Abstract
Background
Hypertension is an important contributor to cardiovascular disease (CVD) in breast cancer (BC) survivors; however, research on blood pressure (BP) and CVD outcomes in BC survivors is limited.
Objectives
The purpose of this study was to better characterize the association between BP and CVD in a large, longitudinal cohort of BC patients.
Methods
Women with invasive BC diagnosed from 2005 to 2013 at Kaiser Permanente Northern California were matched 1:5 to women without BC. Patient data were obtained from electronic health records. Multivariable Cox regression and penalized spline models were used to explore the linear and nonlinear relationship of systolic blood pressure (SBP) and diastolic blood pressure (DBP) on CVD outcomes.
Results
BC cases (n = 12,713) and controls (n = 55,886) had median follow-up of 9.6 years (IQR: 5.0-11.9 years). Women with BC had a mean age of 60.6 years; 64.8% were non-Hispanic White. For ischemic heart disease (IHD), every 10 mmHg increase in SBP and DBP was associated with 1.23 (95% CI: 1.14-1.33) and 1.10 (95% CI: 0.98-1.24) risk, respectively, in women with BC. For stroke, every 10 mmHg increase in SBP and DBP was associated with a 1.45 (95% CI: 1.34-1.58) and 1.91 (95% CI: 1.68-2.18) risk, respectively. A U-shaped relationship was observed between heart failure/cardiomyopathy and BP. The associations between BP and risk of IHD, stroke, and any primary CVD were not statistically different comparing women with BC to controls, but risks varied by BC status for heart failure/cardiomyopathy (P for interaction = 0.01).
Conclusions
Women with and without BC showed similar risks for IHD, stroke, and any primary CVD suggesting similar BP targets should be pursued regardless of BC survivorship status.
Key words: blood pressure, cardiovascular outcomes, breast cancer, hypertension, cardio-oncology
Central Illustration
The number of cancer survivors in the United States (US) is increasing. In 2022, there were more than 18 million cancer survivors in the US and that number is estimated to grow.1 As this cohort ages, noncancer comorbidities increasingly affect the long-term health of survivors. One important comorbidity is cardiovascular disease (CVD), which is the leading cause of noncancer death in cancer survivors.2,3
Hypertension is a known risk-factor for CVD and more prevalent in cancer survivors than in matched controls.4,5 Not only does hypertension increase the risk of mortality, but it is also the most powerful predictor of cardiac events when compared to other traditional CVD risk factors in cancer survivors.4,5 Among women with breast cancer (BC), hypertension represents the most prevalent CVD comorbidity and is more prevalent than in the general population.6, 7, 8 However, there are limited data on the association of blood pressure (BP) and CVD outcomes in BC survivors.
Current recommendations on screening, monitoring, and treatment of hypertension in cancer is based largely on expert-consensus. The American Society of Clinical Oncology released a guideline for the monitoring of cardiac dysfunction in cancer survivors, which states ‘clinicians should regularly evaluate and manage cardiovascular risk factors such as […] hypertension’.9 A 2019 publication in JACC: CardioOncology provides monitoring and management algorithms largely in-line with AHA/ACC 2017 recommendations for the noncancer population but acknowledges that the optimal BP goal for patients with cancer is unknown.10
The Pathways Heart Study is a large prospective cohort study within Kaiser Permanente Northern California (KPNC), an integrated health system, which provides a unique opportunity to study the association of BP and CVD outcomes and address the question of optimal BP management in women with a history of BC.
Methods
Study Sample
The Pathways Heart Study is a prospective cohort study funded by the National Cancer Institute within the integrated KPNC health system, evaluating cardiometabolic risk factors and incident CVD in women with and without BC. KPNC has more than 4.5 million members across the Northern California region with clinical data available from electronic health records (EHRs) and its own cancer registry that is affiliated with the Surveillance, Epidemiology, and End Results program of the National Cancer Institute. Women were eligible to participate in the Pathways Heart Study if they were diagnosed with BC between November 2005 and March 2013, aged 21 years or older, and were active KPNC members for at least 12 months prior to their BC diagnosis. The following additional exclusion criteria were applied: 1) no recorded systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) measures; and 2) having a diagnosis of ischemic heart disease (IHD), stroke, heart failure (HF), and/or cardiomyopathy at baseline. Controls were women without BC matched 5:1 on age and race and ethnicity to a case and used as a comparison group.
The primary objective was to examine the relationship of BP on CVD outcomes in women with BC; however, findings were also estimated in women without BC to provide context and a comparison.
Measures
The exposure of interest was SBP and DBP measures collected during routine clinic visits by automated BP monitors obtained throughout the study period. Since participants could have multiple BP measures collected over the duration of their KPNC membership, a time-averaged SBP and DBP value was calculated using all BP measures available leading up to the first incident CVD, after which measurements were censored.11 This measure was calculated by multiplying a given BP value by the length of time it was observed until the next BP value was recorded. Then, the values were summed and divided by the total observation time. SBP and DBP were evaluated as continuous and categorical (groups of 10-unit increments ranging from 100 to 160 mm Hg for SBP and <60-90 mm Hg for DBP).
The primary outcomes were incident IHD, stroke, and HF/cardiomyopathy. A composite CVD outcome was created and defined as at least one occurrence of one of the 3 primary outcomes. Cardiovascular events were identified from the KPNC EHR based on International Classification of Diseases diagnostic codes and Current Procedural Terminology procedure codes from inpatient, ambulatory, emergency room, and/or hospital visits. Codes for inherited cardiomyopathies were excluded. Cardiovascular events were identified through December 31, 2018.
Covariate data included demographic and health characteristics at the time of BC diagnosis or a reference date for the women without BC. Age was calculated using date of birth and BC diagnosis or reference date. Race and ethnicity were self-reported. Anthropometric measures and health histories recorded in the EHR were used to identify body mass index and smoking behavior. Diabetes was identified from the KPNC Diabetes Registry with diabetes at baseline defined as having a registry entry date prior to the BC diagnosis or reference date. Prevalent dyslipidemia was characterized from International Classification of Diseases diagnostic codes, laboratory reports, or evidence of a prescription for a lipid-lowering medication within 3 years of BC diagnosis or reference date.
Statistical Analysis
Baseline characteristics for women with BC and controls were described using mean ± SD for continuous variables and percentages for categorical variables. Statistically significant differences between BC survivors and controls were evaluated using either the Student’s t-test or the chi-square test. Multivariable Cox proportional hazard models were used to estimate HRs and 95% CIs on the relationship of SBP and DBP on CVD outcomes. HRs were estimated separately for women with BC and controls. For continuous measures of SBP and DBP, restricted cubic splines using 4 knots were used to fit the model since this approach permits evaluation of both linear and nonlinear associations.12,13 When relationships were observed to be nonlinear, HRs and 95% CIs were generated by categories of SBP and DBP. To evaluate whether the association of BP on CVD outcomes differed between women with BC compared to controls, an interaction term of BP and BC status was included in the models. Multiple sensitivity analyses were completed to validate the relationships observed between BP and CVD. First, we examined non-CVD mortality as a competing risk using Fine-Gray models to estimate subdistribution HRs.14 Second, to address the possibility of reverse causation, hazard models were repeated after excluding CVD events occurring in the first year after BC diagnosis. Third, to examine whether cancer treatment confounded the relationships between BP and CVD, hazard models that included adjustment for cancer therapies occurring during the follow-up period were repeated in the BC cohort. Fourth, to account for the possible influence of pre-existing hypertension, hazard models for the BC cohort were stratified by baseline hypertension. Finally, since CVD events may not be mutually independent, a sensitivity analysis censored CVD events after the first primary CVD outcome occurred. All tests were 2-sided and a P value of <0.05 was considered statistically significant. Analyses were conducted using R version 4.0.3.
Results
Baseline Characteristics
A total of 12,713 women with a new diagnosis of invasive BC were matched to 55,886 women without BC (Table 1). Women with BC were on average 61 years old and 65% non-Hispanic White. Compared to controls, women with BC were more likely to be obese, have been a former smoker, have a higher prevalence of baseline diabetes, and be prescribed antihypertensive and anticoagulant medications (P < 0.01).
Table 1.
Baseline Demographic and Clinical Characteristics
| Women With a History of Breast Cancer (n = 12,713) | Matched Controls (n = 55,886) | P Value | |
|---|---|---|---|
| Age at baseline, y | 60.6 ± 12.7 | 60.9 ± 12.6 | <0.01 |
| BMI at baseline | <0.001 | ||
| Underweight (<18.5 kg/m2) | 196 (1.5) | 925 (1.7) | |
| Normal (18.5-24.9 kg/m2) | 4,171 (32.8) | 18,999 (34.0) | |
| Overweight (25-29.9 kg/m2) | 3,993 (31.4) | 17,598 (31.5) | |
| Obese I (30-34.9 kg/m2) | 2,434 (19.1) | 10,108 (18.1) | |
| Obese II+ (≥35 kg/m2) | 1,917 (15.1) | 8,053 (14.4) | |
| Unknown | 2 (0.0) | 203 (0.4) | |
| Racioethnic group | 0.90 | ||
| White | 8,243 (64.8) | 36,101 (64.6) | |
| Black | 913 (7.2) | 4,080 (7.3) | |
| Hispanic | 1,509 (11.9) | 6,815 (12.2) | |
| Asian | 1,891 (14.9) | 8,186 (14.6) | |
| Pacific Islander | 60 (0.5) | 269 (0.5) | |
| Native American or Native Alaskan | 97 (0.8) | 435 (0.8) | |
| Tobacco use at baseline | <0.001 | ||
| Never smoker | 7,558 (59.5) | 36,109 (64.6) | |
| Current smoker | 1,142 (9.0) | 4,838 (8.7) | |
| Former smoker | 3,710 (29.2) | 13,930 (24.9) | |
| Unknown | 303 (2.4) | 1,009 (1.8) | |
| Hypertension | 7,166 (56.4) | 31,217 (55.9) | 0.30 |
| Diabetes mellitus | 2,635 (20.7) | 10,891 (19.5) | <0.01 |
| Dyslipidemia | 6,980 (54.9) | 31,802 (56.9) | <0.01 |
| CV medications | |||
| Antihypertensive | 8,278 (65.1) | 34,971 (57) | <0.01 |
| Statins | 5,811 (45.7) | 26,517 (47.4) | <0.01 |
| Antiplatelet | 437 (3.4) | 2,108 (3.8) | 0.08 |
| Antithrombotic | 1,395 (11.0) | 5,043 (9.0) | <0.01 |
| Cancer therapy | |||
| Anthracyclines | 1,098 (8.6) | - | |
| Endocrine | 9,338 (73.5) | - | |
| Anti-HER2 | 373 (2.9) | - | |
| Radiation therapy | 8,271 (65.1) | - | |
| Time-averaged SBP, mm Hg | 128.1 ± 11.2 | 127.6 ± 11.5 | <0.01 |
| Time-averaged DBP, mm Hg | 72.6 ± 6.9 | 72.2 ± 7.2 | <0.01 |
| Blood measures, mm Hg | 52.3 ± 33.4 | 27.2 ± 23.3 | <0.01 |
Values are mean ± SD or n (%).
BMI = body mass index; CVD = cardiovascular; DBP = diastolic blood pressure; SBP = systolic blood pressure.
Over a median follow-up of 9.6 years (IQR: 5.0-11.9 years), the time-averaged SBP was 128.1 ± 11.2 mm Hg for BC and 127.6 ± 11.5 mm Hg for controls (P < 0.01). The time-averaged DBP was 72.6 ± 6.9 mm Hg and 72.2 ± 7.2 mmHg for participants with BC and controls, respectively (P < 0.001). The mean number of BP measurements obtained over follow-up was 52.3 ± 33.4 in women with BC and 27.2 ± 23.3 in controls. Women in the BC cohort received endocrine (74%), radiation (65%), anthracycline (9%), and anti-HER2 (3%) cancer therapy.
Blood Pressure and CVD
In women with BC, a linear association was observed between SBP and both IHD and stroke (Table 2, Figure 1). In the adjusted proportional hazards model, the risk of IHD was 1.23 (95% CI: 1.14-1.33) per 10 mm Hg increase in SBP. The risk of stroke was 1.45 (95% CI: 1.34-1.58) per 10 mmHg increase in SPB. A U-shaped association was observed between SBP and both HF/cardiomyopathy and the composite CVD outcome. The nadir for SBP and HF/cardiomyopathy risk was 131 mm Hg. Women with BC and a time-averaged SBP of 100 to 109 mm Hg had a 2.08 (95% CI: 1.36-3.20) risk for HF/cardiomyopathy compared to those with a time-averaged SBP of 130 to 139 mm Hg. In comparison, women with a SBP of 150 to 160 mm Hg had a 2.06 (95% CI: 1.45-2.93) risk of HF/cardiomyopathy relative to women with a SBP of 130 to 139 mm Hg. Associations of SBP on risk of IHD and stroke were similar in controls without BC, but differences were observed for the risk of HF/cardiomyopathy (P for interaction = 0.01) (Supplemental Table 1).
Table 2.
Adjusted Cox Spline Models for Systolic Blood Pressure
| Women With a History of Breast Cancer (n = 12,713) |
Matched Controls (n = 55,886) |
|||
|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | |
| Ischemic heart disease | 628 | 1.23 (1.14-1.33) | 3,129 | 1.15 (1.12-1.19) |
| Stroke | 401 | 1.45 (1.34-1.58) | 1,838 | 1.34 (1.29-1.40) |
| HF/cardiomyopathya | 848 | 1.09 (1.02-1.16) | 3,324 | 1.18 (1.15-1.22) |
| Any primary CVD | 1,541 | 1.20 (1.14-1.26) | 6,809 | 1.19 (1.16-1.22) |
| Ischemic heart disease (mm Hg) | ||||
| 100-109 | 11 | 0.87 (0.47-1.61) | 67 | 0.99 (0.77-1.27) |
| 110-119 | 44 | 0.75 (0.54-1.04) | 297 | 0.95 (0.84-1.09) |
| 120-129 | 196 | 1.08 (0.89-1.30) | 941 | 0.99 (0.91-1.08) |
| 130-139 | 232 | Reference | 1,185 | Reference |
| 140-149 | 107 | 1.45 (1.15-1.82) | 468 | 1.34 (1.21-1.50) |
| 150-160 | 30 | 2.81 (1.91-4.11) | 123 | 1.94 (1.61-2.34) |
| Stroke (mm Hg) | ||||
| 100-109 | 9 | 0.97 (0.49-1.94) | 31 | 0.88 (0.61-1.27) |
| 110-119 | 30 | 0.74 (0.50-1.11) | 137 | 0.81 (0.67-0.97) |
| 120-129 | 89 | 0.72 (0.55-0.93) | 491 | 0.91 (0.81-1.02) |
| 130-139 | 165 | Reference | 701 | Reference |
| 140-149 | 67 | 1.19 (0.90-1.58) | 314 | 1.43 (1.25-1.63) |
| 150-160 | 20 | 2.22 (1.40-3.55) | 109 | 2.67 (2.18-3.27) |
| HF/cardiomyopathy (mm Hg) | ||||
| 100-109 | 24 | 2.08 (1.36-3.20) | 45 | 0.97 (0.72-1.32) |
| 110-119 | 74 | 1.20 (0.92-1.55) | 255 | 0.96 (0.83-1.10) |
| 120-129 | 257 | 1.17 (0.99-1.38) | 940 | 0.96 (0.88-1.04) |
| 130-139 | 307 | Reference | 1,321 | Reference |
| 140-149 | 138 | 1.37 (1.12-1.67) | 554 | 1.30 (1.18-1.44) |
| 150-160 | 35 | 2.06 (1.45-2.93) | 147 | 1.98 (1.67-2.35) |
| Any primary CVD (mm Hg) | ||||
| 100-109 | 37 | 1.33 (0.95-1.88) | 131 | 1.05 (0.88-1.26) |
| 110-119 | 132 | 0.99 (0.81-1.20) | 580 | 0.94 (0.86-1.03) |
| 120-129 | 447 | 1.03 (0.91-1.17) | 1,986 | 0.98 (0.92-1.03) |
| 130-139 | 580 | Reference | 2,629 | Reference |
| 140-149 | 245 | 1.30 (1.12-1.51) | 1,072 | 1.34 (1.25-1.44) |
| 150-160 | 64 | 2.27 (1.75-2.95) | 286 | 2.03 (1.80-2.30) |
Adjusted model: Age, race, BMI, smoking status, diabetes, and dyslipidemia.
CVD = cardiovascular disease; DBP = diastolic blood pressure.
Interaction significant (P = 0.01) between BC history and BP on CVD outcome.
Figure 1.
Spline Graphs for SBP and Risk of CVD
Penalized spline graphs are shown for the association of SBP and risk of ischemic heart disease, stroke, HF/cardiomyopathy, and the composite end-point of any primary CVD in women with and without breast cancer. Y-axis: log HR. X-axis: systolic blood pressure in mm Hg. CVD = cardiovascular disease; HF = heart failure; SBP = systolic blood pressure.
A J-shaped association was seen for DBP and both IHD and stroke (Table 3, Figure 2). The risk of IHD with a DBP of 80 to 89 mm Hg was 1.50 (95% CI: 1.14-1.98) compared to the reference group (DBP of 60-69 mm Hg), with a nadir at 69 mm Hg. For stroke, women with a DBP of 80 to 89 mm Hg had 2.56 (95% CI 1.85-3.55) risk compared to the reference group (DBP of 60-69 mm Hg), with nadir at 61 mmHg. A U-shaped association was observed between DBP and HF/cardiomyopathy. The nadir for DBP and HF/cardiomyopathy risk was 73 mm Hg. Women with a DBP of <60 mm Hg had a 1.34 (95% CI: 0.97-1.85) risk of HF/cardiomyopathy compared to women with a DBP of 70 to 79 mm Hg. Women with a DBP of 80 to 90 mm Hg had a HR of 1.54 (95% CI: 1.22-1.94) risk of HF/cardiomyopathy relative to women with a DBP of 70 to 79 mm Hg. HF/cardiomyopathy events represented 47.2% of the total CVD events. A similar U-shaped relationship with DBP was also observed for the composite CVD outcome. The association of DBP on the risk of these CVD outcomes was similar for the controls (Supplemental Table 1).
Table 3.
Adjusted Cox Spline Models for Diastolic Blood Pressure
| Women with a History of Breast Cancer (n = 12,713) |
Matched Controls (n = 55,886) |
|||
|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | |
| Ischemic heart disease | 628 | 1.10 (0.98-1.24) | 3,129 | 1.07 (1.02-1.13) |
| Stroke | 401 | 1.91 (1.68-2.18) | 1,838 | 1.58 (1.48-1.68) |
| HF/cardiomyopathy | 848 | 1.09 (0.99-1.21) | 3,324 | 1.02 (0.97-1.07) |
| Any Primary CVD | 1,541 | 1.25 (1.16-1.34) | 6,809 | 1.17 (1.13-1.21) |
| Ischemic heart disease (mm Hg) | ||||
| <60 | 35 | 1.53 (1.07-2.19) | 190 | 1.31 (1.12-1.53) |
| 60-69 | 214 | Reference | 1,199 | Reference |
| 70-79 | 300 | 1.12 (0.94-1.34) | 1,403 | 0.99 (0.92-1.08) |
| 80-90 | 72 | 1.50 (1.14-1.98) | 309 | 1.25 (1.10-1.42) |
| Stroke (mm Hg) | ||||
| <60 | 19 | 1.37 (0.85-2.22) | 75 | 0.89 (0.70-1.13) |
| 60-69 | 128 | Reference | 648 | Reference |
| 70-79 | 184 | 1.27 (1.01-1.60) | 842 | 0.89 (0.70-1.13) |
| 80-90 | 57 | 2.56 (1.85-3.55) | 241 | 2.27 (1.95-2.64) |
| HF/cardiomyopathy (mm Hg) | ||||
| <60 | 43 | 1.34 (0.97-1.85) | 214 | 1.32 (1.14-1.53) |
| 60-69 | 331 | 1.08 (0.93-1.26) | 1,412 | 1.09 (1.01-1.17) |
| 70-79 | 373 | Reference | 1,382 | Reference |
| 80-90 | 93 | 1.54 (1.22-1.94) | 289 | 1.32 (1.16-1.50) |
| Any primary CVD (mm Hg) | ||||
| <60 | 75 | 1.28 (1.00-1.63) | 374 | 1.13 (1.01-1.26) |
| 60-69 | 561 | 0.97 (0.87-1.09) | 2,640 | 0.97 (0.92-1.02) |
| 70-79 | 699 | Reference | 3,027 | Reference |
| 80-90 | 185 | 0.97 (0.87-1.09) | 704 | 1.43 (1.32-1.55) |
CVD = cardiovascular disease; HF = heart failure.
Adjusted model: Age, race, BMI, smoking status, diabetes, and dyslipidemia.
Figure 2.
Spline Graphs for DBP and Risk of CVD
Penalized spline graphs are shown for the association of DBP and risk of ischemic heart disease, stroke, HF/cardiomyopathy, and the composite end-point of any primary CVD in women with and without breast cancer. Y-axis: log HR. X-axis: diastolic blood pressure in mm Hg. CVD = cardiovascular disease; DBP = diastolic blood pressure; HF = heart failure.
Sensitivity Analyses
The Fine and Gray analysis accounting for the competing risk of non-CVD mortality showed similar results (Supplemental Tables 2 and 3) to the main analysis. For the association of SBP with CVD in women with BC, the subdistribution HR (sHR) was 1.23 (95% CI: 1.13-1.33) per 10 mm Hg increment for IHD and 1.44 (95% CI: 1.30-1.60) per 10 mm Hg increment for stroke. For DBP, the sHR was 1.08 (95% CI: 0.94-1.23) per 10 mm Hg increment for IHD and 1.79 (95% CI: 1.49-2.15) per 10 mm Hg increment for stroke. Relative to the referent group (DBP of 70-79 mm Hg), the highest HF/cardiomyopathy risk was at the highest increment of 80 to 89 mm Hg (sHR 1.36, 95% CI: 1.08-1.70).
For the reverse causality analysis, results were also similar when CVD events in the first year after BC diagnosis were excluded (Supplemental Tables 4 and 5). Results adjusting for cancer therapies were also consistent with the main analysis (Supplemental Tables 6 and 7), as was the findings that censored CVD events after the first primary outcome (Supplemental Tables 8 and 9). Finally, when women with BC were stratified by the prevalence of baseline hypertension, an increased risk was observed for SBP and all CVD outcomes and for DBP and ischemic stroke (Supplemental Tables 10 and 11).
Discussion
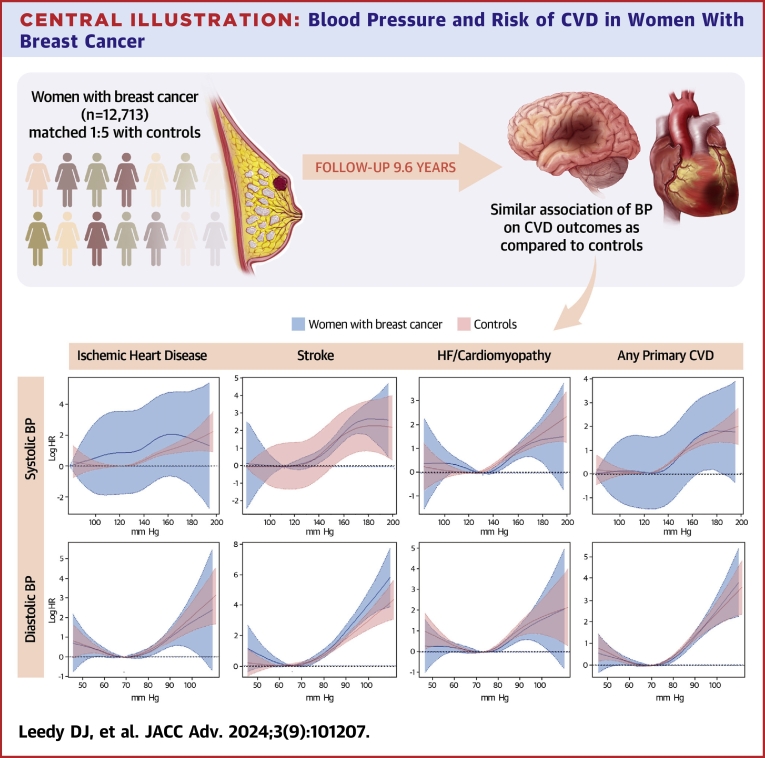
Among women with BC, the present study found that in the time-period following BC diagnosis, SBP had a positive linear association with IHD and stroke, and DBP had a J-shaped association with IHD and stroke (Central Illustration). In addition, SBP and DBP showed a U-shaped relationship for incident HF/cardiomyopathy, suggesting increased risk for this condition at both low and high BP measures. BC survivors with a history of hypertension at baseline had higher risks of the CVD outcomes than BC survivors without baseline hypertension. Most of our findings were consistently observed for women both with and without BC, indicating preventative approaches to reduce risk of CVD via BP should be implemented regardless of BC status.
Central Illustration.
Blood Pressure and Risk of CVD in Women With Breast Cancer
Penalized spline graphs are shown for the association of SBP and DBP with risk of ischemic heart disease, stroke, HF/cardiomyopathy, and the composite end-point of any primary CVD in women with breast cancer (blue) as compared to controls (red). Y-axis: log HR. X-axis: blood pressure in mm Hg. CVD = cardiovascular disease; DBP = diastolic blood pressure; HF = heart failure; SBP = systolic blood pressure.
The risk of CV death increases with time, most apparent 7 years after treatment,15,16 and is the leading cause of death in BC survivors over the age of 65 years.17 However, the effect of BP on the risk for incident CVD in BC survivors, other than as the dichotomous risk of the presence/absence of hypertension, is not well-understood. One study found the presence of hypertension at the time of BC diagnosis imparted an increased 2-year risk of CVD, including CVD death (HR: 1.55, 95% CI: 1.03-2.32).18 Another study found that in BC patients who survived >2 years, hypertension at baseline or new-onset conferred roughly twice the risk of CV death.19 Hypertension is also known to increase the risk of developing HF after treatment with HER2(+) inhibitors and anthracyclines.20,21
The association between BP and CVD risk is complex; it varies based on the study sample and the BP metric used. In the general population, SBP and CVD risk have been described as having a continuous and graded relationship, either linear or log-linear.22, 23, 24 In cohort studies with elevated cardiovascular risk, a U-shaped relationship between SBP and CVD has been described, including individual end-points of all-cause mortality, myocardial infarction, and HF.25, 26, 27, 28 DBP and CVD risk, however, is frequently described as having a ‘J-curve’ relationship with the lowest DBP also having an elevated risk, particularly in patients with prevalent coronary artery disease.29, 30, 31, 32 This effect appears to be partly attenuated after controlling for age and SBP and is not consistently found in all studies.23,29,33
The association between SBP and IHD and stroke in women with BC was observed to be linear in the present study. This linear association is consistent with findings from the general population, reinforcing incremental risk of systolic hypertension on incident IHD and stroke.22, 23, 24 In our analysis, the lower event rates for IHD and stroke limited the statistical power of these observations compared to incident HF/cardiomyopathy. In contrast, a U-shaped association was seen between SBP and incident HF/cardiomyopathy in women with BC, with risk nadiring at 131/73 mm Hg. Case controls demonstrated a similar U-shaped association for SBP and HF/cardiomyopathy, making this curve unlikely to be an effect of cancer treatment and was unchanged after controlling for cancer therapies in the BC cohort. A U-shaped association between SBP and CVD has been previously described in studies, especially in higher risk populations such as in the ALL-HAT study.25, 26, 27, 28 Reverse causality, where the outcome precedes the effect (eg, HF leading to low SBP), must also be considered. It has been proposed to explain the association between low SBP and mortality in the elderly.34 However, our reverse causality analysis excluding events during the first year post-BC diagnosis does not suggest BP was influenced due to the presence of CVD comorbidities. We observed a J-shaped association with DBP and both IHD and stroke in the BC and non-BC cohorts. Low DBP is associated with arterial stiffness and theorized to lead to decreased coronary and cerebral perfusion, which lends biologic plausibility to this finding.35,36 This theory has been used to explain the J-shape curve of DBP and IHD in patients with coronary artery disease or increased CVD risk factors.31 Similar to SBP, the BC cohort had a U-shaped association between DBP and HF/cardiomyopathy in addition to the composite endpoints.
The BC and control cohorts had similar shaped splines (eg, linear, non-linear) for all outcomes. When comparing the BC and controls cohorts, results were largely identical. These findings are important as optimal BP targets for BC survivors have not been previously well-described. Our study suggests that despite the complexity of the associations of SBP or DBP with cardiovascular events, the risk may be similar for BC and non-BC cohorts. Hence, BC survivors and their treating physicians should continue to target optimal BP management as outlined in the most recent guidelines for the general population.37
Limitations to this study include low relative event rates for IHD and stroke, which biased the composite CVD outcome towards the HF/cardiomyopathy outcome. Additionally, the BC cohort may have received more intensive monitoring due to their cancer diagnosis, introducing surveillance bias. Further, these results pertain to women with BC and are not generalizable to other cancer subtypes. Lastly, this was an observational analysis describing associations and should not be implied to describe causation.
Our study has multiple strengths. One, we had a large sample size with nearly 15,000 cases matched 1:5 to controls, which provided power to detect differences between groups. Additionally, the cohort is embedded within an integrated health care network providing rich access to data including comorbidities, longitudinal data, and events and minimized risk for loss-to-follow-up. We had longitudinal BP measurements in patients and modeled our exposure with time-averaged SBP and DBP rather than baseline SBP or DBP only. Also, this cohort is racially diverse with >35% minority composition. Lastly, this study investigates a clinical question that had not previously been studied—the effect of SBP and DBP following BC diagnosis on CVD outcomes.
Conclusions
Women with and without BC showed similar risks across the spectrum of CVD outcomes. Increased risk was conferred in women with history of hypertension. These findings suggest similar BP targets should be pursued regardless of BC survivorship status with particular attention to BC survivors with history of hypertension. Future, prospective studies are needed to confirm these findings.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Hypertension is a major contributor to cardiovascular disease in the general population and is prevalent among breast cancer survivors. Blood pressure impact on cardiovascular disease outcomes may be similar in women with versus without breast cancer.
TRANSLATIONAL OUTLOOK: Optimal blood pressure treatment targets as outlined in guidelines should be applied in women regardless of breast cancer survivorship status.
Funding support and author disclosure
The Pathways Heart Study is supported by the National Cancer Institute (R01 CA214057, U01CA195565). This publication was partially supported by a grant from Alpha Phi. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all Pathways Heart Study staff, and most importantly, the study participants.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA A Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Zaorsky N.G., Churilla T.M., Egleston B.L., et al. Causes of death among cancer patients. Ann Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens A.C., Liu Q., Neglia J.P., et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armenian S.H., Xu L., Ky B., et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Land L.H., Dalton S.O., Jorgensen T.L., Ewertz M. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol. 2012;81:196–205. doi: 10.1016/j.critrevonc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Danese M.D., O'Malley C., Lindquist K., Gleeson M., Griffiths R.I. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23:1756–1765. doi: 10.1093/annonc/mdr486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancik R., Wesley M.N., Ries L.A., Havlik R.J., Edwards B.K., Yates J.W. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 9.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J.B., Geara A.S., Hogan J.J., Townsend R.R. Hypertension in cancer patients and survivors: Epidemiology, diagnosis, and management. JACC CardioOncol. 2019;1:238–251. doi: 10.1016/j.jaccao.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice J.A., Wu C.O. Nonparametric mixed effects models for unequally sampled noisy curves. Biometrics. 2001;57:253–259. doi: 10.1111/j.0006-341x.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 12.Govindarajulu U.S., Malloy E.J., Ganguli B., Spiegelman D., Eisen E.A. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5 doi: 10.2202/1557-4679.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier J., Wu Q.V., Gooley T.A. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant. 2020;55:675–680. doi: 10.1038/s41409-019-0679-x. [DOI] [PubMed] [Google Scholar]
- 14.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Gernaat S.A.M., Ho P.J., Rijnberg N., et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164:537–555. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X., Ji J., Dai X., et al. Association of cardiovascular disease risk factors with late cardiotoxicity and survival in HER2-positive breast cancer survivors. Clin Cancer Res. 2021;27:5343–5352. doi: 10.1158/1078-0432.CCR-20-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor A.E., Schmaltz C.L., Jackson-Thompson J., Visvanathan K. Comorbidities and the risk of cardiovascular disease mortality among racially diverse patients with breast cancer. Cancer. 2021;127:2614–2622. doi: 10.1002/cncr.33530. [DOI] [PubMed] [Google Scholar]
- 20.Pinder M.C., Duan Z., Goodwin J.S., Hortobagyi G.N., Giordano S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 21.Suter T.M., Ewer M.S. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 22.Kannel W.B., Vasan R.S., Levy D. Is the relation of systolic blood pressure to risk of cardiovascular disease continuous and graded, or are there critical values? Hypertension. 2003;42:453–456. doi: 10.1161/01.HYP.0000093382.69464.C4. [DOI] [PubMed] [Google Scholar]
- 23.Rapsomaniki E., Timmis A., George J., et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psaty B.M., Furberg C.D., Kuller L.H., et al. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161:1183–1192. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 25.Itoga N.K., Tawfik D.S., Montez-Rath M.E., Chang T.I. Contributions of systolic and diastolic blood pressures to cardiovascular outcomes in the ALLHAT study. J Am Coll Cardiol. 2021;78:1671–1678. doi: 10.1016/j.jacc.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Yano Y., Rakugi H., Bakris G.L., et al. On-treatment blood pressure and cardiovascular outcomes in older adults with isolated systolic hypertension. Hypertension. 2017;69:220–227. doi: 10.1161/HYPERTENSIONAHA.116.08600. [DOI] [PubMed] [Google Scholar]
- 27.Lim N.K., Park H.Y., Kim W.H., Mancia G., Cho M.C. The U-shaped association between achieved blood pressure and risk of cardiovascular events and mortality in elderly and younger patients. J Hypertens. 2020;38:1559–1566. doi: 10.1097/HJH.0000000000002434. [DOI] [PubMed] [Google Scholar]
- 28.Bohm M., Schumacher H., Teo K.K., et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389:2226–2237. doi: 10.1016/S0140-6736(17)30754-7. [DOI] [PubMed] [Google Scholar]
- 29.Flint A.C., Conell C., Ren X., et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 30.Messerli F.H., Panjrath G.S. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. doi: 10.1016/j.jacc.2009.05.073. [DOI] [PubMed] [Google Scholar]
- 31.Khan N.A., Rabkin S.W., Zhao Y., et al. Effect of lowering diastolic pressure in patients with and without cardiovascular disease: analysis of the SPRINT (systolic blood pressure intervention trial) Hypertension. 2018;71:840–847. doi: 10.1161/HYPERTENSIONAHA.117.10177. [DOI] [PubMed] [Google Scholar]
- 32.Vidal-Petiot E., Ford I., Greenlaw N., et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 33.Kannel W.B., Wilson P.W., Nam B.H., D'Agostino R.B., Li J. A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol. 2004;94:380–384. doi: 10.1016/j.amjcard.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 34.Sattar N., Preiss D. Reverse causality in cardiovascular epidemiological research: more common than imagined? Circulation. 2017;135:2369–2372. doi: 10.1161/CIRCULATIONAHA.117.028307. [DOI] [PubMed] [Google Scholar]
- 35.Webb A.J.S. Progression of arterial stiffness is associated with midlife diastolic blood pressure and transition to late-life hypertensive phenotypes. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEvoy J.W., Chen Y., Rawlings A., et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:2199–2269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.