Abstract
Atmospheric cold plasma (ACP) presents a promising method for the sterilization of coconut milk and exhibits a modifying effect on coconut globulin (CG), the primary allergen in coconut milk. This study investigated the potential role of ACP treatment in mitigating the allergenic properties of coconut milk by examining changes in protein structure. ACP treatment induced structural alterations in CG, disrupting binding sites with immunoglobulin E (IgE). Consequently, this led to a reduction in the affinity between CG and IgE, evidenced by a decrease in Ka from 2.17 × 104/M to 0.64 × 104/M, thereby diminishing IgE-mediated allergic reactions. The findings from allergenic and cellular models further corroborated that ACP treatment decreased the allergenicity of CG by 55.18%, while inhibiting degranulation and the release of allergic mediators. This study presents an innovative methodology for producing hypoallergenic coconut milk, thereby expanding the applicability of ACP technology within the food industry.
Keywords: Spatial structure, Affinity, Binding capacity, Hydrophobic group, Allergenicity reduction
Graphical abstract

Highlights
-
•
ACP treatment led to the transformation of CG secondary and tertiary structure.
-
•
The change in CG structure induced by ACP led to a decrease in affinity with IgE.
-
•
ACP-treated CG showed a decrease in allergic reactions.
1. Introduction
The consumption of coconut (Cocos nucifera L.), a prominent tropical fruit, along with its derivatives such as coconut extract and coconut milk, is widely consumed in various regions as a staple component of daily diets. Being classified within the tree nut family, coconuts possess the potential to trigger severe allergic reactions, including cardiovascular and respiratory symptoms (Warren, Sehgal, Nimmagadda, & Gupta, 2023). Coconut is recognized as a significant food allergen by the USA Food and Drug Administration (FDA), with its primary allergenic components derived from coconut globulins (CG), specifically the 7S and 11S globulins (Polk, Dinakarpandian, Nanda, Barnes, & Dinakar, 2016). Notably, CG demonstrates a considerable degree of sequence homology with epitopes identified in globulin-type tree nut allergens, such as Car i 4, Ana o 2, and Cor a 9 (Jin et al., 2017). Although coconut allergies are not as prevalent as nut allergies like those to peanuts, approximately 0.4% of Americans experience severe allergic reactions to coconuts (Warren et al., 2023). It is conceivable that the allergenicity and allergic manifestations associated with coconut could potentially be mitigated by targeting allergenic epitopes in CG during processing, thereby expanding the market for coconut products.
Type I hypersensitivity, the most prevalent type of food allergy, is mediated by immunoglobulin E (IgE) and is frequently associated with the identification of allergenic epitopes by IgE, leading to allergic reactions (Zhang et al., 2024). Modifying the structure of allergens through processes such as heat treatment can result in decreased recognition by IgE, thereby reducing allergenicity (Pi et al., 2024). While heat treatment is a commonly utilized method for reducing allergenicity in food products, it can adversely affect sensory attributes, nutritional value, and overall quality.
In contrast, non-thermal technologies, including high pressure, pulsed electric fields, irradiation, and cold plasma, have shown promise as alternative approaches for mitigating food allergenicity with minimal impact on food composition and properties. High-pressure treatments, in particular, have been effective in diminishing the allergenic properties of proteins found in soy and peach by inducing modifications in their secondary and tertiary structure (Kang, Zhang, Yu, He, & Chen, 2023; Xi & He, 2018). However, these treatments may inadvertently expose allergenic epitopes within the proteins, thereby potentially enhancing their allergenicity. Conversely, pulsed electric field and irradiation treatments have shown limited success in mitigating the allergenicity of various foods, primarily due to their minimal impact on protein structures (Ekezie, Cheng, & Sun, 2018; Shah et al., 2019). In contrast, atmospheric cold plasma (ACP) technology has demonstrated superior efficacy relative to other non-thermal technologies, attributable to its enhanced penetration capabilities and rapid energy generation (Olatunde et al., 2023). The active constituents of ACP have exhibited the ability to cleave peptide bonds, oxidize amino acids, and disrupt the structural integrity of proteins, thereby reducing allergenicity (Olatunde et al., 2023). Numerous studies have explored the potential application of ACP in mitigating the allergenicity of various food products, including shrimp, peanuts, and milk (Cheng, Li, & Sun, 2024; Hsieh & Ting, 2024). However, these investigations have predominantly focused on changes in protein structure and sensitization levels, with relatively limited examination of the binding interactions between proteins and IgE, which are typically assessed through enzyme-linked immunosorbent assay (ELISA). A thorough investigation of the binding affinity and dynamic interactions between allergens and IgE following ACP treatment is essential for elucidating the mechanisms underlying allergenicity reduction.
Our prior research demonstrated ACP treatment was an effective method for enhancing the stability of coconut milk while exerting minimal impact on its quality (Chen, Chen, Fang, Pei, & Zhang, 2024). The objective of this work was to further examine the potential role of ACP treatment in diminishing the allergenicity of coconut milk, with a particular emphasis on assessing structural and allergenic modifications in CG. Therefore, this study aimed to broaden the application of ACP technology. Various analytical techniques, including group content analysis, circular dichroism (CD) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, fluorescence spectroscopy, dynamic light scattering, and transmission electron microscopy (TEM) were employed to examine the structural modifications of CG following ACP treatment. Additionally, this study utilized isothermal titration calorimetry (ITC), surface plasmon resonance (SPR), and quartz crystal microbalance with dissipation (QCM-D) to investigate changes in the binding affinity of IgE to CG. The KU812 cell model was utilized to validate alterations in CG-induced allergic reactions. The findings of this investigation may serve as a valuable resource for future research initiatives and the development of desensitized coconut-based products.
2. Materials and methods
2.1. Materials
Fresh coconuts were procured from local markets in Haikou, China, and promptly processed in the laboratory. Dinitrophenylhydrazine (DNPH), trichloroacetic acid, ethanol, ethyl acetate, guanidine hydrochloride, urea, and Ellman reagent were sourced from Sinopharm (Beijing, China). Phosphotungstic acid, β-mercaptoethanol, Tris, glycine, KBr, and H2SO4 were purchased from Aladdin Reagent (Shanghai, China). Tween-20 and bovine serum albumin (BSA) were obtained from Servicebio (Wuhan, China). Reagents for cell culture were acquired from Gibco (CA, USA).
2.2. Coconut milk preparation, treatments, and protein extracts
Following the methodology described by Chen et al. (2024), fresh coconut kernels were separated from the coconut after the removal of the brown shell. Subsequently, the coconut kernels were mixed with an equal mass of water, subjected to high-shear homogenization (IKA RET, Staufen, Germany) at 10000 rpm for 1 min, and then filtered to obtain coconut milk.
For the ACP treatment, 80 mL of fresh coconut milk was placed into a rectangular container lined with packaging film. The ACP system (BK-130, Phenix Technologies, USA), equipped with two aluminum electrodes, was utilized to treat coconut milk. The treated gap and discharge gap were set at 40 and 10 mm, respectively. The samples were exposed with a carrier gas of air for 30 s, with the ACP voltage adjusted to 50, 60, and 70 kV, respectively.
Post-treatment, the coconut milk was freeze-dried and subsequently pulverized into a powder. The powder was dispersed in a 10-fold volume n-hexane and mixed for 2 h, followed by filtration to eliminate the solvent. The powder was subjected to two repetitions of dispersion in a 10-fold NaCl solution (0.5 mol/L, w/v) for 4 h. Following this, the solution was centrifuged using a Hitachi CR22N centrifuge (Tokyo, Japan) at 10000 g for 20 min at 4 °C. The supernatant was then collected. Subsequently, dialysis was performed for 24 h using a 10 kDa membrane at 4 °C, and the CG was obtained through freeze-drying.
2.3. Determination of carbonyl, free -SH, and S—S bond contents
Prior to analysis, the CG powder was dispersed in a 10 mmol/L phosphate buffer solution (PBS, pH 6.0) at a concentration of 5 mg/mL. For the determination of carbonyl content, a 5 mL sample was mixed with an equal volume of DNPH reagent (10 mmol/L, 5 mL) and incubated at 25 °C for 2 h. Following the addition of 20% trichloroacetic acid, the precipitate was collected via centrifugation (Hitachi CR22N, Tokyo, Japan) at 12000 g for 5 min at 4 °C. Subsequently, the precipitate was washed with an ethanol/ethyl acetate mixture (5 mL/5 mL) and redissolved in 6 mol/L guanidine hydrochloride. The solution was then subjected to centrifugation (Xiangyi TGL-16, Changsha, China) at 1000 g for 3 min at 4 °C, and the supernatant was collected. Absorbance at 370 nm was measured using a spectrophotometer (Puxi General Instrument, Beijing, China). The molar extinction coefficient used for calculations was 22,000 M−1 cm−1.
The quantification of free sulfhydryl (-SH) and disulfide (S—S) bonds was conducted using Ellman's method, as described by Gantumur et al. (2023). The CG solution was diluted to a concentration of 2 mg/mL and subsequently mixed with Ellman's reagent in a volumetric ratio of 100:1. The reaction mixture was incubated in the dark for 1 h. The free -SH content was measured using a spectrophotometer (Puxi General Instrument, Beijing, China) at a wavelength of 412 nm, employing a molar extinction coefficient of 13,600 mol−1 cm−1.
For the determination of S—S bond content, the samples were treated with a solution of urea and guanidine hydrochloride (both at 6 mol/L) containing (2%, v/v) β-mercaptoethanol. The solution was maintained at 25 °C for 1 h, followed by mixing with 12% trichloroacetic acid for 1 h. Precipitates were separated via centrifugation (Hitachi CR22N, Tokyo, Japan) at 10000 g for 10 min. The precipitates were then redissolved in Tris-glycine buffer and Ellman reagent was subsequently added. The results were determined using a spectrophotometer (Puxi General Instrument, Beijing, China) at 412 nm, according to the following equation:
| (1) |
where C1 represents the total -SH content and C2 represents the free -SH content.
2.4. Circular dichroism spectroscopy
The samples were diluted to achieve a protein concentration of 0.1 mg/mL. CD spectra were acquired using a Bio-Logic MOS-500 spectrometer (Isere, France), with the scanning range set between 190 and 250 nm. A protein-free sample served as the background to establish the baseline.
2.5. Fourier transform infrared spectroscopy
Freeze-dried CG powder was combined with KBr in a 1:100 weight ratio and compressed into thin tablets. Data collection from 4000 to 400 cm−1 was performed using a Nicolet iS50 spectrometer (Thermo Fisher Scientific, MA, USA). Each spectrum represented the cumulative result of 32 scans, with a resolution of 2 cm−1.
2.6. Intrinsic fluorescence spectroscopy
Prior to measurement, the samples were dissolved in PBS to achieve a concentration of 1 mg/mL. Fluorescence spectra of the samples were acquired using an F4700 fluorescence photometer (Hitachi, Tokyo, Japan) within the wavelength range of 300 to 450 nm.
2.7. Determination of particle size distribution, polydispersity index (PDI), and zeta potential
Particle size distribution, polydispersity index (PDI), and zeta potential were analyzed using a Nano ZS90 instrument (Malvern, Worcestershire, UK). All tests were conducted at 25 °C with a sample concentration of 1 mg/mL. The assays were equilibrated for 120 s, and data from three independent tests assays were collected.
2.8. Transmission electron microscopy
TEM images were obtained using a Talos F200X G2 field emission TEM (Thermo Fisher Scientific, MA, USA). A CG solution (1 mg/mL, 10 μL) was applied to a carbon support film on a copper grid and subsequently stained with 3% phosphotungstic acid for 1 min prior to observation. The carbon support film was thoroughly dried and then loaded into the TEM system.
2.9. Human sera from coconut allergy patients
Sera pooled from five individuals with coconut allergy, obtained from Plasmalab International Co., Ltd. (Everett, WA, USA), were used for potential allergenicity assessment, as detailed in Table S1. The coconut-specific IgE levels ranged from 8.75 to 12.2 kU/L.
2.10. Isothermal titration calorimetry
ITC analysis of CG in combination with IgE was conducted following the methodology of Yi et al. (2023) with some modifications. To achieve the required IgE concentration for ITC testing, total protein was extracted from the sera utilizing the Active Protein Extraction Reagent Kit (Beyotime, Shanghai, China). Subsequently, the IgE concentration was quantified using an ELISA kit (X-Y Biotechnology, Shanghai, China), and the sample was diluted to an IgE concentration of 1 μM. The molecular weight of CG was estimated based on our previous research (Chen et al., 2023a) and diluted to 20 μM with PBS. The tests were conducted using a Nano-ITC instrument (TA Instruments, New Castle, USA). The experimental procedure involved administering 30 injections of 1 μL of CG solution into 300 μL of IgE solution at 180 s intervals at 25 °C. prior to data analysis, the heat of dilution was subtracted. The data were subsequently analyzed using NanoAnalyze Data Analysis software (TA Instruments, New Castle, USA).
2.11. Surface plasmon resonance spectroscopy
SPR analysis was conducted on a Biacore X100 system (Cytiva Lifesciences, Marlborough, USA) to investigate the binding ability between CG and IgE at 25 °C. In this analysis, allergic sera were diluted 10-fold with PBS and injected into a CM5 chip, which was pre-conditioned for 20 min at a flow rate of 1 μL/min. Subsequently, CG solutions of varying concentrations (1–16 nM) were introduced onto the immobilized sensor chip at a flow rate of 5 μL/min. The binding reaction was monitored over time.
2.12. Quartz crystal microbalance with dissipation analysis
The interaction between CG and IgE was further analyzed using a Q-Sense analyzer QCM-D (Biolin, Vasteras, Sweden) at 25 °C. prior to the experiment, the gold-plated chip underwent a cleaning process at 70 °C for 30 min and was subsequently dried under a stream of nitrogen. A five-step protocol was employed to monitor real-time dissipation and frequency variations at a flow rate of 50 μL/min: (A) baseline establishment using PBS; (B) introduction of CG solution over the chip surface; (C) rinsing with PBS; (D) exposure of the chip surface to allergic sera; (E) final rinsing with PBS. The frequency and dissipation data were subsequently analyzed using QSense Dfind software (version 1.2.8, Biolin, Vasteras, Sweden).
2.13. Allergenicity estimation
The allergenicity of CG was assessed using an indirect competitive ELISA method (Zeng et al., 2023). A 96-well plate was coated with the sample solution (100 μL, 50 μg/mL) and incubated at 4 °C for 12 h. Following the PBS-T wash (PBS containing 0.05% Tween-20), the plate was blocked with 1% BSA (w/v, 200 μL/well) for 1 h. Following an additional wash with PBS-T, the wells were incubated with patient sera (diluted in 1:20) from individuals with coconut allergy for 3 h at 37 °C. Subsequently, the plate was washed and incubated with mouse anti-human IgE monoclonal antibody (BioLegend, San Diego, USA) for 1 h at 37 °C. Thereafter, 100 μL of rabbit anti-mouse IgG conjugated with horseradish peroxidase (Servicebio, Wuhan, China) was added and incubated in PBS-T. Following another wash, the plate was treated with tetramethylbenzidine and allowed to react in the dark for 15 min. Finally, the reaction was terminated by the addition of 50 μL of 1 mol/L H2SO4. The absorbance at 450 nm was measured using a microplate reader (BioTek, MA, USA). The allergenicity of ACP-treated CG was quantified relative to untreated CG, which was set as the baseline at 100%.
2.14. KU812 cell culture assay
2.14.1. Cell culture
KU812 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin, maintained at 37 °C in a humidified incubator with 5% CO2.
2.14.2. KU812 sensitization
KU812 cells (1 × 105 cells/mL) were seeded into a 24-well plate and incubated at 37 °C for 24 h. Subsequently, the cells were washed with PBS and sensitized with human serum at a 1:20 dilution. After 24 h of incubation, KU812 cells were subjected to centrifugation at 250 g for 5 min and subsequently washed three times with PBS. The allergen was activated by incubation with CG solution (100 μg/well) for 60 min at 37 °C. Following this, the cells and supernatant were separated by centrifugation and collected. In accordance with the manufacturer's instructions (Beyotime, Shanghai, China), the levels of β-hexosaminidase (β-Hex), histamine (His), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), and interleukin-6 (IL-6) were quantified using specific assay kits. Positive and negative controls were included and processed on the same ELISA plate.
2.15. Statistical analysis
All experiments were conducted with a minimum of three independent replicates. Statistical analysis of the results was performed using SPSS 25.0 software (SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) was employed to identify significant differences.
3. Results and discussion
3.1. Protein structure
The impact of ACP treatment on the allergenicity of coconut milk was initially assessed through ELISA experiments, aimed at evaluating the potential of this technique for reducing allergenicity in coconut milk. As illustrated in Fig. S1, ACP treatment successfully decreased the allergenicity of coconut milk from 100% to 54% at 70 kV. This result suggested that ACP treatment was an effective technique for reducing the allergenicity of coconut milk. CG, the major allergen in coconut milk (Jin et al., 2017), was isolated from ACP-treated coconut milk to validate the mechanism by which ACP treatment reduced allergenicity.
The content of carbonyl and sulfhydryl groups are important parameters for reflecting the oxidation degree of proteins, thereby evaluating the structural changes in proteins after ACP treatment (Nyaisaba et al., 2019). As illustrated in Fig. 1a, the carbonyl content of CG exhibited a significantly increase with the escalation of ACP treatment voltage. The active species generated by ACP facilitated the chemical modification of specific side-chain amino acids, culminating in the formation of carbonyl derivatives. The -SH group, a reactive moiety within the protein, served as an indicator of protein oxidation. The intramolecular or intermolecular cross-linking of sulfhydryl groups led to a decrease in free -SH content and the formation of S—S bonds. The ACP treatment resulted in the oxidation of sulfur-containing groups in proteins, as evidenced by a decrease in free-SH content and an increase in S—S content, as illustrated in Fig. 1b. This phenomenon was consistent with findings reported by Yu et al. (2023).
Fig. 1.
Protein structure of CG treated by ACP at 0, 50, 60, and 70 kV: (a) carbonyl content; (b) free -SH and S—S bond content; (c) CD spectra; (d) secondary structure content; (e) FTIR spectra; (f) fluorescence spectra. Different lowercase letters indicate significant differences (p < 0.05).
The modifications in the CG structure at the secondary structure level induced by ACP treatment were analyzed using CD spectroscopy. As depicted in Fig. 1c, untreated CG exhibited a strong positive peak at 195 nm, along with two negative peaks at 208 and 222 nm, indicative of a protein rich in α-helix structures (Venkataratnam, Sarangapani, Cahill, & Ryan, 2019). The negative peak observed at approximately 214 nm is indicative of the β-sheet structure in proteins (Dong, Fan, Ma, Du, & Xiang, 2021). With the application of increasing ACP treatment voltage, the absolute values of these peaks diminished, potentially corresponding to the expansion of CG structure. Deconvolution analysis of the CD spectra facilitated the calculation of the percentage of secondary structure content in CG (Fig. 1d). The structural expansion of CG following ACP treatment was associated with a transition from α-helix and β-sheet conformations to random coil structures. The results from the CD spectra indicated that ACP treatment induced the expansion of the CG structure, with the ordered secondary structures being replaced by random coil. This structural alteration may be associated with the disruption of allergenic sites.
FTIR spectroscopy was utilized to examine the modifications in CG groups following ACP treatment, as illustrated in Fig. 1e. The peaks observed in the regions of 1700–1600 cm−1 and 1600–1500 cm−1 correspond to the amide I bands (C O group vibrations) and amide II bands (N—H and C—N group vibrations) within CG, respectively (Chen et al., 2023b). The perturbation of amide I bands is typically associated with alterations in protein secondary structure. Observable shifts in absorption peaks within this spectral region suggested modifications in protein secondary structure, corroborating findings from circular dichroism spectroscopy. Absorption in the amide II band primarily arose from the bending vibrations of protein NH bonds (Zafar, Asefi, Siahpoush, Roufegarinejad, & Alizadeh, 2023). Furthermore, bands within the 1320 to 1230 cm−1 range corresponded to the amide III band, which originated from the bending vibrations of N—H groups and stretching vibrations of C—N groups (Sharafodin & Soltanizadeh, 2023). Although the amide II and amide III bands exhibit lower sensitivity to protein structure compared to the amide I bands, they can still provide a general estimate of changes in protein secondary structure (Queiroz et al., 2021). Following ACP treatment, shifts were observed in the amide II and amide III bands of CG, indicating that ACP treatment disrupted the natural secondary structure of CG. Additionally, the broad peak observed at 3500–3200 cm−1 corresponded to the amide A band, indicative of the formation of intermolecular and intramolecular hydrogen bonds involving accessible O—H and N—H groups. Following ACP treatment, the expansion of the CG structure facilitated the exposure of functional groups, resulting in the formation of additional hydrogen bonds between and within CG molecules. This structural change resulted in a noticeable shift towards higher wavenumbers in the amide A band. Peaks was observed in the 3000–2800 cm−1 range in proteins were attributed to the stretching vibrations of C—H bonds (Ghobadi, Varidi, Koocheki, & Varidi, 2021). These characteristic peaks of CG also exhibited alterations following ACP treatment, suggesting modifications in the structure of aliphatic amino acids on protein surface. These structural transforms might consequently impact the hydrophobic region present on the protein surface.
The tertiary structure of CG following ACP treatment was investigated through the measurement of fluorescence spectra. Protein intrinsic fluorescence, primarily generated by aromatic amino acids such as tryptophan, is highly sensitive to the microenvironment, making it a valuable indicator for characterizing protein structural changes (Li et al., 2017). The fluorescence intensity of CG exhibited a decline with increasing ACP treatment voltage (Fig. 1f). Specifically, the maximum relative fluorescence intensity of CG decreased by 25.6% at 70 kV. As an aromatic and hydrophobic amino acid, tryptophan residues exhibit a decrease in fluorescence intensity when exposed to a highly polar environment (Yu et al., 2020). In addition, following ACP treatment, a red-shift in the maximum emission wavelength was observed. This indicated that ACP treatment induced alterations in the tertiary structure of CG, resulting in the exposure of hydrophobic groups.
In summary, the application of ACP treatment led to the unfolding of CG and induced changes in its spatial conformation, potentially diminishing or eliminating its ability to bind to allergenic sites and thereby reducing its allergenic potential.
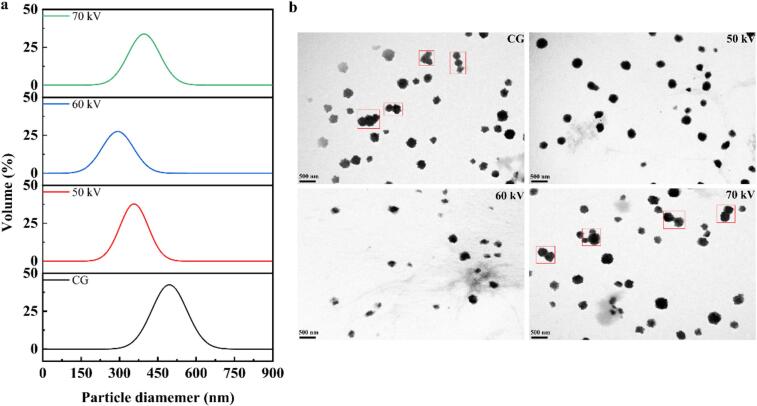
3.2. Particle size, PDI, zeta potential, and TEM
The particle size and PDI of the protein were evaluated using a laser particle size analyzer. Following ACP treatment at varying voltages, the particle size, PDI, distribution, and TEM images of CG are presented in the Fig. 2 and Table S2. A single distribution peak and smaller particle size were observed in CG following ACP treatment at 50 and 60 kV. Simultaneously, the zeta potential of CG decreased from −16.00 to −18.65 mV, indicating that the charged groups in CG were exposed on the molecular surface following ACP treatment. This exposure likely enhanced electrostatic repulsion, thereby promoting the dispersion of CG molecules in solution. TEM images corroborated these findings, revealing a reduction in the degree of CG aggregation and the presence of more monodisperse and small particles. These observations are consistent with the findings of Mehr and Koocheki (2023). However, it was noteworthy that excessive voltage exerted a detrimental effect on the dispersion of CG. Following ACP treatment at 70 kV, the particle size of CG increased and its distribution broadened, concomitant with a reduction in electrostatic repulsion. This outcome was attributed to the excessive exposure of CG hydrophobic groups, which enhanced the hydrophobic interactions between molecules, thereby inducing particle aggregation. TEM imaging corroborated this phenomenon, revealing an increased presence of CG aggregates. These findings manifested that ACP treatment at moderate voltage levels facilitated the dispersion of CG in solution.
Fig. 2.
Particle size distribution (a) and microstructure (b) of CG treated by ACP at 0, 50, 60, and 70 kV.
3.3. Isothermal titration calorimetry
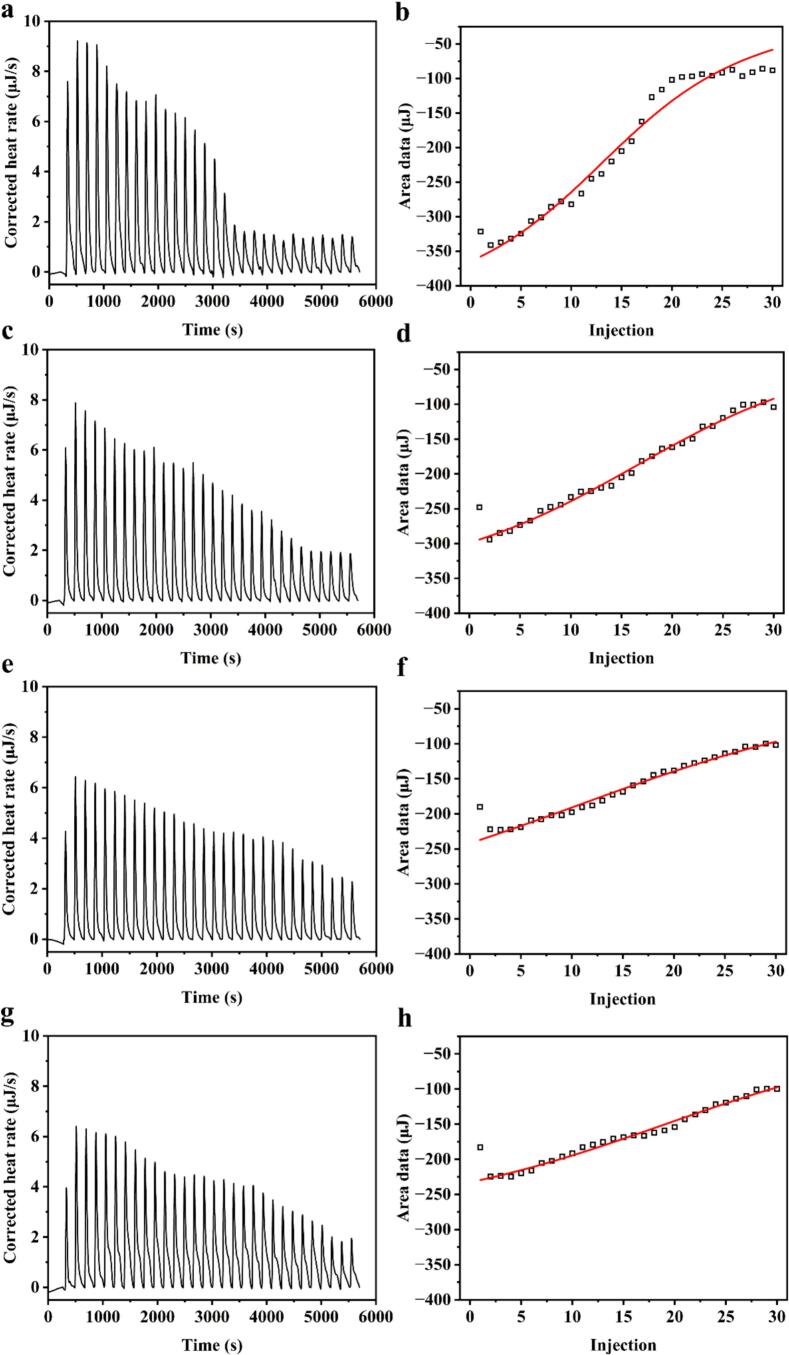
Allergic reactions commonly arise from the release of mediators triggered by the interaction between specific IgE antibodies and allergens in patients. The affinity between IgE and the allergen significantly influences the intensity of the physiological response and the severity of symptoms experienced by the patient. The binding affinity, stoichiometry, and thermodynamic parameters of the interaction between IgE and CG were assessed using ITC, a technique based on thermodynamic principles (Wu et al., 2020). The ITC titration curves and the corresponding fitting results for CG and IgE, following ACP treatment under varying voltage conditions, are presented in Fig. 3 and Table 1, respectively. The calorimetric titration curve indicated that the interaction between CG and IgE, with the untreated CG-IgE complex reaching equilibrium after titration to 30 drops. In addition, the association constant (Ka) of CG binding to IgE was determined to be 2.17 × 104/M, signifying a strong binding affinity between CG and IgE. Notably, the peak intensity of CG binding to IgE diminished following ACP treatment and exhibited a negative correlation with voltage, suggesting a reduction in interaction. This phenomenon was further substantiated by the results of Ka obtained after fitting, particularly at 70 kV, where the Ka value decreased to 0.64 × 104/M. Insight into the driving forces of the interaction between the two molecules could be gleaned by analyzing the signs of enthalpy (ΔH) and entropy (ΔS). The consistent positive and negative signs of these thermodynamic parameters across all binding processes of CG and IgE indicated that the negative ΔH and positive ΔS values corresponded to the involvement of hydrophobic interactions and electrostatic attractions in the binding process (Liao, Dumas, Elaissari, & Gharsallaoui, 2023). Consequently, these interactions simultaneously supported and competed with one another, accelerating the accumulation of CG at adjacent binding sites. Treatment with ACP significantly obstructed the inherent binding process between CG and IgE, resulting in a substantial decrease in the absolute values of ΔH and ΔG. These results indicated that the structural alteration of CG induced by ACP treatment resulted in the elimination of the binding site for IgE and a diminished affinity for the antibody.
Fig. 3.
Thermogram (left) and binding isotherm (right) of CG treated by ACP at 0, 50, 60, and 70 kV. (a) and (b): 0; (c) and (d): 50 kV; (e) and (f): 60 kV; (g) and (h): 70 kV.
Table 1.
Thermodynamic parameters of interaction between IgE and CG (1–16 nM) after ACP treatment at 0, 50, 60, and 70 kV.
| sample | n | Ka (×104/M) | Kd (×10−4 M) | ΔH (kJ/mol) | -TΔS (kJ/mol) | ΔG (kJ/mol) |
|---|---|---|---|---|---|---|
| 0 | 0.59 ± 0.04b | 2.17 ± 0.05a | 0.46 ± 0.01c | −87.51 ± 3.77c | 61.91 ± 1.80d | −25.60 ± 4.57b |
| 50 kV | 0.75 ± 0.10a | 1.07 ± 0.17b | 0.95 ± 0.15b | −74.26 ± 2.45b | 50.76 ± 1.50c | −23.50 ± 1.82ab |
| 60 kV | 0.85 ± 0.08a | 0.69 ± 0.04c | 1.45 ± 0.07a | −63.14 ± 2.47a | 42.47 ± 0.84b | −20.67 ± 1.65a |
| 70 kV | 0.88 ± 0.10a | 0.64 ± 0.06c | 1.57 ± 0.15a | −59.24 ± 1.44a | 38.33 ± 0.79a | −20.90 ± 1.01a |
Different lowercase letters indicate significant differences (p < 0.05).
3.4. Surface plasmon resonance
SPR, recognized for its high sensitivity in optical sensing, operates by detecting the interaction between light and free electrons within the chip, thereby enabling the continuous monitoring of minute variations in the effective refractive index of the chip surface (Benede et al., 2022). During this interaction, the refraction angle changes with the association and dissociation of molecules, permitting real-time study of biomolecular interactions (Zhou et al., 2024). Given its heightened sensitivity compared to ITC technology, SPR was used in this study to further investigate the kinetic characteristics of CG-IgE binding. The variations in the SPR signal, arising from the interaction between CG and IgE immobilized on the chip surface, are depicted in Fig. 4a-d. The binding and dissociation phases were maintained for 120 and 480 s, respectively. Across a range of CG concentrations (1–16 nM), the binding ability between CG and IgE demonstrated analogous SPR spectra, indicating a consistent binding mechanism. The significant increase in the SPR response value during CG binding to IgE process, concurrent with concentration rise from 1 to 16 nM, suggested a positive correlation between antibody binding strength and antigen concentration. Conversely, following ACP treatment at 16 nM, the SPR response value of CG decreased from 24.40 RU to 22.43, 18.31, and 18.01 RU, respectively, indicating variations in binding affinity between CG and IgE under different ACP voltage conditions. Additionally, the association rate constant (Kon) and the dissociation rate constant (Koff) are shown in Fig. 4a-d after fitting and calculation. These constants, Kon and Koff, elucidated the formation rate and dissociation time of CG and IgE complexes, respectively (Mottin, Razan, Nogues, & Jullien, 2021). Untreated CG exhibited a high affinity for IgE, with Kon and Koff values of 1.62 × 105 M−1 s−1 and 3.74 × 10−4 s−1, respectively. The primary allergens in coconut comprise 7S globulin and 11S globulin, which are also the predominant components of CG (Jin et al., 2017). In the documented instances of coconut allergy, the serum of numerous patients exhibited elevated levels of IgE, which interacts with 7S and 11S globulin (Soyer, Ozen, Tiras, & Dallar, 2010). The pronounced affinity between these components in CG and IgE in the serum of allergic individuals elicited in a swift response in the SPR spectra. Notably, ACP treatment effectively reduced the Kon from 1.62 to 1.37, 1.07, and 1.02 M−1 s−1 and increased Koff from 3.74 to 5.65, 8.17, and 8.97 s−1, representing a decrease in the affinity between CG and IgE. The results obtained from SPR demonstrated that ACP could effectively reduce IgE-mediated CG allergy from a kinetic standpoint.
Fig. 4.
SPR sensor grams of interaction between IgE and CG (1–16 nM) after ACP treatment at 0 (a), 50 (b), 60 (c), and 70 kV (d); The frequency shift and dissipation shift of IgE binding to CG after treated with ACP at 0 (e), 50 (f), 60 (g), and 70 kV (h).
3.5. Quartz crystal microbalance with dissipation
QCM, a highly sensitive acoustic shearing technique, is adept at monitoring temporal mass variations as a polymer layer forms at the solid-liquid interface. This technology operates on the principle that a quartz crystal oscillates at a specific frequency when an external voltage is applied. Any alteration in oscillation frequency, resulting from the addition or removal of molecules on the chip surface due to binding, is recorded, thereby providing insight into the corresponding changes in mass (Suthar et al., 2023). Conventional QCM could evaluate the surface mass density by recording frequency shifts and applying the Sauerbrey equation. In contrast, QCM-D simultaneously measures frequency and dissipation shifts, thereby providing insights into surface mass and viscoelasticity of interface layer (Plikusiene, Maciulis, Ramanavicius, & Ramanaviciene, 2022).
The temporal variations in dissipation and frequency shifts on the gold chip surface during the interaction between CG and IgE are shown in Fig. 4e-h. A five-stage protocol was employed to elucidate the dynamic interaction between CG and IgE: (A) baseline measurement; (B) adsorption of CG on the chip surface; (C) desorption of loosely bound CG; (D) interaction between CG and IgE; (E) desorption of loosely bound IgE. The immobilization of the protein on the gold chip is contingent upon the interaction between hydrophobic groups and the chip surface. Treatment with ACP, particularly at 60 and 70 kV, facilitated the exposure of hydrophobic groups of CG, thereby enhancing its immobilization on the gold chip surface. The process resulted in a decrease in frequency and an increase in dissipation. The adsorption of IgE on the chip surface was affected by the CG content and the affinity between CG and IgE. Following ACP treatment, although the adsorption of CG on the chip surface increased, the adsorption amount of IgE decreased significantly, indicating a restoration of dissipation and frequency shift. Table S3 illustrates the temporal change in adsorption mass per unit area after fitting. Following ACP treatment, the IgE-binding mass declined from 3.56 to 3.23, 2.29, and 2.24 μg/cm2, respectively. These findings further demonstrated that the structural alterations of CG induced by the ACP treatment diminished the interaction between CG and IgE, potentially mitigating CG-induced allergic responses.
3.6. Allergenicity of CG
At least three putative sensitizing epitope hotspots were identified within the 7S and 11S globulins of coconut, suggesting that CG possesses the capacity to elicit severe allergic reactions (Jin et al., 2017). Consistent with these predictions, Fig. 5a illustrates that CG exhibited high allergenicity and robust binding to specific IgE in sera, paralleling the observations made in coconut milk. The allergenicity of CG was markedly reduced from 100% to 55.18% (at 70 kV) following ACP treatment with increasing treatment voltage. The reactive species generated by ACP were able to interact with the amino acid side chains of the CG molecule, leading to alterations in its secondary and tertiary structures. This structural transformation likely involved the disruption of specific sites within the CG molecule that are capable of binding to IgE in allergic sera. Consequently, the number of binding sites between CG and IgE was diminished, indicating a reduction in binding affinity. Excessive ACP treatment at 70 kV could induce aggregation among CG molecules, as evidenced by our previous study (Chen et al., 2023b). These structural alterations may obscure the allergenic epitopes of CG, thereby preventing further degradation of these epitopes by ACP treatment. Consequently, no significant difference in the allergenicity of CG was observed following ACP treatment at 70 kV and 60 kV.
Fig. 5.
a: Relative allergenicity of CG after ACP treatment at 0, 50, 60, and 70 kV; release content of β-hexosaminidase (b), histamine (c), TNF-α (d), IL-4 (e), and IL-6 (f) of KU812 cell after sensitized by CG after ACP treatment at 0, 50, 60, and 70 kV. Different lowercase letters indicate significant differences (p < 0.05).
3.7. Evaluation of potential sensitization based on KU812 cells
Food allergy usually manifest as IgE-mediated type I allergic reactions, characterized by cellular degranulation and the rapid release of a substantial quantity of allergic mediators (Lu et al., 2022). KU812 cells, which express high-affinity fragments of IgE receptors, can release allergic mediators such as β-Hexosaminidase (β-Hex), histamine (His), tumor necrosis factor (TNF), and interleukins (IL) upon IgE activation (Wu et al., 2022). Consequently, KU812 cells are frequently utilized to assess the degree of sensitization induced by allergens. The levels of degranulation mediators and allergic mediators are illustrated in Fig. 5b-f. β-Hex, a glycosidase, is released during degranulation of KU812 cells. His serves as a biomarker of degranulation, and its release rate can be used to quantify the extent of cell degranulation (Cheng, Li, & Sun, 2023). Untreated CG resulted in a pronounced allergic reaction, characterized by a 57.17% release of β-Hex and a His concentration of 15.43 ng/mL. Following ACP treatment, the extent of CG-induced degranulation in KU812 cells was markedly reduced, as evidenced by decreased levels of β-Hex and His. Specifically, with increasing ACP treatment voltage, the release of β-Hex and His declined to 37.64% and 9.14 ng/mL, respectively. However, no significant difference was observed between the effects of ACP treatments at 60 and 70 kV on CG-induced degranulation in KU812 cells. KU812 cells produce allergic mediators that are central to the pathophysiology of IgE-mediated conditions such as asthma, rhinitis, and dermatitis (Nurmatov et al., 2017). TNF-α, IL-4, and IL-6 are characteristic allergic mediators produced by KU812 cells and their release indicates the degree of sensitization induced by the allergen (Zhang et al., 2023). Upon application of ACP (70 kV) to CG, the levels of TNF-α, IL-4, and IL-6 released from KU812 cells decreased from 264.33, 2.51, and 116.87 pg/mL to 174.60, 1.23, and 64.00 pg/mL, respectively. These results suggested that ACP treatment mitigated the allergic responses of KU812 cells by reducing the affinity of CG for IgE.
4. Conclusion
In conclusion, the findings of this study substantiate the efficacy of ACP treatment as a technology for mitigating the allergenicity of coconut milk. Following ACP treatment, the structure of CG, the primary allergen in coconut milk, underwent unfolding, leading to the destruction of allergenic epitopes specifically recognized by IgE. This structural alteration was corroborated through ITC, SPR, and QCM-D, which collectively demonstrated a reduced affinity of ACP-treated CG for IgE. Consequently, the IgE-mediated allergic response was attenuated following ACP treatment, as evidenced by a decrease in allergenicity and a concomitant reduction in degranulation and release of allergic mediators in cellular models. This study presents a promising approach for the development of hypoallergenic coconut milk products utilizing ACP technology.
CRediT authorship contribution statement
Yang Chen: Writing – review & editing, Writing – original draft, Visualization, Methodology, Conceptualization. Yile Chen: Methodology. Tian Li: Software, Methodology. Jiamei Wang: Resources. Weimin Zhang: Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The research was funded by the National Natural Science Foundation of China (Grant No. 32260609). We extend our sincere gratitude to the China Scholarship Council and Home for Researchers (www.home-for-researchers.com) for their invaluable support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101732.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Benede S., Lozano-Ojalvo D., Cristobal S., Costa J., D’Auria E., Velickovic T.C., et al. New applications of advanced instrumental techniques for the characterization of food allergenic proteins. Critical Reviews in Food Science and Nutrition. 2022;62(31):8686–8702. doi: 10.1080/10408398.2021.1931806. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Y.L., Fang Y.J., Pei Z.S., Zhang W.M. Coconut milk treated by atmospheric cold plasma: Effect on quality and stability. Food Chemistry. 2024;430 doi: 10.1016/j.foodchem.2023.137045. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Y.L., Jiang L.Z., Yang Z.H., Fang Y.J., Zhang W.M. Shear emulsification condition strategy impact high internal phase Pickering emulsions stabilized by coconut globulin-tannic acid: Structure of protein at the oil-water interface. LWT - Food Science and Technology. 2023;187:115283. doi: 10.1016/j.lwt.2023.115283. [DOI] [Google Scholar]
- Chen Y., Yao M.Y., Yang T.Y., Fang Y.J., Xiang D., Zhang W.M. Changes in structure and emulsifying properties of coconut globulin after the atmospheric pressure cold plasma treatment. Food Hydrocolloids. 2023;136 doi: 10.1016/j.foodhyd.2022.108289. [DOI] [Google Scholar]
- Cheng J.H., Li J., Sun D.W. Effects of dielectric barrier discharge cold plasma on structure, surface hydrophobicity and allergenic properties of shrimp tropomyosin. Food Chemistry. 2023;409 doi: 10.1016/j.foodchem.2022.135316. [DOI] [PubMed] [Google Scholar]
- Cheng J.H., Li J.L., Sun D.W. In vivo biological analysis of cold plasma on allergenicity reduction of tropomyosin in shrimp. Food Chemistry. 2024;432 doi: 10.1016/j.foodchem.2023.137210. [DOI] [PubMed] [Google Scholar]
- Dong S.S., Fan L.M., Ma Y.F., Du J., Xiang Q.S. Inactivation of polyphenol oxidase by dielectric barrier discharge (DBD) plasma: Kinetics and mechanisms. LWT - Food Science and Technology. 2021;145 doi: 10.1016/j.lwt.2021.111322. [DOI] [Google Scholar]
- Ekezie F.G.C., Cheng J.H., Sun D.W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends in Food Science & Technology. 2018;74:12–25. doi: 10.1016/j.tifs.2018.01.007. [DOI] [Google Scholar]
- Gantumur M.A., Sukhbaatar N., Shi R.J., Hu J.L., Bilawal A., Qayum A., et al. Structural, functional, and physicochemical characterization of fermented whey protein concentrates recovered from various fermented-distilled whey. Food Hydrocolloids. 2023;135 doi: 10.1016/j.foodhyd.2022.108130. [DOI] [Google Scholar]
- Ghobadi M., Varidi M.J., Koocheki A., Varidi M. Effect of heat treatment on the structure and stability of grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum nanoparticles. International Journal of Biological Macromolecules. 2021;182:26–36. doi: 10.1016/j.ijbiomac.2021.03.170. [DOI] [PubMed] [Google Scholar]
- Hsieh K.C., Ting Y.W. Atmospheric cold plasma reduces Ara h 1 antigenicity in roasted peanuts by altering the protein structure and amino acid profile. Food Chemistry. 2024;441 doi: 10.1016/j.foodchem.2023.138115. [DOI] [PubMed] [Google Scholar]
- Jin T.C., Wang C., Zhang C.Y., Wang Y., Chen Y.W., Guo F., et al. Crystal structure of cocosin, a potential food allergen from coconut (Cocos nucifera) Journal of Agricutural and Food Chemistry. 2017;65(34):7560–7568. doi: 10.1021/acs.jafc.7b02252. [DOI] [PubMed] [Google Scholar]
- Kang W.H., Zhang J.K., Yu N., He L., Chen Y. Effect of ultrahigh-pressure treatment on the structure and allergenicity of peach allergenic proteins. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136227. [DOI] [PubMed] [Google Scholar]
- Li J.G., Xiang Q.S., Liu X.F., Ding T., Zhang X.S., Zhai Y.S., et al. Inactivation of soybean trypsin inhibitor by dielectric-barrier discharge (DBD) plasma. Food Chemistry. 2017;232:515–522. doi: 10.1016/j.foodchem.2017.03.167. [DOI] [PubMed] [Google Scholar]
- Liao W., Dumas E., Elaissari A., Gharsallaoui A. The formation mechanism of multilayer emulsions studied by isothermal titration calorimetry and dynamic light scattering. Food Hydrocolloids. 2023 doi: 10.1016/j.foodhyd.2022.108275. [DOI] [Google Scholar]
- Lu Q.L., Zuo L.L., Wu Z.H., Li X., Tong P., Wu Y., et al. Characterization of the protein structure of soymilk fermented by Lactobacillus and evaluation of its potential allergenicity based on the sensitized-cell model. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130569. [DOI] [PubMed] [Google Scholar]
- Mehr H.M., Koocheki A. Effects of short-term and long-term cold plasma treatment on the color, structure, and Pickering foaming properties of grass pea protein particles. Food Hydrocolloids. 2023;143 doi: 10.1016/j.foodhyd.2023.108846. [DOI] [Google Scholar]
- Mottin D., Razan F., Nogues C., Jullien M.C. Out-of-equilibrium measurements of kinetic constants on a biosensor. Analytical Chemistry. 2021;93(19):7266–7274. doi: 10.1021/acs.analchem.1c00543. [DOI] [PubMed] [Google Scholar]
- Nurmatov U., Dhami S., Arasi S., Pajno G.B., Fernandez-Rivas M., Muraro A., et al. Allergen immunotherapy for IgE-mediated food allergy: A systematic review and meta-analysis. Allergy. 2017;72(8):1133–1147. doi: 10.1111/all.13124. [DOI] [PubMed] [Google Scholar]
- Nyaisaba B.M., Miao W.H., Hatab S., Siloam A., Chen M.L., Deng S.G. Effects of cold atmospheric plasma on squid proteases and gel properties of protein concentrate from squid (Argentinus ilex) mantle. Food Chemistry. 2019;291:68–76. doi: 10.1016/j.foodchem.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Olatunde O.O., Hewage A., Dissanayake T., Aluko R.E., Karaca A.C., Shang N., et al. Cold atmospheric plasma-induced protein modification: Novel nonthermal processing technology to improve protein quality, functionality, and allergenicity reduction. Comprehensive Reviews in Food Science and Food Safety. 2023;22:2197–2234. doi: 10.1111/1541-4337.13144. [DOI] [PubMed] [Google Scholar]
- Pi X.W., Peng Z.Y., Liu J.F., Jiang Y.Q., Wang J.R., Fu G.M., et al. Sesame allergy: Mechanisms, prevalence, allergens, residue detection, effects of processing and cross-reactivity. Critical Reviews in Food Science and Nutrition. 2024;64(10):2847–2862. doi: 10.1080/10408398.2022.2128031. [DOI] [PubMed] [Google Scholar]
- Plikusiene I., Maciulis V., Ramanavicius A., Ramanaviciene A. Spectroscopic ellipsometry and quartz crystal microbalance with dissipation for the assessment of polymer layers and for the application in biosensing. Polymers. 2022;14:1056. doi: 10.3390/polym14051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk B.I., Dinakarpandian D., Nanda M., Barnes C., Dinakar C. Association of tree nut and coconut sensitizations. Annals of Allergy, Asthma & Immunology. 2016;117(4):412–416. doi: 10.1016/j.anai.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Queiroz L.S., Regnard M., Jessen F., Mohammadifar M.A., Sloth J.J., Petersen H.O., et al. Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae. International Journal of Biological Macromolecules. 2021;186:714–723. doi: 10.1016/j.ijbiomac.2021.07.081. [DOI] [PubMed] [Google Scholar]
- Shah F., Shi A.M., Ashley J., Kronfel C., Wang Q., Maleki S.J., et al. Peanut allergy: Characteristics and approaches for mitigation. Comprehensive Reviews in Food Science and Food Safety. 2019;18:1361–1387. doi: 10.1111/1541-4337.12472. [DOI] [PubMed] [Google Scholar]
- Sharafodin H., Soltanizadeh N. Potential application of DBD plasma technique for modifying structural and physicochemical properties of soy protein isolate. Food Hydrocolloids. 2023;122 doi: 10.1016/j.foodhyd.2021.107077. [DOI] [Google Scholar]
- Soyer O.U., Ozen C., Tiras U., Dallar Y. Anaphylaxis in a neonate caused by ceftazidime. Allergy. 2010;65(11):1486–1487. doi: 10.1111/j.1398-9995.2010.02373.x. [DOI] [PubMed] [Google Scholar]
- Suthar J., Alvarez-Fernandez A., Osarfo-Mensah E., Angioletti-Uberti S., Williams G.R., Guldin S. Amplified EQCM-D detection of extracellular vesicles using 2D gold nanostructured arrays fabricated by block copolymer self-assembly. Nanoscale Horizons. 2023;8(4):460–472. doi: 10.1039/d2nh00424k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataratnam H., Sarangapani C., Cahill O., Ryan C.B. Effect of cold plasma treatment on the antigenicity of peanut allergen Ara h 1. Innovative Food Science and Emerging Technologies. 2019;52:368–375. doi: 10.1016/j.ifset.2019.02.001. [DOI] [Google Scholar]
- Warren C.M., Sehgal S., Nimmagadda S.R., Gupta R. Prevalence and burden of coconut allergy in the United States. Annals of Allergy, Asthma & Immunology. 2023;131:645–652. doi: 10.1016/j.anai.2023.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Mei S., Duan R., Geng F., Wu W.X., Li X., et al. How black tea pigment theaflavin dyes chicken eggs: Binding affinity study of theaflavin with ovalbumin. Food Chemistry. 2020;303 doi: 10.1016/j.foodchem.2019.125407. [DOI] [PubMed] [Google Scholar]
- Wu Y.T., Lu Y.Y., Huang Y.H., Lin H., Chen G.Z., Chen Y., et al. Glycosylation reduces the allergenicity of turbot (Scophthalmus maximus) parvalbumin by regulating digestibility, cellular mediators release and Th1/Th2 immunobalance. Food Chemistry. 2022;382 doi: 10.1016/j.foodchem.2022.132574. [DOI] [PubMed] [Google Scholar]
- Xi J., He M.X. High hydrostatic pressure (HHP) effects on antigenicity and structural properties of soybean β-conglycinin. Journal of Food Science and Technology. 2018;55(2):630–637. doi: 10.1007/s13197-017-2972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X., Gao S.X., Gao X., Zhang X., Xia G.H., Liu Z.Y., et al. Glycolipids improve the stability of liposomes: The perspective of bilayer membrane structure. Food Chemistry. 2023;412 doi: 10.1016/j.foodchem.2023.135517. [DOI] [PubMed] [Google Scholar]
- Yu J.J., Ji H., Chen Y., Zhang Y.F., Zheng X.C., Li S.H., et al. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. International Journal of Biological Macromolecules. 2020;158:1194–1203. doi: 10.1016/j.ijbiomac.2020.04.255. [DOI] [PubMed] [Google Scholar]
- Yu J.J., Zhang Z.Y., Lin X.N., Ji Y.Q., Zhang R.R., Ji H., et al. Changes in the structure and hydration properties of high-temperature peanut protein induced by cold plasma oxidation. International Journal of Biological Macromolecules. 2023;253 doi: 10.1016/j.ijbiomac.2023.127500. [DOI] [PubMed] [Google Scholar]
- Zafar H.S., Asefi N., Siahpoush V., Roufegarinejad L., Alizadeh A. Preparation of egg white powder using electrohydrodynamic drying method and its effect on quality characteristics and functional properties. Food Chemistry. 2023;426 doi: 10.1016/j.foodchem.2023.136567. [DOI] [PubMed] [Google Scholar]
- Zeng J.H., Zou J.Z., Zhao J.L., Lin K., Zhang L.W., Yi H.X., et al. Chymosin pretreatment accelerated papain catalysed hydrolysis for decreasing casein antigenicity by exposing the cleavage site at tyrosine residues. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134777. [DOI] [PubMed] [Google Scholar]
- Zhang M.L., Lu Q.L., Bai J., Gao J.Y., Wu Z.H., Li X., et al. Evaluation of the potential anti-soybean allergic activity of different forms of Lactobacillus delbrueckii subsp. bulgaricus based on cell model in vitro. Food & Function. 2023;14(2):746–758. doi: 10.1039/d2fo02189g. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Geng Q., Song M., Li X., Yang A.S., Tong P., et al. The structure and potential allergenicity of peanut allergen monomers after roasting. Food & Function. 2024;15(5):2577–2586. doi: 10.1039/d3fo05351b. [DOI] [PubMed] [Google Scholar]
- Zhou T.M., Liu H., Diao X.Y., Zhao Q., Duan J.Y., Henry I.I., et al. Molecular interaction between myofibrillar protein and beta-carotene during heating. Food Chemistry. 2024;435 doi: 10.1016/j.foodchem.2023.137588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.