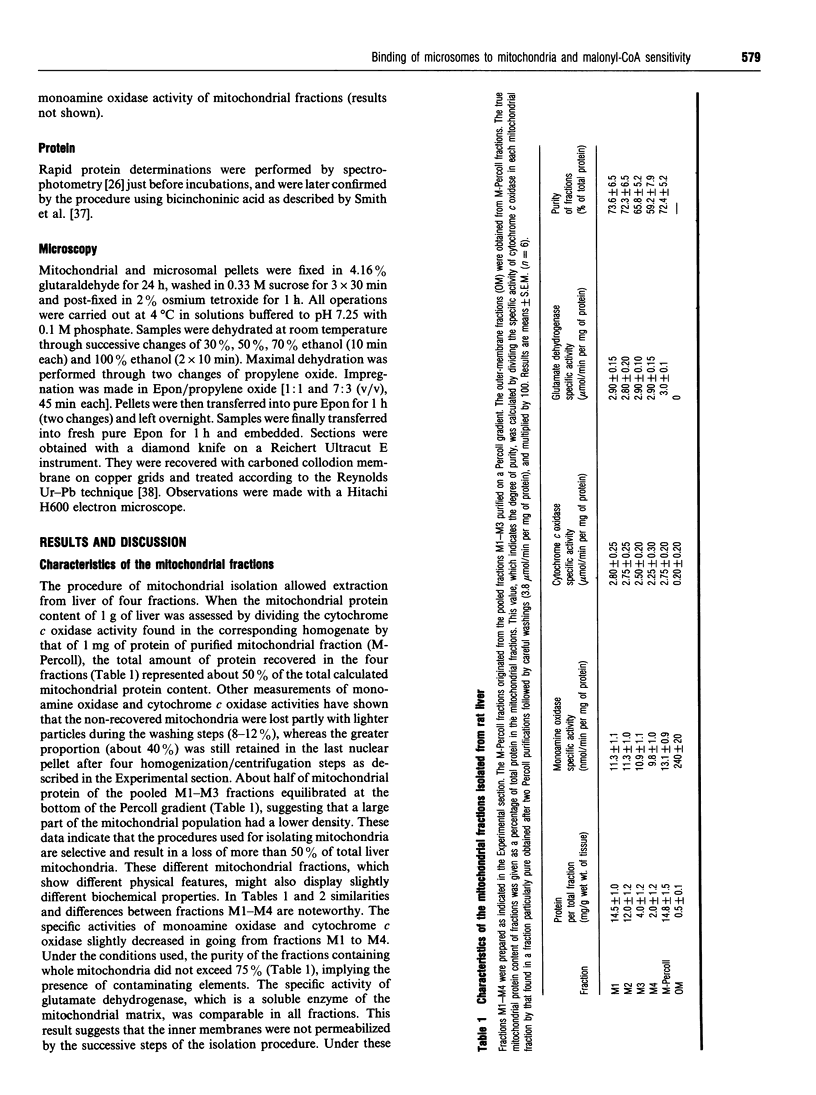
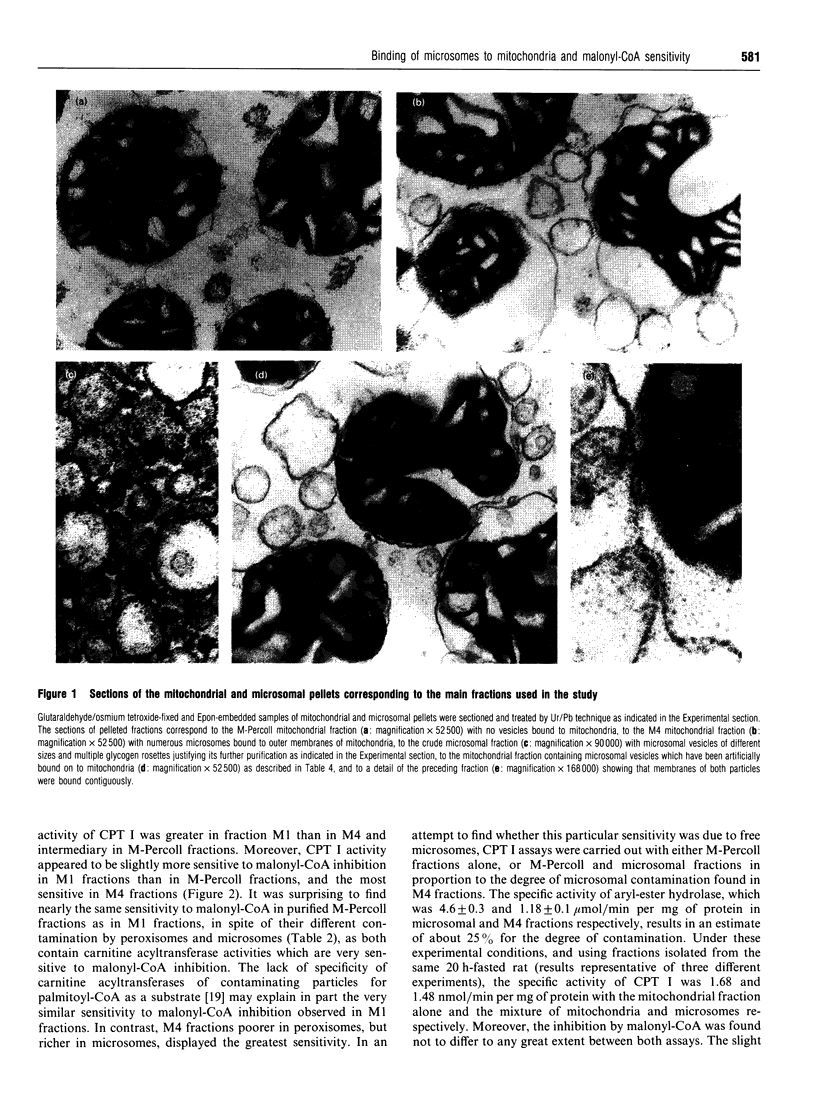
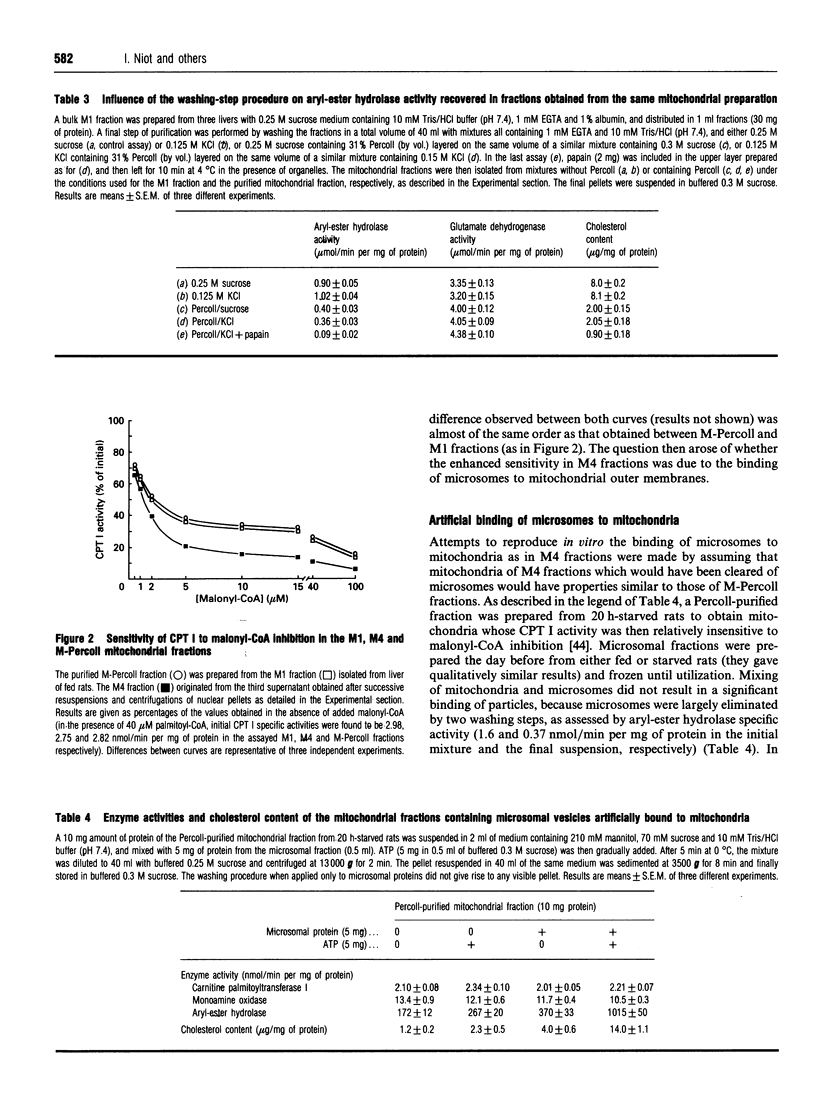
Abstract
Liver mitochondrial fractions as normally isolated contain only 10-20% of total mitochondria and may not be representative of the whole mitochondrial population. This study was designed to evaluate the dependence of the sensitivity of carnitine palmitoyl-transferase I (CPT I) to malonyl-CoA inhibition in mitochondrial fractions that are not normally studied. Four fractions prepared from rat liver were found to be contaminated to different extents by microsome vesicles, on the basis of marker-enzyme activities and micrographic data. Purification of mitochondrial fractions on a Percoll gradient decreased to some extent the microsomal contamination, which was due in part to the existence of close bonds between microsomes and the outer membranes of mitochondria. A greater degree of contamination of mitochondrial fractions by microsomes was correlated with a greater sensitivity of CPT I to malonyl-CoA inhibition. Attempts were made to enhance the sensitivity of CPT I to malonyl-CoA with the use of microsomes. Measurements performed by adding mitochondria and microsomes in the same CPT I assay failed to demonstrate any significant enhancement of malonyl-CoA inhibition. However, addition of ATP to a mixture of mitochondria and microsomes was shown to trigger the binding of both particles, as assessed by enzymic and micrographic data, and to increase the sensitivity of CPT I to malonyl-CoA inhibition. These results demonstrated that the binding of microsomes to mitochondria, unlike the simple mixing of both particles, was capable of altering the sensitivity of CPT I to malonyl-CoA. The data also suggest that this process could be of physiological importance, owing to the frequency of contiguous zones between mitochondria and endoplasmic reticulum observed in sections of intact liver cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P., Paumgartner D., Muellener D., Losa G., Weibel E. R. Integrated stereological and biochemical studies on hepatocytic membranes. I.V. Heterogeneous distribution of marker enzymes on endoplasmic reticulum membranes in fractions. J Cell Biol. 1980 Jun;85(3):577–586. doi: 10.1083/jcb.85.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. Palmityl-CoA: carnitine O-palmityltransferase in the mitochondrial oxidation of palmityl-CoA. Eur J Biochem. 1967 Jun;1(4):427–433. doi: 10.1007/978-3-662-25813-2_58. [DOI] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Clouet P., Henninger C., Niot I., Boichot J., Bezard J. Short term treatment by fenofibrate enhances oxidative activities towards long-chain fatty acids in the liver of lean Zucker rats. Biochem Pharmacol. 1990 Nov 1;40(9):2137–2143. doi: 10.1016/0006-2952(90)90246-h. [DOI] [PubMed] [Google Scholar]
- Clouet P., Niot I., Bézard J. Pathway of alpha-linolenic acid through the mitochondrial outer membrane in the rat liver and influence on the rate of oxidation. Comparison with linoleic and oleic acids. Biochem J. 1989 Nov 1;263(3):867–873. doi: 10.1042/bj2630867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick J. P., Ramsay R. R. L-carnitine acyltransferase in intact peroxisomes is inhibited by malonyl-CoA. Biochem J. 1989 Sep 15;262(3):801–806. doi: 10.1042/bj2620801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993 Mar 15;268(8):5817–5822. [PubMed] [Google Scholar]
- Gambert P., Lallemant C., Archambault A., Maume B. F., Padieu P. Assessment of serum cholesterol by two methods: gas-liquid chromatography on a capillary column and chemical ionization-mass fragmentography with isotopic dilution of [3,4-13C] cholesterol as internal standard. J Chromatogr. 1979 Jan 1;162(1):1–6. doi: 10.1016/s0378-4347(00)82057-5. [DOI] [PubMed] [Google Scholar]
- Ghadiminejad I., Saggerson E. D. The relationship of rat liver overt carnitine palmitoyltransferase to the mitochondrial malonyl-CoA binding entity and to the latent palmitoyltransferase. Biochem J. 1990 Sep 15;270(3):787–794. doi: 10.1042/bj2700787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán M., Castro J., Maquedano A. Ethanol feeding to rats reversibly decreases hepatic carnitine palmitoyltransferase activity and increases enzyme sensitivity to malonyl-CoA. Biochem Biophys Res Commun. 1987 Dec 16;149(2):443–448. doi: 10.1016/0006-291x(87)90387-1. [DOI] [PubMed] [Google Scholar]
- Kolodziej M. P., Zammit V. A. Sensitivity of inhibition of rat liver mitochondrial outer-membrane carnitine palmitoyltransferase by malonyl-CoA to chemical- and temperature-induced changes in membrane fluidity. Biochem J. 1990 Dec 1;272(2):421–425. doi: 10.1042/bj2720421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L., REMMERT L. F. An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation. J Biol Chem. 1959 Sep;234:2459–2464. [PubMed] [Google Scholar]
- Leighton F., Poole B., Lazarow P. B., De Duve C. The synthesis and turnover of rat liver peroxisomes. I. Fractionation of peroxisome proteins. J Cell Biol. 1969 May;41(2):521–535. doi: 10.1083/jcb.41.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. Heterogeneous distribution of glucose 6-phosphatase in rat liver microsomal fractions as shown by adaptation of a cytochemical technique. Biochem J. 1973 May;134(1):69–78. doi: 10.1042/bj1340069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly K., Bugaisky G. E., Umeda P. K., Bieber L. L. The medium-chain carnitine acyltransferase activity associated with rat liver microsomes is malonyl-CoA sensitive. Arch Biochem Biophys. 1990 Jul;280(1):167–174. doi: 10.1016/0003-9861(90)90532-4. [DOI] [PubMed] [Google Scholar]
- Lund H. Carnitine palmitoyltransferase: characterization of a labile detergent-extracted malonyl-CoA-sensitive enzyme from rat liver mitochondria. Biochim Biophys Acta. 1987 Mar 13;918(1):67–75. doi: 10.1016/0005-2760(87)90010-5. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., McGroarty E. J., Bieber L. L., Tolbert N. E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973 May 25;248(10):3426–3432. [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- McGarry J. D., Sen A., Esser V., Woeltje K. F., Weis B., Foster D. W. New insights into the mitochondrial carnitine palmitoyltransferase enzyme system. Biochimie. 1991 Jan;73(1):77–84. doi: 10.1016/0300-9084(91)90078-f. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Spycher M. A., Meyer U. A. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim Biophys Acta. 1981 Aug 20;646(2):283–297. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Spycher M. A., Meyer U. A. Isolation of a subfraction of rough endoplasmic reticulum closely associated with mitochondria. Evidence for its role in cytochrome P450 synthesis. Exp Cell Res. 1978 Feb;111(2):479–483. doi: 10.1016/0014-4827(78)90197-0. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Merritt W. D., Lembi C. A. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73(1):43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S. V., Murthy M. S., Noël H. Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochim Biophys Acta. 1986 Jun 27;877(2):223–230. doi: 10.1016/0005-2760(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Montisano D., Eisner D., Cascarano J. The physical association between rat liver mitochondria and rough endoplasmic reticulum. I. Isolation, electron microscopic examination and sedimentation equilibrium centrifugation analyses of rough endoplasmic reticulum-mitochondrial complexes. Exp Cell Res. 1980 Aug;128(2):343–352. doi: 10.1016/0014-4827(80)90070-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J Cell Biol. 1977 Mar;72(3):714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakkestad J. A., Bremer J. The outer carnitine palmitoyltransferase and regulation of fatty acid metabolism in rat liver in different thyroid states. Biochim Biophys Acta. 1983 Feb 7;750(2):244–252. doi: 10.1016/0005-2760(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkl A., Fahimi H. D. Isolation and characterization of peroxisomes from the liver of normal untreated rats. Eur J Biochem. 1985 Jun 3;149(2):257–265. doi: 10.1111/j.1432-1033.1985.tb08920.x. [DOI] [PubMed] [Google Scholar]
- Woeltje K. F., Kuwajima M., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. II. Use of detergents and antibodies. J Biol Chem. 1987 Jul 15;262(20):9822–9827. [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Altered release of carnitine palmitoyltransferase activity by digitonin from liver mitochondria of rats in different physiological states. Biochem J. 1985 Sep 1;230(2):389–394. doi: 10.1042/bj2300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Kolodziej M. P. Target size analysis by radiation inactivation of carnitine palmitoyltransferase activity and malonyl-CoA binding in outer membranes from rat liver mitochondria. Biochem J. 1989 Oct 1;263(1):89–95. doi: 10.1042/bj2630089. [DOI] [PMC free article] [PubMed] [Google Scholar]