Abstract
Background
Prior studies have incompletely assessed whether the development of cardiometabolic risk factors (CVDRF) (hypertension, hyperlipidemia, and diabetes mellitus) mediates the association between anxiety and depression (anxiety/depression) and cardiovascular disease (CVD).
Objectives
The authors aimed to evaluate the following: 1) the association between anxiety/depression and incident CVDRFs and whether this association mediates the increased CVD risk; and 2) whether neuro-immune mechanisms and age and sex effects may be involved.
Methods
Using a retrospective cohort design, Mass General Brigham Biobank subjects were followed for 10 years. Presence and timing of anxiety/depression, CVDRFs, and CVD were determined using ICD codes. Stress-related neural activity, chronic inflammation, and autonomic function were measured by the assessment of amygdalar-to-cortical activity ratio, high-sensitivity CRP, and heart rate variability. Multivariable regression and mediation analyses were employed.
Results
Among 71,214 subjects (median age 49.6 years; 55.3% female), 27,048 (38.0%) developed CVDRFs during follow-up. Pre-existing anxiety/depression associated with increased risk of incident CVDRF (OR: 1.71 [95% CI: 1.59-1.83], P < 0.001) and with a shorter time to their development (β = −0.486 [95% CI: −0.62 to −0.35], P < 0.001). The development of CVDRFs mediated the association between anxiety/depression and CVD events (log-odds: 0.044 [95% CI: 0.034-0.055], P < 0.05). Neuro-immune pathways contributed to the development of CVDRFs (P < 0.05 each) and significant age and sex effects were noted: younger women experienced the greatest acceleration in the development of CVDRFs after anxiety/depression.
Conclusions
Anxiety/depression accelerate the development of CVDRFs. This association appears to be most notable among younger women and may be mediated by stress-related neuro-immune pathways. Evaluations of tailored preventive measures for individuals with anxiety/depression are needed to reduce CVD risk.
Key words: amygdala, cardiometabolic, mental health, neuro immune, prevention, sex differences
Central Illustration
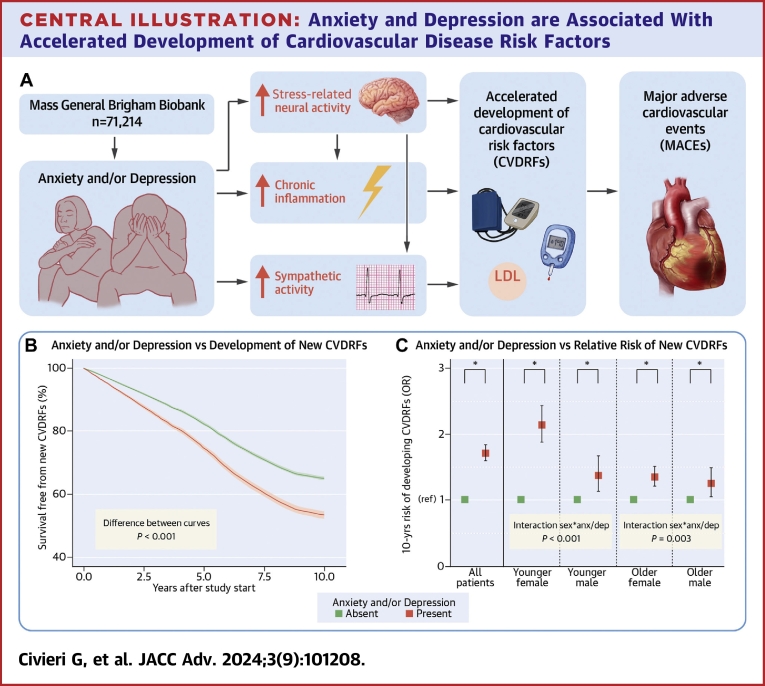
Stress-related psychiatric conditions, such as anxiety and depression (anxiety/depression), have emerged as independent risk factors for cardiovascular disease (CVD).1,2 Although several mechanisms linking psychiatric conditions to CVD have been proposed,1 a simple yet potentially important mediator linking anxiety/depression to CVD remains under-investigated: an increase in the risk of developing major traditional cardiometabolic CVD risk factors (CVDRF) (hypertension (HTN), dyslipidemia (HLD), and diabetes mellitus (DM)). Notably, while prior work has shown that anxiety/depression are associated, mainly cross-sectionally, with individual CVDRFs,3, 4, 5, 6, 7 no studies have specifically assessed the relationship between anxiety/depression and the risk of developing any CVDRFs, when all major CVDRFs are assessed concurrently. Moreover, previous studies have not evaluated how anxiety/depression may impact the timing of the development of CVDRFs or the degree to which an accelerated gain in CVDRFs explains the heightened CVD risk associated with anxiety/depression. Furthermore, it remains unknown whether age and sex may influence these potential relationships.
Neuro-immune pathways, including stress-related neural activity, systemic inflammation, and autonomic nervous system activity, have been proposed to mediate the association between stress and increased CVD risk.8 Advanced imaging with 18F-fluorodeoxyglucose positron emission tomography/computed tomography has enabled, for example, evaluation of neural mechanisms by which psychosocial stress and psychiatric conditions, including anxiety/depression, trigger the development of CVD9,10 and CVDRFs, including HTN11 and DM.12 The impact of psychosocial stress on the development of CVDRFs through autonomic nervous system dysfunction and chronic inflammation has also been partially investigated.13, 14, 15, 16, 17, 18 As such, these tools are well-suited to evaluate the biological pathways linking anxiety/depression to the risk of developing CVDRFs.
Accordingly, using a large well-characterized biobank cohort, we sought to test the hypotheses that: 1) anxiety/depression accelerate the development of CVDRFs (independently of potential confounders); 2) the development of CVDRFs contributes to the increased CVD risk associated with anxiety/depression; and 3) neuro-immune pathways mediate the link between anxiety/depression and development of CVDRFs. Furthermore, because prior observations demonstrate important age and sex effects for the CVD risk associated with anxiety/depression19, 20, 21, 22 as well as with traditional CVDRFs,23 we also investigated whether age and sex modified the association between anxiety/depression and development of CVDRFs.
Methods
Study sample
Individuals (age ≥18 years) enrolled and consented into the Mass General Brigham (MGB) Biobank, a biorepository that recruits participants through the MGB Network,24 were studied. The MGB Human Research Committee approved the study protocol.
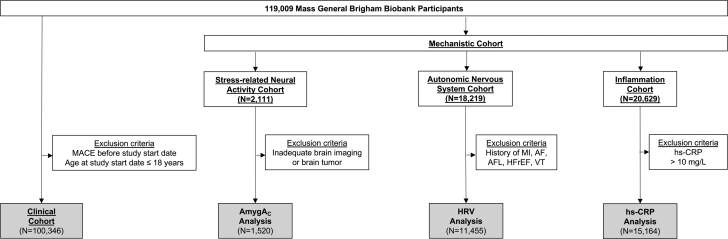
Our retrospective cohort study used data collected in the MGB Biobank until December 20, 2020 (data lock date), which is therefore considered the date of the last follow-up. Within the study population, we identified the following: 1) a larger clinical cohort with clinical data; and 2) smaller mechanistic cohorts with neuro-immune data (Figure 1). Within the clinical cohort, we investigated the relationships between anxiety/depression, development of CVDRFs, and the subsequent risk of major adverse cardiovascular disease events (MACE). To evaluate 10-year event rates, we defined December 20, 2010 as the study start date. Patients with MACE before the study start date were excluded. Additional details about the clinical cohort are reported in Supplemental Figure 1. In the mechanistic cohorts, we performed exploratory analyses about the role of stress-related neural activity, autonomic nervous system function, and chronic inflammation (as components of neuro-immune pathways) in the development of CVDRF.
Figure 1.
Study Design
AmygAc = amygdalar to ventromedial prefrontal cortical activity ratio; AF = atrial fibrillation; AFL = atrial flutter; HFrEF = heart failure with reduced ejection fraction; HRV = heart rate variability; hs-CRP = high sensitivity C-reactive protein; MACE = major adverse cardiovascular event; MI = myocardial infarction; VT = ventricular tachycardia.
Psychiatric conditions
For all patients, data were collected regarding the diagnoses of anxiety/depression using International Classification of Disease (ICD-10 codes (Supplemental Table 1). We assessed the impact of depression, anxiety, and of the combined group depression and/or anxiety (ie, 1 or both conditions versus none) (Supplemental Figure 1). Given the established relationship between anxiety/depression,25 depression and/or anxiety was predefined as the primary study exposure variable. To reduce the risk of reverse causality, we only considered diagnoses of anxiety/depression prior to the study start date (ie the first day of the 10-year observation period, December 20, 2010), and patients with new diagnoses of anxiety/depression after study start date were excluded from analysis. In a sensitivity analysis used to confirm the validity of ICD codes, we also used natural language processing (NLP) to confirm the ICD-derived diagnosis of depression. Additional details are provided in the Supplemental Methods.
Cardiovascular risk factors
Development of a new CVDRF was the primary outcome of this study. Development of a new CVDRF was defined as a new diagnosis of HTN, HLD, or DM after the study start date, all identified using ICD-10 codes (Supplemental Table 1). The period from the start of the observation period to diagnosis of a new CVDRF (ie, the time between the study start date and the date of diagnosis of a new CVDRF) was used to identify the time required to develop a new CVDRF.
Covariables
For all participants, the presence of factors predisposing to CVDRFs was extracted from electronic health record data. Covariables were defined a-priori based on existing literature26,27 and were derived either from MGB Biobank records or from a voluntary health survey completed by participants upon enrollment in the Biobank.24 Sex was defined as biological sex. Physical activity was derived from the voluntary health survey for a subset of participants. Body mass index and smoking status (current or prior smoking) were derived from either voluntary health surveys or MGB Biobank records for individuals who did not complete the survey. Insomnia was defined using the relevant ICD-10 code (G47.0). Antidepressant therapy (ie, selective serotonin reuptake inhibitors or serotonin and norepinephrine reuptake inhibitors) was derived from MGB Biobank records. Socioeconomic variables (ie, employment status and educational attainment) were derived from the voluntary health survey, while median income was derived using zip code-level census data. Additional details are in the Supplemental Methods.
Adverse cardiovascular events
MACE were defined according to Framingham Heart Study criteria.28 MACE data were obtained from the medical records using ICD-10 codes; the MACE composite endpoint included the following: myocardial infarction, unstable angina, heart failure, stroke, transient ischemic attack, coronary revascularization, peripheral vascular disease, and peripheral revascularization. Further details are reported in Supplemental Table 2. Incident MACE were assessed for the 10-year period from the study start date to the first event date, death, or last follow-up. To avoid reverse causation, patients with MACE before study start date were excluded.
Neuro-immune measures
Stress-related neural activity was measured as amygdalar metabolic activity relative to cortical activity (amygdalar to cortical activity ratio: AmygAC) as previously described.9,29, 30, 31, 32, 33 As described previously, cortical activity was assessed in the ventromedial prefrontal cortex, given its role in regulating the limbic system.29, 30, 31,34 Clinically available high-sensitivity C-reactive protein (hs-CRP) was used as a surrogate biomarker for chronic inflammation. Autonomic nervous system activity was evaluated using heart rate variability (HRV) derived from clinical electrocardiograms. Additional details are available in the Supplemental Methods.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, version 28, IBM corporation) and statistical package R (version 4.3.1, R Foundation for Statistical Computing). Given the impact of the pre-existing CVDRFs on the further development of CVDRFs, all analyses using development of CVDRFs as outcome were adjusted for pre-existing CVDRFs. Multivariable logistic regressions and multivariable linear regressions were employed to assess the relationships between pre-existing anxiety/depression (ie, anxiety/depression before study start date) and, respectively, the risk of developing new CVDRFs and the time required to develop new CVDRFs. Implementation of both logistic and linear regression models allowed us to fully discriminate between the probability of developing new CVDRFs and the time required for their development. The cumulative impact of anxiety/depression on the time required to develop a new CVDRF was also assessed. Adjusted survival curves were obtained using the R-package ‘adjustedCurves’ (version 0.10.1). Traditional mediation analysis, using SPSS PROCESS macro v4.2, was performed to test whether the number of CVDRFs developed mediates the link between anxiety/depression and incident MACE. Variables involved in the mediation model were carefully selected to suggest causal effects. In the mechanistic cohorts, multivariable Cox regression was used to investigate the impact of neuro-immune pathways on the development of new CVDRFs. Participants with missing data were excluded from corresponding analyses (pairwise deletion). A 2-sided P value <0.05 was used to define statistical significance for all analyses. Additional details about statistical analysis and different sensitivity analyses are given in the Supplemental Methods.
Results
Clinical cohort
Of 119,009 MGB Biobank participants, 100,346 were included in the clinical cohort. Of these, 71,214 were included in the anxiety and/or depression analysis (Supplemental Figure 1).
Individuals with (versus without) anxiety and/or depression were more likely to be female and to have a history of smoking, higher body mass index, insomnia, HTN, DM, or HLD. Median age in women was lower than in men (48.80 [IQR: 34.80-59.61] versus 52.97 [IQR: 40.78-62.42] years, P < 0.001). Additional details of the baseline characteristics are presented in Table 1.
Table 1.
Baseline Characteristic of the Clinical Cohort by the Presence of Anxiety and/or Depression
| All Patients |
Patients Without Pre-Existing CVDRFs |
|||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (N = 71,214) | Baseline Anxiety and/or Depression |
P Value Comparing Groups | All Patients (N = 49,774) | Baseline Anxiety and/or Depression |
P Value Comparing Groups | |||
| Present (n = 14,744) | Absent (n = 56,470) | Present (n = 5,454) | Absent (n = 44,320) | |||||
| Age, y | 49.61 (35.39-60.21) | 50.75 (39.99-60.36) | 50.74 (36.43-61.19) | <0.001 | 45.91 (30.78-57.12) | 41.61 (30.66-51.09) | 47.90 (32.44-58.77) | <0.001 |
| Female | 39,401 (55.3%) | 10,358 (70.2%) | 29,043 (51.4%) | <0.001 | 30,728 (42.1%) | 1,320 (24.2%) | 20,774 (46.9%) | <0.001 |
| Race (non-White) | 10,521 (14.8%) | 2,360 (16.0%) | 8,161 (14.5%) | <0.001 | 7,547 (15.2%) | 936 (17.2%) | 6,611 (14.9%) | <0.001 |
| Ethnicity (Hispanic) | 2,079 (2.9%) | 798 (5.4%) | 1,281 (2.3%) | <0.001 | 1,334 (2.7%) | 312 (5.7%) | 1,022 (2.3%) | <0.001 |
| Smokinga | 24,287 (39.3%) | 6,424 (48.0%) | 17,863 (36.9%) | <0.001 | 24,551 (39.4%) | 2,250 (46.3%) | 13,314 (35.8%) | <0.001 |
| BMI, kg/m2 | 26.76 (23.64-30.81) | 27.55 (24.02-32.20) | 26.58 (23.56-30.43) | <0.001 | 26.31 (23.20-30.21) | 26.17 (22.96-30.54) | 26.32 (23.24-30.17) | 0.586 |
| Recommended physical activitya | 22,817 (71.5%) | 3,964 (63.6%) | 18,853 (73.4%) | <0.001 | 22,265 (71.4%) | 1,415 (66.1%) | 14,504 (73.8%) | <0.001 |
| Insomnia | 2,466 (3.5%) | 1,835 (12.4%) | 631 (1.1%) | <0.001 | 733 (1.0%) | 390 (7.2%) | 163 (0.4%) | <0.001 |
| Therapy with SSRI or SNRIb | 9,988 (14.0%) | 8,731 (59.2%) | 1,257 (2.2%) | <0.001 | 3,418 (7.8%) | 2,828 (66.7%) | 590 (1.5%) | <0.001 |
| Pre-existing CVDRFs | ||||||||
| Any CVDRF | 27,520 (27.2%) | 9,290 (63.0%) | 12,150 (21.5%) | <0.001 | NA | |||
| Pre-existing HTN | 14,247 (20.0%) | 6,373 (43.2%) | 7,874 (13.9%) | <0.001 | ||||
| Pre-existing HLD | 16,686 (23.4%) | 7,315 (49.6%) | 9,371 (16.6%) | <0.001 | ||||
| Pre-existing DM | 4,390 (6.2%) | 2,190 (14.9%) | 2,195 (3.9%) | <0.001 | ||||
| Number of pre-existing CVDRFs | ||||||||
| 0 | 49,774 (69.9%) | 5,454 (37.0%) | 44,320 (78.5%) | <0.001 | NA | |||
| 1 | 10,322 (14.5%) | 4,129 (28.0%) | 6,193 (11.0%) | <0.001 | ||||
| 2 | 8,353 (11.7%) | 3,729 (25.3%) | 4,624 (8.2%) | <0.001 | ||||
| 3 | 2,765 (3.9%) | 1,432 (9.7%) | 1,333 (2.4%) | <0.001 | ||||
Values are median (IQR) or n (%). Boldface values represent statistical significance (P < 0.05).
BMI = body mass index; CVDRF = cardiometabolic cardiovascular risk factor; DM = diabetes mellitus; HLD = hyperlipidemia; HTN = hypertension; SSRI = selective serotonin reuptake inhibitors; SNRI = serotonin and norepinephrine reuptake inhibitors.
Data are derived from health surveys that are not available for all patients. % refers to the total number of participants for whom we have survey data. Physical activity is defined based on WHO-recommended exercise levels (≥500 METs/week). CVDRFs: hypertension, hyperlipidemia, and diabetes mellitus.
Anxiety, depression, and development of CVDRFs
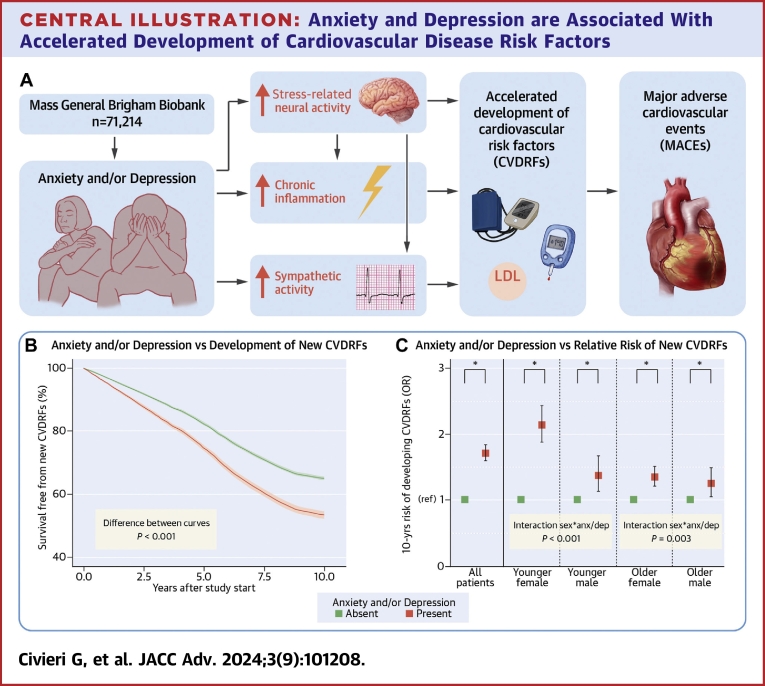
Among 71,214 participants in the anxiety and/or depression analysis, 27,048 (38.0%) developed a new CVDRF during follow-up. Participants with pre-existing anxiety and/or depression had significantly higher 10-year risk of developing a new CVDRF, a finding that persisted after adjusting for demographic characteristics and health behaviors (primary model; OR: 1.71 [95% CI: 1.59 to 1.83], P < 0.001) (Table 2). Similar results were observed for anxiety and depression individually, after adjustment for antidepressant medications (Table 2) and in subjects with both anxiety and depression (OR: 1.98 [95% CI: 1.77-2.21], P < 0.001). Survival free from the development of new CVDRFs was significantly greater in patients without depression/anxiety (difference of proportion free from new CVDRFs at 10 years: 11.6% [95% CI: 10.2-13.0%], P < 0.001) (Central Illustration B). In additional analyses, where those with pre-existing CVDRFs were excluded or the date of Biobank consent was employed as start of the observation period, the findings were generally similar (Supplemental Tables 3 and 4). Also, exclusion of participants with post-traumatic stress disorder from the pool of participants with anxiety disorders yielded similar results, both for the combined predictor anxiety and/or depression (OR: 1.56 [95% CI: 1.44-1.68], P < 0.001) and for the single predictor anxiety (OR: 1.56 [95% CI: 1.43-1.71], P < 0.001). Anxiety/depression also significantly heightened the risk of developing each of the CVDRFs tested individually (Supplemental Table 5).
Table 2.
Impact of Anxiety and/or Depression on the 10-Yrs Risk of and Timing of Developing CVDRFs
| Predictor | Covariables | 10 Years Risk of Developing ≥1 CVDRFs |
Time to Develop One CVDRF |
||||
|---|---|---|---|---|---|---|---|
| N | OR (95% CI) | P Value | N | β (95% CI) | P Value | ||
| Depression | Pre-existing CVDRFs | 77,805 | 1.298 (1.238-1.361) | <0.001 | 30,956 | −0.451 (−0.453 to −0.360) | <0.001 |
| Age, sexa | 77,804 | 1.847 (1.756-1.942) | <0.001 | 30,955 | −0.591 (−0.684 to −0.497) | <0.001 | |
| Smokingb | 67,511 | 1.763 (1.671-1.859) | <0.001 | 27,525 | −0.551 (−0.648 to −0.453) | <0.001 | |
| Lifestyle factorsb | 34,554 | 1.576 (1.454-1.709) | <0.001 | 13,338 | −0.473 (−0.623 to −0.322) | <0.001 | |
| All the above (Primary Model) | 34,522 | 1.569 (1.447-1.701) | <0.001 | 13,334 | −0.471 (−0.622 to −0.321) | <0.001 | |
| SSRI or SNRIc | 29,819 | 1.393 (1.226-1.582) | <0.001 | 11,324 | −0.303 (−0.547 to −0.060) | 0.015 | |
| Anxiety | Pre-existing CVDRFs | 72,482 | 1.195 (1.139-1.254) | <0.001 | 28,489 | −0.418 (−0.513 to −0.322) | <0.001 |
| Age, sexa | 72,481 | 1.789 (1.699-1.884) | <0.001 | 28,488 | −0.567 (−0.665 to −0.469) | <0.001 | |
| Smokingb | 62,684 | 1.706 (1.616-1.801) | <0.001 | 25,279 | −0.538 (−0.640 to −0.436) | <0.001 | |
| Lifestyle factorsb | 32,284 | 1.654 (1.522-1.796) | <0.001 | 12,366 | −0.488 (−0.644 to −0.332) | <0.001 | |
| All the above (Primary Model) | 32,252 | 1.646 (1.515-1.788) | <0.001 | 12,361 | −0.483 (−0.639 to −0.326) | <0.001 | |
| SSRI or SNRIc | 27,759 | 1.397 (1.247-1.564) | <0.001 | 10,352 | −0.298 (−0.520 to −0.076) | 0.009 | |
| Anxiety and/or depression | Pre-existing CVDRFs | 71,214 | 1.316 (1.263-1.373) | <0.001 | 27,048 | −0.433 (−0.525 to −0.361) | <0.001 |
| Age, sexa | 71,213 | 1.921 (1.836-2.009) | <0.001 | 27,047 | −0.579 (−0.664 to −0.494) | <0.001 | |
| Smokingb | 61,727 | 1.826 (1.741-1.916) | <0.001 | 24,054 | −0.539 (−0.628 to −0.450) | <0.001 | |
| Lifestyle factorsb | 31,768 | 1.714 (1.595-1.842) | <0.001 | 11,731 | −0.490 (−0.624 to −0.355) | <0.001 | |
| All the above (Primary Model) | 31,739 | 1.707 (1.589-1.835) | <0.001 | 11,728 | −0.486 (−0.621 to −0.351) | <0.001 | |
| SSRI or SNRIc | 28,288 | 1.440 (1.294-1.603) | <0.001 | 10,353 | −0.300 (−0.511 to −0.089) | 0.005 | |
Boldface values represent statistical significance (P < 0.05).
β = unstandardized coefficient; CVDRF = cardiometabolic cardiovascular risk factor; SSRI = selective serotonin reuptake inhibitors; SNRI = serotonin and norepinephrine reuptake inhibitors.
Analysis adjusted for the pre-existing CVDRFs.
Analysis adjusted for the pre-existing CVDRFs, age, and sex.
Analysis adjusted for all the components of the primary model. Lifestyle factors: body mass index, insomnia, and physical activity. CVDRFs: hypertension, hyperlipidemia, and diabetes mellitus.
Central Illustration.
Anxiety and Depression are Associated With Accelerated Development of Cardiovascular Disease Risk Factors
(A) Anxiety and/or depression are associated with an accelerated development of new CVDRFs. Increased stress-related neural activity, chronic inflammation, and sympathetic activity are associated with anxiety and/or depression, interact with each other, and might represent the mechanistic background of the association between psychiatric conditions and development of new CVDRF. Finally, the development of new CVDRF represents an important mediator in the relationship between anxiety, depression, and MACE. (B) Depression and/or anxiety significantly reduce the survival free from the development of new CVDRF (area between the curves=0.718 [95% CI: 0.627-0.807], P < 0.001). (C) Younger females suffer a significantly greater influence of anxiety and/or depression on the development of CVDRFs than other age and sex subgroups. Analyses are adjusted for the components of the primary model. Error bars represent 95% CI. ∗P < 0.05; anx/dep = anxiety and/or depression; CVDRF = cardiometabolic cardiovascular risk factor (hypertension, hyperlipidemia, and diabetes mellitus); ns = not significant; MACE = major adverse cardiovascular event.
In a sensitivity analysis using only depression diagnoses confirmed by NLP (either ICD positive and NLP positive or ICD negative and NLP negative), the impact of depression on the development of new CVDRFs was even stronger (OR: 1.94 [95% CI: 1.75-2.15], P < 0.001).
Anxiety, depression, and timing of the development of CVDRFs
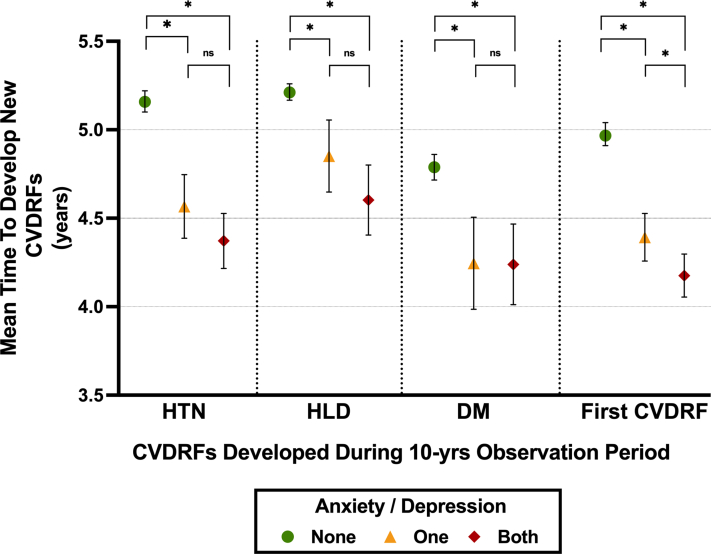
The median time from the study start date to the diagnosis of a new CVDRF was 4.80 years (IQR: 2.68-6.92 years). Those with pre-existing anxiety and/or depression developed a new CVDRF sooner than individuals without, even after adjustment for components of the primary model (β = −0.486 years [95% CI: −0.62 to −0.35], P < 0.001) and for antidepressant medications (Table 2). Moreover, a cumulative effect of anxiety/depression on the timing of the development of a new CVDRF was observed (Figure 2). Anxiety/depression additionally were associated with an accelerated development of each of the CVDRFs tested individually (Supplemental Table 6). Sensitivity analyses excluding individuals with pre-existing CVDRFs, or using consent date as study start date, showed similar findings (Supplemental Tables 3 and 4).
Figure 2.
Anxiety and Depression Influence the Timing of Development of CVDRFs
Among subject (n = 27,048) who develop CVDRF, those with anxiety and/or depression develop them sooner than patients without. The presence of both conditions has a significant effect compared to the presence of only one. Time is expressed as mean time within each group, with error bars representing 95% confidence interval. ∗P < 0.05; HTN = hypertension; HLD = hyperlipidemia; DM = diabetes mellitus; CVDRF = cardiometabolic cardiovascular risk factor (hypertension, hyperlipidemia, diabetes mellitus); ns = non-significant.
Relationship between anxiety, depression, development of CVDRFs, and incident MACE
The distribution of MACE and MACE subtypes is reported in Supplemental Table 7. Anxiety, depression, number of CVDRFs developed, and timing of CVDRF development were independently associated with increased risk of MACE (Supplemental Table 8). Survival free from incident MACE was significantly greater in patients without depression/anxiety (difference of proportion free from new MACE at 10 years: 2.3% [95% CI: 0.5%-3.2%], P < 0.001) (Supplemental Figure 2). Mediation analysis showed that, overall, the number of CVDRFs gained explained 17.7% of the association between anxiety/depression and MACE, independently from demographic factors and health behaviors (Table 3).
Table 3.
Development of CVDRFs Mediates the Association Between Anxiety and/or Depression and 10-Year Risk of MACE in the Overall Population and Across Subgroups Stratified by Age and Sex
| Predictor | Mediator | Outcome | Subgroup | N | Direct Pathway (ie, excluding CVDRFs developed) |
Indirect Pathway (ie, mediated by CVDRFs developed) |
|||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | Percentage | P Value | |||||
| Anxiety and/or depression | Number of CVDRFs developed | MACE | All patientsa | 30,248 | 0.2040 (0.0928-0.3152) | 0.003 | 0.0440 (0.0342-0.0547) | 17.7% | <0.05 |
| Younger femaleb | 8,159 | 0.1912 (−0.1318;0.5143) | 0.2459 | 0.0544 (0.0143-0.0924) | 22.1% | <0.05 | |||
| Younger maleb | 5,268 | 0.2128 (−0.1290;0.5546) | 0.2223 | 0.0402 (0.0104-0.0754) | 16.6% | <0.05 | |||
| Older femaleb | 9,844 | 0.1504 (−0.0108;0.3115) | 0.0674 | 0.0207 (0.0067-0.0.358) | 12.1% | <0.05 | |||
| Older maleb | 6,977 | 0.0357 (−0.1637;0.2352) | 0.7257 | 0.0069 (−0.0152;0.0293) | NA | >0.05 | |||
Boldface values represent statistical significance (P < 0.05).
CVDRF = cardiometabolic cardiovascular risk factor; MACE = major adverse cardiovascular event; β = log-odds.
Analysis is adjusted for the components of the primary model (age, sex, pre-existing CVDRFs, lifestyle factors, and smoking)
Analyses are adjusted for pre-existing CVDRFs, lifestyle factors, and smoking. Lifestyle factors: body mass index, insomnia, and physical activity. CVDRFs: hypertension, hyperlipidemia, diabetes mellitus. “Younger” and “older” was defined according to the median age for each sex. For patients who develop CVDRFs, only MACE occurring after development of the new CVDRFs are considered.
In sensitivity analyses evaluating coronary endpoints (myocardial infarction, unstable angina, and coronary revascularization), anxiety/depression were associated with increased risk of coronary events (OR: 1.25, [95% CI: 1.08-1.44], P = 0.02). The development of CVDRFs explained 41.7% of the association between anxiety/depression and coronary events (indirect pathway: log odds 0.0853 [95% CI: 0.0677-0.1032]; P < 0.05).
Age and sex effects in the relationships between anxiety, depression, CVDRFs, and MACE
Significant age and sex interactions were noted for anxiety/depression-attributable development of CVDRFs (P < 0.001 and P < 0.001, respectively) (Table 4). Thereafter, the population was stratified according to age and sex; median age was used to define “younger” versus “older” (ie 48.80 years for women and 52.97 years for men). Baseline characteristics of these groups are reported in Supplemental Table 9. Overall, younger females experienced the highest anxiety/depression-associated risk of developing CVDRFs (OR: 2.14 [95% CI: 1.87-2.44], P < 0.001, Central Illustration C). Adjustment for socioeconomic variables did not significantly change the results (Supplemental Figure 3). Notably, in absolute terms (ie, the proportion of patients who developed a new CVDRF), the development of a new CVDRF was more frequent among older males, as expected. However, the greatest impact of anxiety and/or depression on the absolute risk of developing a new CVDRF was observed among younger females (+106.8%; P < 0.05) (Supplemental Figure 4).
Table 4.
Age and Sex Determine Differences in 10-Yr Risk of Developing Cardiometabolic Cardiovascular Risk Factors
| Predictor | Age Groups |
Sex Groups |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age < Mediana |
Age ≥ Mediana |
Interaction with Ageb |
Femalec |
Malec |
Interaction With Sexd |
|||||
| 10-Year Risk of Developing ≥1 CVDRFs | ||||||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | P Value | |
| Depression | 1.472 (1.311-1.653) | <0.001 | 1.128 (1.012-1.256) | 0.029 | <0.001 | 1.597 (1.452-1.757) | <0.001 | 1.396 (1.195-1.631) | <0.001 | 0.002 |
| Anxiety | 1.504 (1.336-1.693) | <0.001 | 1.120 (1.004-1.249) | 0.043 | <0.001 | 1.665 (1.510-1.836) | <0.001 | 1.488 (1.272-1.742) | <0.001 | 0.002 |
| Anxiety and/or Depression | 1.596 (1.441-1.769) | <0.001 | 1.200 (1.091-1.319) | <0.001 | <0.001 | 1.785 (1.637-1.945) | <0.001 | 1.467 (1.285-1.675) | <0.001 | <0.001 |
Boldface values represent statistical significance (P < 0.05).
CVDRF = cardiometabolic cardiovascular risk factor.
All analyses are adjusted for sex and for the other components of the primary model (pre-existing CVDRFs, lifestyle factors, and smoking).
The interaction terms = predictor∗age [≥versus <median].
All analyses are adjusted for age and for the other components of the primary model (pre-existing CVDRFs, lifestyle factors, and smoking).
Interaction term = predictor∗sex. Lifestyle factors: body mass index, insomnia, and physical activity. CVDRFs: hypertension, hyperlipidemia, diabetes mellitus.
The impact of anxiety/depression on the timing of development of CVDRFs was also greater among younger women (Supplemental Figure 5), though the interaction term was not-significant (P for interaction = 0.129).
Younger females showed the highest risk for MACE related to anxiety/depression (Supplemental Figure 6). Accordingly, we sought to test whether differences in the development of CVDRFs contributed to the observed age and sex variations of MACE risk. Within each age/sex subgroup, the highest mediated effect (ie, the proportion of the total effect of depression/anxiety on MACE risk mediated by the development of CVDRFs) was found in younger females, among whom the number of CVDRFs developed accounted for 22.1% of the association between psychiatric conditions and MACE (Table 3).
Activity within neuro-immune pathways in the relationship between anxiety, depression, and development of CVDRFs
Next, we investigated potential pathways involved in the relationship between anxiety/depression and development of CVDRFs. Notably, in the smaller “mechanistic cohorts”, pre-existing anxiety/depression were similarly associated with an increased risk of developing new CVDRFs (Supplemental Table 10).
AmygAC was assessed in 1,520 study participants (49.6% females, median age 63.94 years [IQR: 54.81-71.40 years], 50.0% with pre-existing anxiety/depression). Higher AmygAC was associated with an increased risk of developing new CVDRF after adjusting for other predisposing factors (adjusted HR 1.13, [95% CI 1.01-1.27], P = 0.033) (Supplemental Table 11). This effect was mainly driven by the higher risk of developing HTN and DM.
We additionally assessed hs-CRP and HRV, in 15,164 (61.3% females, median age 57.23 yrs [44.04-67.24], 47.1% with pre-existing anxiety/depression) and 11,455 (56.5% females, median age 58.85 yrs [46.97-67.85], 53.4% with pre-existing anxiety/depression) individuals, respectively. Both hs-CRP and HRV associated with increased risk of developing new CVDRFs (Supplemental Table 11).
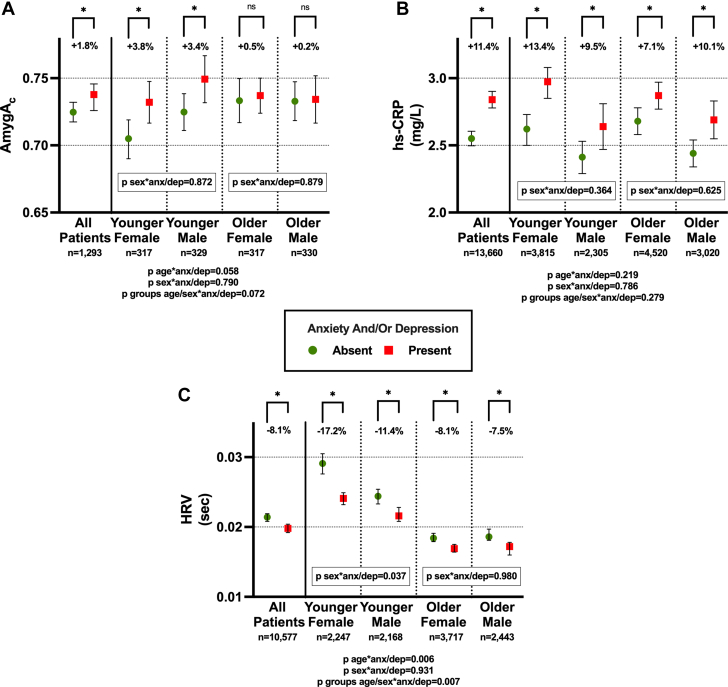
Given the age and sex differences reported in the clinical cohort, we assessed the impact of anxiety/depression on neuro-immune pathways across different age and sex groups. Though significant age and sex differences were found only for autonomic nervous system function, the impact of anxiety/depression on neuro-immune pathways was overall more pronounced in younger women (Figure 3).
Figure 3.
Age and Sex Modify the Relationship Between Psychiatric Conditions and Neuro-immune Pathways
(A) In younger participants, the presence of pre-existing anxiety and/or depression tends to determine a greater increase in AmygAc (P for interaction = 0.058). (B) Although younger females have the greatest relative increase in hs-CRP levels when pre-existing depression/anxiety is present, neither age nor sex significantly modified the impact of depression/anxiety on hs-CRP. (C) In younger participants, the presence of pre-existing anxiety and/or depression determined a significantly greater increase in HRV (P for interaction = 0.06). Among these, the effect is stronger in female (P for interaction = 0.037). Mean values of AmygAc, HRV, and CRP are reported. Error bars represent 95% CI. Age is divided according to the median age of each study cohort. ∗P < 0.05; anx/dep = anxiety and/or depression; AmygAc = amygdalar to ventromedial prefrontal cortical activity ratio; HRV = heart rate variability; hs-CRP = high sensitivity C-reactive protein; ns = not significant
Discussion
The study demonstrates that anxiety/depression are significantly associated with an accelerated development of new CVDRFs, independently of potential confounders such as health behaviors and medications prescribed for anxiety/depression. Further, we observed that neuro-immune pathways associated with stress contribute to the anxiety/depression-associated development of CVDRFs. Furthermore, the link between anxiety/depression and MACE was mediated by the development of CVDRFs. Notably, these effects were particularly pronounced in younger women, suggesting a potential mechanism to explain prior observations that the CVD risks of anxiety/depression are greatest among younger women.19, 20, 21 Taken together, these findings provide novel insight into the complex relationship between anxiety/depression and CVD and highlight the need for comprehensive interventions targeting mental health and CVDRFs to reduce the risk of CVD associated with anxiety/depression.
Anxiety/depression increase the risk for and accelerate the development of cardiovascular risk factors
While previous studies have separately, and primarily cross-sectionally, examined the association between anxiety/depression and individual CVDRFs,3, 4, 5, 6, 7 our study investigated the collective impact of these mental health conditions on the development of these risk factors. Further, in contrast to prior studies that focused on associations between anxiety/depression and the risk of CVD itself,1,2 our study specifically examines the rate at which individuals acquire CVDRFs. Our findings demonstrate a significant impact of anxiety/depression on CVDRF development that is independent from lifestyle factors and common35 antidepressant medications.
Additionally, prior work suggests that the duration of exposure to CVDRFs plays an important role in CVD risk.36 To our knowledge, the current study is unique in examining the influence of anxiety/depression on the timing of CVDRF acquisition. We found that anxiety/depression are independently and synergistically associated with an accelerated acquisition of new CVDRFs. The earlier development of CVDRFs has important implications, as it extends the duration of exposure to these risk factors and, ultimately, contributes to adverse CVD outcomes.
Moreover, the current study provides insights into mechanisms through which anxiety/depression may contribute to the development of CVDRFs. Although the exact underlying pathophysiological mechanisms connecting anxiety/depression to the development of CVDRFs are not fully understood, several factors have been hypothesized.1 These include heightened stress-related neural activity,11,12 autonomic nervous system dysfunction,13 chronic inflammation,14,15,18 and behavioral mechanisms such as diet, smoking, insomnia, and physical inactivity.26,27 Our findings support these hypotheses and suggest that heightened neuro-immune pathways associated with anxiety/depression (such as stress-related neural activity,10 autonomic nervous system dysfunction,16 and inflammation15,17,18) associate with the development of new CVDRFs.
Development of CVDRFs mediates the effects of anxiety/depression on cardiovascular events
Anxiety/depression are independent risk factors for the occurrence of MACE.1,2 Herein, we observed that anxiety/depression leads to MACE in part via development of CVDRFs, independently from demographics and health behaviors. Moreover, we observed that the greatest mediated effect was among younger women. This, together with the other findings, suggests that anxiety/depression may exert relatively larger effects in neuro-immune pathways that enhance the development of CVDRFs in this subgroup, which in turn mediate the development of MACE. This could provide a potential mechanism explaining the high CVD risk associated with anxiety/depression in younger female.19, 20, 21 Our findings might have important clinical implications. Indeed, earlier screening for CVDRFs among individuals with anxiety or depression might reduce CVD risks.
Age and sex modify the relationship between anxiety/depression and cardiovascular risk factors
Recently, the prevalence of anxiety/depression has increased in young adults.37,38 Concurrently, a rising burden of CVDRFs in young adults has been reported,39 especially in females,40 which translates into an increased incidence of CVD among these individuals.40 As a possible connection to these prior epidemiological observations, we found that anxiety/depression are more strongly associated with the acquisition of new CVDRFs among younger women.
Several factors can explain our findings of larger impact of anxiety/depression on cardiometabolic risk among younger individuals and women.22,41,42 In women and in younger individuals, anxiety/depression tend to be more severe43,44 and may associate with greater biological consequences.45,46 In our study, the greatest impact of anxiety/depression on neuro-immune pathways of stress was found among younger women, potentially contributing to the higher relative risk of developing CVDRFs. It should be noted, however, that younger women without anxiety/depression tend to have the healthiest values for AmygAC, HRV, and CRP. Nevertheless, in this group, anxiety/depression induced a catch-up phenomenon with neuro-immune values that approach those of men and older women, thus explaining the heightened relative risk of developing new CVDRFs and the subsequent heightened relative CVD risk.
Study limitations
This study carries the inherent limitations of a retrospective observational design, and causality cannot be derived from our results. Though we accounted for many pertinent confounders, residual confounding cannot be excluded. Moreover, data about physical activity are not available for all patients and adjustment for this covariable reduced sample size. Mortality was also not investigated. Diagnoses were based on ICD codes, which may be subject to misclassification However, in a sensitivity analysis using diagnoses of depression obtained via natural language processing, similar results were obtained. Further, the ICD codes do not provide data on disease severity. However, we provide data on the co-occurrence of depression and anxiety, which may represent a surrogate for affective disorder disease severity47 and has been shown to be associated with a heightened risk of affective disease-related adverse health conditions.48 Additionally, patients with anxiety/depression might seek medical attention more frequently than those without psychological maladies. This factor might contribute to the increased new diagnosis of CVDRFs noted among individuals with anxiety/depression. Still, it would not explain the link between the accelerated gain in CVDRFs and the development of incident MACE. We also used dates of the first prescription of antidepressants and do not know for how long this therapy was continued or patients' adherence to these medications. Moreover, we did not evaluate the role of anxiolytic medications or psychological interventions: additional studies on this aspect are needed.49 Further, data about the number of patients lost during follow-up is unavailable; we posit that patients without new ICD codes during the follow-up were less likely to have experienced new diagnoses or events. This could have eventually led to an underestimation (but not an overestimation) of the association between anxiety/depression and the development of CVDRFs. Lastly, mechanistic analyses were performed in a sample of participants for whom such data were available as part of clinical care. Therefore, ascertainment bias cannot be excluded.
Conclusions
This study demonstrates that anxiety and depression are associated with an accelerated development of CVDRFs, independently of health behaviors and medications. This development of CVDRFs significantly contributes to the observed heightened CVD risk and appears to be mediated by neuro-immune mechanisms. Notably, important age and sex effects exist, whereby the impact of anxiety/depression on neuro-immune mechanisms and the development of CVDRFs appears to be greatest in younger women. Together, these findings help clarify the pathogenic links between anxiety, depression, and CVD and underscore a need for tailored screening and preventive measures among individuals with anxiety and depression.
Funding support and author disclosures
Dr Osborne has received consulting fees from WCG Clinical for unrelated work; and is supported in part by NIH K23HL151909 and AHA 23SCISA1143491. Dr Tawakol’s institution has received grant support from Lung Biotechnologies for unrelated work. Dr Seligowski is supported in part by NIH MH125920. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Important neuroimmune pathways underlie the association between anxiety, depression, and cardiovascular risk. Specific treatment of these pathways may reduce the cardiovascular risk associated with anxiety and depression.
COMPETENCY IN PATIENT CARE: A close screening of cardiovascular risk factors should be performed in subjects with anxiety and depression. Younger female, although generally being at low risk of developing cardiovascular risk factors, are particularly susceptible to the cardiometabolic effects of anxiety and depression,
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables and a figure, please see the online version of this paper.
Appendix A. Supplementary data
References
- 1.Carney R.M., Freedland K.E. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14:145–155. doi: 10.1038/nrcardio.2016.181. [DOI] [PubMed] [Google Scholar]
- 2.Roest A.M., Martens E.J., de Jonge P., Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Golden S.H., Williams J.E., Ford D.E., et al. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2004;27:429–435. doi: 10.2337/diacare.27.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Santos-Veloso M.A.O., Melo MISL de, Cavalcanti R.A.N., Bezerra L.S., Chaves-Markman Â.V., Lima SG de. Prevalence of depression and anxiety and their association with cardiovascular risk factors in Northeast Brasil primary care patients. Rev Assoc Med Bras. 2019;65:801–809. doi: 10.1590/1806-9282.65.6.801. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W.-Y., Nan N., He Y., et al. Prevalence of depression and anxiety symptoms and their associations with cardiovascular risk factors in coronary patients. Psychol Health Med. 2022;28:1275–1287. doi: 10.1080/13548506.2022.2104885. [DOI] [PubMed] [Google Scholar]
- 6.Maatouk I., Herzog W., Böhlen F., et al. Association of hypertension with depression and generalized anxiety symptoms in a large population-based sample of older adults. J Hypertens. 2016;34:1711–1720. doi: 10.1097/HJH.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 7.Honigberg M.C., Ye Y., Dattilo L., et al. Low depression frequency is associated with decreased risk of cardiometabolic disease. Nat Cardiovasc Res. 2022;1:125–131. doi: 10.1038/s44161-021-00011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborne M.T., Shin L.M., Mehta N.N., Pitman R.K., Fayad Z.A., Tawakol A. Disentangling the links between psychosocial stress and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawakol A., Ishai A., Takx R.A., et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezue K.N., Zureigat H., Abohashem S., et al. Abstract 13714: stress-related neural pathways mediate the link between depression/anxiety disorders and cardiovascular disease. Circulation. 2021;144 [Google Scholar]
- 11.Grewal Simran, Mezue Kenechukwu, Abohashem Shady, et al. Abstract 14748: genetic and neurobiological factors link chronic stress to earlier onset hypertension. Circulation. 2022;146 [Google Scholar]
- 12.Osborne M.T., Ishai A., Hammad B., et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology. 2019;100:32–40. doi: 10.1016/j.psyneuen.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J., Chang Y., Kim Y., Shin H., Ryu S. Ten-second heart rate variability, its changes over time, and the development of hypertension. Hypertension. 2022;79:1308–1318. doi: 10.1161/HYPERTENSIONAHA.121.18589. [DOI] [PubMed] [Google Scholar]
- 14.Calle M.C., Fernandez M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–191. doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sgoifo A., Carnevali L., Alfonso M., de los A.P., Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015;18:343–352. doi: 10.3109/10253890.2015.1045868. [DOI] [PubMed] [Google Scholar]
- 17.Michopoulos V., Powers A., Gillespie C.F., Ressler K.J., Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42:254–270. doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccarino V., Johnson B.D., Sheps D.S., et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarino V., Badimon L., Bremner J.D., et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. 2020;41:1687–1696. doi: 10.1093/eurheartj/ehy913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.N., Yun J.-S., Ko S.-H., et al. Impacts of gender and lifestyle on the association between depressive symptoms and cardiovascular disease risk in the UK Biobank. Sci Rep. 2023;13 doi: 10.1038/s41598-023-37221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolderen K.G., Strait K.M., Dreyer R.P., et al. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. JAHA. 2015;4 doi: 10.1161/JAHA.114.001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein J.M., Hale T., Foster S.L., Tobet S.A., Handa R.J. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology. 2019;44:59–70. doi: 10.1038/s41386-018-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millett E.R.C., Peters S.A.E., Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363 doi: 10.1136/bmj.k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutin N.T., Schecter S.B., Perez E.F., et al. The Evolution of a large biobank at mass general Brigham. J Pers Med. 2022;12:1323. doi: 10.3390/jpm12081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fawcett J., Kravitz H.M. Anxiety syndromes and their relationship to depressive illness. J Clin Psychiatry. 1983;44:8–11. [PubMed] [Google Scholar]
- 26.Bonnet F., Irving K., Terra J.-L., Nony P., Berthezène F., Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Gan Y., Zhou X., et al. Insomnia and the risk of hypertension: a meta-analysis of prospective cohort studies. Sleep Med Rev. 2021;56 doi: 10.1016/j.smrv.2020.101403. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino R.B., Vasan R.S., Pencina M.J., et al. General cardiovascular risk Profile for Use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 29.Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezue K., Osborne M.T., Abohashem S., et al. Reduced stress-related neural Network activity mediates the effect of Alcohol on cardiovascular risk. J Am Coll Cardiol. 2023;81:2315–2325. doi: 10.1016/j.jacc.2023.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radfar A., Abohashem S., Osborne M.T., et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur Heart J. 2021;42:1898–1908. doi: 10.1093/eurheartj/ehab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang D.O., Eo J.S., Park E.J., et al. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: a prospective serial 18F-FDG-PET/CT imaging assessment. Eur Heart J. 2021;42:1883–1895. doi: 10.1093/eurheartj/ehaa1095. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.-M., Lee R., Kim Y., et al. Impact of metabolic activity of Vertebra and amygdala on stroke Recurrence: a prospective cohort study. Circ: Cardiovascular Imaging. 2023;16 doi: 10.1161/CIRCIMAGING.122.014544. [DOI] [PubMed] [Google Scholar]
- 34.Zureigat H., Osborne M.T., Abohashem S., et al. Effect of stress-related neural pathways on the cardiovascular Benefit of physical activity. J Am Coll Cardiol. 2024;83:1543–1553. doi: 10.1016/j.jacc.2024.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y., Kataoka Y., Ostinelli E.G., Cipriani A., Furukawa T.A. National prescription patterns of antidepressants in the treatment of adults with major depression in the US between 1996 and 2015: a population representative survey based analysis. Front Psychiatry. 2020;11:35. doi: 10.3389/fpsyt.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Pletcher M.J., Vittinghoff E., et al. Association between cumulative low-Density Lipoprotein Cholesterol exposure during young adulthood and Middle age and risk of cardiovascular events. JAMA Cardiol. 2021;6:1406–1413. doi: 10.1001/jamacardio.2021.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin R.D., Weinberger A.H., Kim J.H., Wu M., Galea S. Trends in anxiety among adults in the United States, 2008-2018: Rapid increases among young adults. J Psychiatr Res. 2020;130:441–446. doi: 10.1016/j.jpsychires.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodwin R.D., Dierker L.C., Wu M., Galea S., Hoven C.W., Weinberger A.H. Trends in U.S. Depression prevalence from 2015 to 2020: the Widening treatment Gap. Am J Prev Med. 2022;63:726–733. doi: 10.1016/j.amepre.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal R., Yeh R.W., Joynt Maddox K.E., Wadhera R.K. Cardiovascular risk factor prevalence, treatment, and Control in US adults aged 20 to 44 Years, 2009 to March 2020. JAMA. 2023;329:899. doi: 10.1001/jama.2023.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora S., Stouffer G.A., Kucharska-Newton A.M., et al. Twenty Year Trends and sex differences in young adults Hospitalized with acute myocardial infarction: the ARIC community surveillance study. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaccarino V., Bremner J.D. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J.E., Holsen L.M., Ironside M., et al. Neural response to stress differs by sex in young adulthood. Psychiatry Res Neuroimaging. 2023;332 doi: 10.1016/j.pscychresns.2023.111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank E., Carpenter L.L., Kupfer D.J. Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry. 1988;145:41–45. doi: 10.1176/ajp.145.1.41. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg J.H., Breckenridge J.N., Sheikh J.I. Age differences in symptoms of depression and anxiety: examining behavioral medicine outpatients. J Behav Med. 2003;26:119–132. doi: 10.1023/a:1023030605390. [DOI] [PubMed] [Google Scholar]
- 45.Morris A.A., Zhao L., Ahmed Y., et al. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73:462–468. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia R.G., Mareckova K., Holsen L.M., et al. Impact of sex and depressed mood on the central regulation of cardiac autonomic function. Neuropsychopharmacology. 2020;45:1280–1288. doi: 10.1038/s41386-020-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmeijer-Sevink M.K., Batelaan N.M., van Megen H.J.G.M., et al. Clinical relevance of comorbidity in anxiety disorders: a report from The Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2012;137:106–112. doi: 10.1016/j.jad.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Bobo W.V., Grossardt B.R., Virani S., St Sauver J.L., Boyd C.M., Rocca W.A. Association of depression and anxiety with the Accumulation of chronic conditions. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart J.C., Perkins A.J., Callahan C.M. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76:29–37. doi: 10.1097/PSY.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.