Abstract
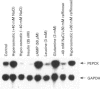
Exposure of isolated perfused rat livers to hypo-osmotic (225 mosmol/l) perfusion media for 3 h led to a decrease of about 60% in mRNA levels for phosphoenolpyruvate carboxy-kinase (PEPCK) compared with normo-osmotic (305 mosmol/l) perfusions. Conversely, PEPCK mRNA levels increased about 3-fold during hyperosmotic (385 mosmol/l) perfusions. The anisotonicity effects were not explained by changes in the intracellular cyclic AMP (cAMP) concentration or by changes of the extracellular Na+ or Cl- activity. Similar effects of aniso-osmolarity on PEPCK mRNA levels were found in cultured rat hepatoma H4IIE.C3 cells, the experimental system used for further characterization of the effect. Whereas during the first hour of anisotonic exposure no effects on PEPCK mRNA levels were detectable, near-maximal aniso-osmolarity effects were observed within the next 2-3 h. PEPCK mRNA levels increased sigmoidally with the osmolarity of the medium, and the anisotonicity effects were most pronounced upon modulation of osmolarity between 250 and 350 mosmol/l. The aniso-osmolarity effects on PEPCK mRNA were not affected in presence of Gö 6850, protein kinase C inhibitor. cAMP increased the PEPCK mRNA levels about 2.3-fold in normo-osmotic media, whereas insulin lowered the PEPCK mRNA levels to about 8%. The effects of cAMP and insulin were also observed during hypo-osmotic and hyperosmotic exposure, respectively, but the anisotonicity effects were not abolished in presence of the hormones. The data suggest that hepatocellular hydration affects hepatic carbohydrate metabolism also over a longer term by modulating PEPCK mRNA levels. This is apparently unrelated to protein kinase C or alterations of cAMP levels. The data strengthen the view that cellular hydration is an important determinant for cell metabolic function by extending its regulatory role in carbohydrate metabolism to the level of mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Arebalo R. E., Tormanen C. D., Hardgrave J. E., Noland B. J., Scallen T. J. In vivo regulation of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase: immunotitration of the enzyme after short-term mevalonate or cholesterol feeding. Proc Natl Acad Sci U S A. 1982 Jan;79(1):51–55. doi: 10.1073/pnas.79.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquet A., Gaussin V., Bollen M., Stalmans W., Hue L. Mechanism of activation of liver acetyl-CoA carboxylase by cell swelling. Eur J Biochem. 1993 Nov 1;217(3):1083–1089. doi: 10.1111/j.1432-1033.1993.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Baquet A., Meijer A. J., Hue L. Hepatocyte swelling increases inositol 1,4,5-trisphosphate, calcium and cyclic AMP concentration but antagonizes phosphorylase activation by Ca2(+)-dependent hormones. FEBS Lett. 1991 Jan 14;278(1):103–106. doi: 10.1016/0014-5793(91)80094-j. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Fillat C., Valera A., Bosch F. Epidermal growth factor inhibits phosphoenolpyruvate carboxykinase gene expression in rat hepatocytes in primary culture. FEBS Lett. 1993 Mar 8;318(3):287–291. doi: 10.1016/0014-5793(93)80530-8. [DOI] [PubMed] [Google Scholar]
- Finkenzeller G., Newsome W., Lang F., Häussinger D. Increase of c-jun mRNA upon hypo-osmotic cell swelling of rat hepatoma cells. FEBS Lett. 1994 Mar 7;340(3):163–166. doi: 10.1016/0014-5793(94)80129-0. [DOI] [PubMed] [Google Scholar]
- Gaussin V., Baquet A., Hue L. Cell shrinkage follows, rather than mediates, the short-term effects of glucagon on carbohydrate metabolism. Biochem J. 1992 Oct 1;287(Pt 1):17–20. doi: 10.1042/bj2870017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Haddad P., Haeussinger D., Lang F. Cell volume regulation in liver. Ren Physiol Biochem. 1988 May-Oct;11(3-5):202–220. doi: 10.1159/000173163. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Granner D., Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem. 1990 Jun 25;265(18):10173–10176. [PubMed] [Google Scholar]
- Gurney A. L., Park E. A., Giralt M., Liu J., Hanson R. W. Opposing actions of Fos and Jun on transcription of the phosphoenolpyruvate carboxykinase (GTP) gene. Dominant negative regulation by Fos. J Biol Chem. 1992 Sep 5;267(25):18133–18139. [PubMed] [Google Scholar]
- Hallbrucker C., vom Dahl S., Lang F., Gerok W., Häussinger D. Modification of liver cell volume by insulin and glucagon. Pflugers Arch. 1991 Jun;418(5):519–521. doi: 10.1007/BF00497781. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume and hormone action. Trends Pharmacol Sci. 1992 Oct;13(10):371–373. doi: 10.1016/0165-6147(92)90114-l. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stoll B., vom Dahl S., Theodoropoulos P. A., Markogiannakis E., Gravanis A., Lang F., Stournaras C. Effect of hepatocyte swelling on microtubule stability and tubulin mRNA levels. Biochem Cell Biol. 1994 Jan-Feb;72(1-2):12–19. doi: 10.1139/o94-003. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Weiss L., Sies H. Activation of pyruvate dehydrogenase during metabolism of ammonium ions in hemoglobin-free perfused rat liver. Eur J Biochem. 1975 Apr 1;52(3):421–431. doi: 10.1111/j.1432-1033.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Lang F., Stehle T., Häussinger D. Water, K+, H+, lactate and glucose fluxes during cell volume regulation in perfused rat liver. Pflugers Arch. 1989 Jan;413(3):209–216. doi: 10.1007/BF00583532. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- McGrane M. M., Yun J. S., Patel Y. M., Hanson R. W. Metabolic control of gene expression: in vivo studies with transgenic mice. Trends Biochem Sci. 1992 Jan;17(1):40–44. doi: 10.1016/0968-0004(92)90426-a. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Baquet A., Gustafson L., van Woerkom G. M., Hue L. Mechanism of activation of liver glycogen synthase by swelling. J Biol Chem. 1992 Mar 25;267(9):5823–5828. [PubMed] [Google Scholar]
- Minton A. P., Colclasure G. C., Parker J. C. Model for the role of macromolecular crowding in regulation of cellular volume. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J., Granner D. K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Saha N., Stoll B., Lang F., Häussinger D. Effect of anisotonic cell-volume modulation on glutathione-S-conjugate release, t-butylhydroperoxide metabolism and the pentose-phosphate shunt in perfused rat liver. Eur J Biochem. 1992 Oct 1;209(1):437–444. doi: 10.1111/j.1432-1033.1992.tb17307.x. [DOI] [PubMed] [Google Scholar]
- Santell L., Rubin R. L., Levin E. G. Enhanced phosphorylation and dephosphorylation of a histone-like protein in response to hyperosmotic and hypoosmotic conditions. J Biol Chem. 1993 Oct 5;268(28):21443–21447. [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Schulz W. A., Eickelmann P., Hallbrucker C., Sies H., Häussinger D. Increase of beta-actin mRNA upon hypotonic perfusion of perfused rat liver. FEBS Lett. 1991 Nov 4;292(1-2):264–266. doi: 10.1016/0014-5793(91)80880-c. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos P. A., Stournaras C., Stoll B., Markogiannakis E., Lang F., Gravanis A., Häussinger D. Hepatocyte swelling leads to rapid decrease of the G-/total actin ratio and increases actin mRNA levels. FEBS Lett. 1992 Oct 26;311(3):241–245. doi: 10.1016/0014-5793(92)81111-x. [DOI] [PubMed] [Google Scholar]
- Tohyama Y., Kameji T., Hayashi S. Mechanisms of dramatic fluctuations of ornithine decarboxylase activity upon tonicity changes in primary cultured rat hepatocytes. Eur J Biochem. 1991 Dec 18;202(3):1327–1331. doi: 10.1111/j.1432-1033.1991.tb16507.x. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Cimbala M. A., Felz K., Monahan J. E., Leis J. P., Hanson R. W. Identification of a DNA clone to phosphoenolpyruvate carboxykinase (GTP) from rat cytosol. Alterations in phosphoenolpyruvate carboxykinase RNA levels detectable by hybridization. J Biol Chem. 1981 Oct 25;256(20):10224–10227. [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Dahl S., Hallbrucker C., Lang F., Gerok W., Häussinger D. A non-invasive technique for cell volume determination in perfused rat liver. Biol Chem Hoppe Seyler. 1991 Jun;372(6):411–418. doi: 10.1515/bchm3.1991.372.1.411. [DOI] [PubMed] [Google Scholar]