dependent iron-ascorbate and lipid-peroxyl-radical-generating linoleic acid 13-monohydroperoxide (LOOH)-activated haemoglobin systems, as measured by thiobarbituric-acid-reactive substance. Carcinine and carnosine are good scavengers of OH. radicals, as detected by iron-dependent radical damage to the sugar deoxyribose. This suggests that carnosine and carcinine are able to scavenge free radicals or donate hydrogen ions. The iodometric, conjugated diene and t.l.c. assessments of lipid hydroperoxides (13-monohydroperoxide linoleic acid and phosphatidylcholine hydroperoxide) showed their efficient reduction and deactivation by carnosine and carcinine (10-25 mM) in the liberated and bound-to-artificial-bilayer states. This suggests that the peroxidase activity exceeded that susceptible to direct reduction with glutathione peroxidase. Imidazole, solutions of beta-alanine, or their mixtures with peptide moieties did not show antioxidant potential. Free L-histidine and especially histamine stimulated iron (II) salt-dependent LPO. Due to the combination of weak metal chelating (abolished by EDTA), OH. and lipid peroxyl radicals scavenging, reducing activities to liberated fatty acid and phospholipid hydroperoxides, carnosine and carcinine appear to be physiological antioxidants able to efficiently protect the lipid phase of biological membranes and aqueous environments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Dobson G. P., Hoeger U., Parkhouse W. S. Role of histidine-related compounds to intracellular buffering in fish skeletal muscle. Am J Physiol. 1985 Oct;249(4 Pt 2):R449–R454. doi: 10.1152/ajpregu.1985.249.4.R449. [DOI] [PubMed] [Google Scholar]
- Arnould J. M., Frentz R. Mise en evidence, isolement et structure chimique d'une substance caracteristique du coeur de Carcinus maenas (L.): la beta-alanylhistamine. Comp Biochem Physiol C. 1975 Jan 1;50(1):59–66. [PubMed] [Google Scholar]
- Aruoma O. I., Laughton M. J., Halliwell B. Carnosine, homocarnosine and anserine: could they act as antioxidants in vivo? Biochem J. 1989 Dec 15;264(3):863–869. doi: 10.1042/bj2640863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev M. A. Antioxidant activity of L-carnosine, a natural histidine-containing dipeptide in crystalline lens. Biochim Biophys Acta. 1989 Aug 22;1004(3):363–371. doi: 10.1016/0005-2760(89)90085-4. [DOI] [PubMed] [Google Scholar]
- Babizhayev M. A., Costa E. B. Lipid peroxide and reactive oxygen species generating systems of the crystalline lens. Biochim Biophys Acta. 1994 Feb 22;1225(3):326–337. doi: 10.1016/0925-4439(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Babizhayev M. A., Deyev A. I. Lens opacity induced by lipid peroxidation products as a model of cataract associated with retinal disease. Biochim Biophys Acta. 1989 Jul 17;1004(1):124–133. doi: 10.1016/0005-2760(89)90222-1. [DOI] [PubMed] [Google Scholar]
- Babizhayev M. A., Deyev A. I., Linberg L. F. Lipid peroxidation as a possible cause of cataract. Mech Ageing Dev. 1988 Jul;44(1):69–89. doi: 10.1016/0047-6374(88)90080-2. [DOI] [PubMed] [Google Scholar]
- Babizhayev M. A. The biphasic effect of calcium on lipid peroxidation. Arch Biochem Biophys. 1988 Nov 1;266(2):446–451. doi: 10.1016/0003-9861(88)90276-7. [DOI] [PubMed] [Google Scholar]
- Boldyrev A. A., Dupin A. M., Batrukova M. A., Bavykina N. I., Korshunova G. A., Shvachkin YuP A comparative study of synthetic carnosine analogs as antioxidants. Comp Biochem Physiol B. 1989;94(2):237–240. doi: 10.1016/0305-0491(89)90339-8. [DOI] [PubMed] [Google Scholar]
- Boldyrev A. A., Dupin A. M., Bunin AYa, Babizhaev M. A., Severin S. E. The antioxidative properties of carnosine, a natural histidine containing dipeptide. Biochem Int. 1987 Dec;15(6):1105–1113. [PubMed] [Google Scholar]
- Boldyrev A. A., Dupin A. M., Pindel E. V., Severin S. E. Antioxidative properties of histidine-containing dipeptides from skeletal muscles of vertebrates. Comp Biochem Physiol B. 1988;89(2):245–250. doi: 10.1016/0305-0491(88)90218-0. [DOI] [PubMed] [Google Scholar]
- Boldyrev A. A., Severin S. E. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul. 1990;30:175–194. doi: 10.1016/0065-2571(90)90017-v. [DOI] [PubMed] [Google Scholar]
- Crush K. G. Carnosine and related substances in animal tissues. Comp Biochem Physiol. 1970 May 1;34(1):3–30. doi: 10.1016/0010-406x(70)90049-6. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Some prevalent biomolecules as defenses against singlet oxygen damage. Photochem Photobiol. 1988 Mar;47(3):357–362. doi: 10.1111/j.1751-1097.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Koller E., Slee R. G., Koster J. F. Possible involvement of the lipid-peroxidation product 4-hydroxynonenal in the formation of fluorescent chromolipids. Biochem J. 1986 Oct 15;239(2):405–409. doi: 10.1042/bj2390405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. W., Fisher H. Carnosine, histidine, and wound healing. Surgery. 1982 Jan;91(1):56–60. [PubMed] [Google Scholar]
- Flancbaum L., Brotman D. N., Fitzpatrick J. C., Van Es T., Kasziba E., Fisher H. Existence of carcinine, a histamine-related compound, in mammalian tissues. Life Sci. 1990;47(17):1587–1593. doi: 10.1016/0024-3205(90)90188-w. [DOI] [PubMed] [Google Scholar]
- Gercken G., Bischoff H., Trotz M. Myokardprotektion durch eine Carnosin-gepufferte kardioplegische Lösung. Arzneimittelforschung. 1980;30(12):2140–2143. [PubMed] [Google Scholar]
- Gille J. J., Pasman P., van Berkel C. G., Joenje H. Effect of antioxidants on hyperoxia-induced chromosomal breakage in Chinese hamster ovary cells: protection by carnosine. Mutagenesis. 1991 Jul;6(4):313–318. doi: 10.1093/mutage/6.4.313. [DOI] [PubMed] [Google Scholar]
- Grossmann A., Wendel A. Non-reactivity of the selenoenzyme glutathione peroxidase with enzymatically hydroperoxidized phospholipids. Eur J Biochem. 1983 Oct 3;135(3):549–552. doi: 10.1111/j.1432-1033.1983.tb07687.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochem J. 1984 Dec 15;224(3):761–767. doi: 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINSHAW L. B., JORDAN M. M., VICK J. A. Histamine release and endotoxin shock in the primate. J Clin Invest. 1961 Sep;40:1631–1637. doi: 10.1172/JCI104385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Aruoma O. I. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987 Aug 15;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Ault K. T. Scavenging of singlet molecular oxygen by imidazole compounds: high and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem Photobiol. 1990 Jan;51(1):59–66. doi: 10.1111/j.1751-1097.1990.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Hartman Z., Hartman P. E. Copper and cobalt complexes of carnosine and anserine: production of active oxygen species and its enhancement by 2-mercaptoimidazoles. Chem Biol Interact. 1992 Sep 28;84(2):153–168. doi: 10.1016/0009-2797(92)90076-w. [DOI] [PubMed] [Google Scholar]
- Hicks M., Gebicki J. M. A spectrophotometric method for the determination of lipid hydroperoxides. Anal Biochem. 1979 Nov 1;99(2):249–253. doi: 10.1016/s0003-2697(79)80003-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Shii D., Segami T., Kojima R., Suzuki Y. Preventive actions of N-(3-aminopropionyl)-L-histidinato zinc (Z-103) through increases in the activities of oxygen-derived free radical scavenging enzymes in the gastric mucosa on ethanol-induced gastric mucosal damage in rats. Jpn J Pharmacol. 1992 Jul;59(3):267–274. doi: 10.1254/jjp.59.267. [DOI] [PubMed] [Google Scholar]
- Jackson M. C., Kucera C. M., Lenney J. F. Purification and properties of human serum carnosinase. Clin Chim Acta. 1991 Feb 15;196(2-3):193–205. doi: 10.1016/0009-8981(91)90073-l. [DOI] [PubMed] [Google Scholar]
- Johnson P., Fedyna J. S., Schindzielorz A., Smith C. M., Kasvinsky P. J. Regulation of muscle phosphorylase activity by carnosine and anserine. Biochem Biophys Res Commun. 1982 Dec 15;109(3):769–775. doi: 10.1016/0006-291x(82)92006-x. [DOI] [PubMed] [Google Scholar]
- Johnson P., Hammer J. L. Effects of L-1-methyl-histidine and the muscle dipeptides carnosine and anserine on the activities of muscle calpains. Comp Biochem Physiol B. 1989;94(1):45–48. doi: 10.1016/0305-0491(89)90008-4. [DOI] [PubMed] [Google Scholar]
- KAHLSON G., NILSSON K., ROSENGREN E., ZEDERFELDT B. Wound healing as on rate of histamine formation. Lancet. 1960 Jul 30;2(7144):230–234. doi: 10.1016/s0140-6736(60)91426-4. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S. Initiation of membranal lipid peroxidation by activated metmyoglobin and methemoglobin. Arch Biochem Biophys. 1985 Mar;237(2):314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- Kohen R., Misgav R., Ginsburg I. The SOD like activity of copper:carnosine, copper:anserine and copper:homocarnosine complexes. Free Radic Res Commun. 1991;12-13 Pt 1:179–185. doi: 10.3109/10715769109145784. [DOI] [PubMed] [Google Scholar]
- Kohen R., Yamamoto Y., Cundy K. C., Ames B. N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A. 1988 May;85(9):3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova S. G., Russanova I. E., Russanov E. M. Do the copper complexes of histamine, histidine and of two H2-antagonists react with O2-? Free Radic Res Commun. 1991;12-13 Pt 1:215–220. doi: 10.3109/10715769109145789. [DOI] [PubMed] [Google Scholar]
- Kühn H., Schewe T., Rapoport S. M. The stereochemistry of the reactions of lipoxygenases and their metabolites. Proposed nomenclature of lipoxygenases and related enzymes. Adv Enzymol Relat Areas Mol Biol. 1986;58:273–311. doi: 10.1002/9780470123041.ch7. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Peppers S. C., Kucera-Orallo C. M., George R. P. Characterization of human tissue carnosinase. Biochem J. 1985 Jun 15;228(3):653–660. doi: 10.1042/bj2280653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane N., McMurray J., O'Dowd J. J., Dargie H. J., Miller D. J. Synergism of histidyl dipeptides as antioxidants. J Mol Cell Cardiol. 1991 Nov;23(11):1205–1207. doi: 10.1016/0022-2828(91)90077-y. [DOI] [PubMed] [Google Scholar]
- Margolis F. L. Carnosine in the primary olfactory pathway. Science. 1974 May 24;184(4139):909–911. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- O'Dowd J. J., Robins D. J., Miller D. J. Detection, characterisation, and quantification of carnosine and other histidyl derivatives in cardiac and skeletal muscle. Biochim Biophys Acta. 1988 Nov 17;967(2):241–249. doi: 10.1016/0304-4165(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Ohtsuka C., Takahashi H. Brain function estimated from the ratio of glutamine to homocarnosine levels in cerebrospinal fluid. Brain Dev. 1983;5(5):509–511. doi: 10.1016/s0387-7604(83)80084-9. [DOI] [PubMed] [Google Scholar]
- Parker C. J., Jr, Ring E. A comparative study of the effect of carnosine on myofibrillar-ATPase activity of vertebrate and invertebrate muscles. Comp Biochem Physiol. 1970 Dec 1;37(3):413–419. doi: 10.1016/0010-406x(70)90569-4. [DOI] [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Damage to proteins, enzymes, and amino acids by peroxidizing lipids. Arch Biochem Biophys. 1966 Jan;113(1):5–8. doi: 10.1016/0003-9861(66)90150-0. [DOI] [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Polymerization of proteins induced by free-radical lipid peroxidation. Arch Biochem Biophys. 1966 Jan;113(1):150–155. doi: 10.1016/0003-9861(66)90168-8. [DOI] [PubMed] [Google Scholar]
- Salim-Hanna M., Lissi E., Videla L. A. Free radical scavenging activity of carnosine. Free Radic Res Commun. 1991;14(4):263–270. doi: 10.3109/10715769109088955. [DOI] [PubMed] [Google Scholar]
- Schubert J., Watson J. A., Baecker J. M. Formation of a histidine-peroxide adduct by H2O2 or ionizing radiation on histidine: chemical and microbiological properties. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jan 17;14(6):577–583. doi: 10.1080/09553006914551771. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Muakkassah-Kelly S. F., Montestruque S. The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxides. Arch Biochem Biophys. 1983 Jun;223(2):441–452. doi: 10.1016/0003-9861(83)90608-2. [DOI] [PubMed] [Google Scholar]
- Shi X., Dalal N. S., Kasprzak K. S. Generation of free radicals from lipid hydroperoxides by Ni2+ in the presence of oligopeptides. Arch Biochem Biophys. 1992 Nov 15;299(1):154–162. doi: 10.1016/0003-9861(92)90257-w. [DOI] [PubMed] [Google Scholar]
- Spector A. The search for a solution to senile cataracts. Proctor lecture. Invest Ophthalmol Vis Sci. 1984 Feb;25(2):130–146. [PubMed] [Google Scholar]
- Thomas J. P., Girotti A. W. Photooxidation of cell membranes in the presence of hematoporphyrin derivative: reactivity of phospholipid and cholesterol hydroperoxides with glutathione peroxidase. Biochim Biophys Acta. 1988 Oct 14;962(3):297–307. doi: 10.1016/0005-2760(88)90259-7. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Girotti A. W. Reactivity of photochemically-generated lipid hydroperoxides in cell membranes with glutathione peroxidase. Photochem Photobiol. 1989 Feb;49(2):153–156. doi: 10.1111/j.1751-1097.1989.tb04089.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Maiorino M., Ursini F., Girotti A. W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990 Jan 5;265(1):454–461. [PubMed] [Google Scholar]
- Ursini F., Bindoli A. The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):255–276. doi: 10.1016/0009-3084(87)90053-3. [DOI] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Hutchison D. C., Baum H. The antioxidant action of human extracellular fluids. Effect of human serum and its protein components on the inactivation of alpha 1-antiproteinase by hypochlorous acid and by hydrogen peroxide. Biochem J. 1987 Apr 1;243(1):219–223. doi: 10.1042/bj2430219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Miki T., Orii Y., Takeshita M. Specific inhibition of redox-linked proton pump activity of cytochrome oxidase by oleate hydroperoxide and involvement of ferrocytochrome c in the catabolism of hydroperoxide. Biochem Int. 1990;21(1):33–40. [PubMed] [Google Scholar]
- Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim Biophys Acta. 1991 Nov 14;1115(1):15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]


