Abstract
Inflammatory bowel diseases (IBD) significantly contribute to high mortality globally and negatively affect patients' qualifications of life. The gastrointestinal tract has unique anatomical characteristics and physiological environment limitations. Moreover, certain natural or synthetic anti-inflammatory drugs are associated with poor targeting, low drug accumulation at the lesion site, and other side effects, hindering them from exerting their therapeutic effects. Colon-targeted drug delivery systems represent attractive alternatives as novel carriers for IBD treatment. This review mainly discusses the treatment status of IBD, obstacles to drug delivery, design strategies of colon-targeted delivery systems, and perspectives on the existing complementary therapies. Moreover, based on recent reports, we summarized the therapeutic mechanism of colon-targeted drug delivery. Finally, we addressed the challenges and future directions to facilitate the exploitation of advanced nanomedicine for IBD therapy.
Keywords: Colon-targeted drug delivery system, Inflammatory bowel disease, Design strategy, Treatment mechanism, Advanced complementary therapy
Graphical abstract
Colon-targeted drug delivery systems are used to locally deliver drugs for improving inflammatory bowel disease efficacy. Design strategies, applications and therapeutic mechanisms are reviewed, and challenges and prospects are discussed. Schematic illustration of the design of colon-targeted drug delivery system in treatment of inflammatory bowel disease.

1. Introduction
1.1. Treatment status of inflammatory bowel diseases
Within the human body, the intestine is the most complex and greatest immunological organ. Continuous exposure to intestinal contents elicits a prolonged self-protection response, causing intestinal inflammation [1]. Inflammatory bowel diseases (IBD), which include Crohn's disease and ulcerative colitis (UC), cause complications such as bloody diarrhea and abdominal pain. It can seriously compromise the quality of life, significantly increasing the hazard index of colorectal cancer (CRC) and imposing a life-long medical strain for mountains of patients around the world [2]. CRC ranks third in incidence and second in mortality from the disease, as reported by a 2020 WHO assessment [3], which causes persistent ulcers and epithelial damage. CRC is increasing worldwide, especially in young people [4].
According to the global drug research and development data on IBD provided by the patsnap database (https://synapse.zhihuiya.com/) and the pharnexcloud database (https://pharma.bcpmdata.com/), as of the statistical time, a total of 1137 new drugs are under development and 142 of them have been approved for listing. At present, the new drugs developed are mainly small molecule drugs and monoclonal antibodies, and other types include synthetic peptides, live bacterial preparations, fusion proteins, and bispecific antibodies (Fig. 1A). Traditional drugs used to treat IBD include aminosalicylic acid, corticosteroids, immunosuppressants, and monoclonal antibodies [5]. Despite being first-line medications, 5-aminosalicylic acid (5-ASA) and glucocorticoids have certain side effects. Novel biotherapies, such as infliximab, adalimumab, golimumab, vedolizumab, and ustekinumab, have been developed. However, these therapies are not only expensive but also associated with increasing rates of drug resistance [6]. In addition, the top 10 targets of listed IBD drugs are TNF-α, GR, α4β7, JAK1, IL-12 & IL-23, TYK2, NLRP3, FXR, S1PR1 and TNF. Recent studies have focused on the above targets, targeting the cytokine network through the use of multiple cytokine blockers (e.g., JAK inhibitors upadacitinib [7] approved by the FDA as the first oral treatment for moderately to severely active Crohn's disease in 2023) or multiple cytokine blockers simultaneously (e.g., anti-TNF and anti-IL-23 antibodies) [8]. This may provide important hints for active ingredient screening and targeted delivery of cargoes for IBD treatment
Fig. 1.
Treatment status of IBD. (A) The top 10 types of novel medication categories for IBD treatment and their development stages. (B) Multiple factors contribute to the pathophysiology of IBD.
By investigating the information from the clinical trial database (clinicaltrials.gov) and the data related to IBD therapeutic agents collected by Drugs @ FDA database (https://www.fda.gov/drugs), FDA has not yet approved any colon-targeting agents for the treatment of IBD; only some liposome preparations are approved to enter the clinical trial cohort, such as Peg-Liposomal Prednisolone Sodium Phosphate (Nanocort ®) (NCT01039103) and Nano Calciferol (Vitamin D) (NCT05733117). Therefore, a series of valuable colon-targeted delivery systems (CTDDs) need to be further developed to meet the needs of pharmaceutical preparations and clinical treatment.
As IBD is intensely bound with the microbial flora and immune system, understanding the pathological mechanism is crucial for developing a rational drug delivery design [9]. IBD is eventually caused by tissue damage and ulcers brought on by the recruiting and invasion of immunological cells, the release of proinflammatory cytokines, and the buildup of reactive oxygen species (ROS) [10]. The pathological mechanisms of IBD include the following: (1) Impaired mucus layer and intestinal barrier function induced by genetic, dietary, drug and other factors; (2) Disorder of the mucosal immune system composed of intestinal-associated lymphoid tissue and lamina propria involving multiple inflammatory cell infiltration and cytokine secretion; (3) Intestinal microbial dysbiosis mainly including the diversity and abundance [11] (Fig. 1B). IBD can be effectively treated using local gastrointestinal-specific delivery therapeutic agents, comprising antigens, immunomodulatory intestinal barrier boosters, and intestinal microbiome transplantation. The emergence of novel nanomaterials has made oral nanomedicine a desirable alternative since it increases the drug's local concentration, effectiveness, and bioavailability while avoiding the toxicity and unfavorable effects associated with high dosages [12,13].
1.2. Obstacles to drug delivery
IBD treatment includes drug therapy, surgical treatment, and fecal transplantation. However, surgery does not cure the condition because many people experience post-operative recurrence, which worsens their quality of life and results in more intestinal damage [14]. Although fecal microbiota transplantation has been proven effective [15], preventing the introduction of pathogens through other techniques is challenging, and its safety and standardization are still being explored. Currently, the mainstay of drug therapy for IBD remains oral, intravenous, and enema administration. In addition, intravenous administration, which has been used in treating moderately to severely active IBD that highly improves drug bioavailability and avoids first-pass effects and gastrointestinal irritation, is also prone to side effects, including systemic toxicity, while requiring higher criteria for the physicochemical properties of the nanopreparations [16]. Rectal administration is a practical alternative for the treatment of mild and moderate IBD with the advantage of bypassing hepatic first-pass effects but with some drawbacks, such as low compliance, short intestinal retention time, and limited proximal intestinal treatment sites [17]. Currently, oral drug therapy is preferred because of satisfactory patient compliance. Oral CTDDs improve the availability of drugs in the colon and their biodistribution in the body by limiting their absorption in the rest parts of the gastrointestinal tract (GIT)of the body [18]. Therefore, this drug delivery technology can effectively and safely treat IBD. Table 1 lists some examples of representative studies.
Table 1.
Publications on representative CTDDs for the treatment of IBD.
| Targeting response mode | Drug | Formulation name | Formulation | Response mode conditions | Route | Disease model | Mechanism of action | Refs |
|---|---|---|---|---|---|---|---|---|
| pH- and redox-responsive | Ginsenoside Rh2 | Rh2/LA-UASP NPs | Grafting A. sinensis polysaccharide with urocanic acid and α-LA | pH 5.5 and 10 mM GSH | I.V. | RAW264.7 cells induced with LPS(1 μg/ml), 3.5 % DSS induced mice | STAT3/miR-214 signal pathway, ROS, TNF-α and IL-1β ↓, IL-10↑ | [22] |
| pH- and charge-responsive | Ag+ | HA-SH-Ag/Alginate-Ca microspheres (HAMs) | Ag+ utilized to crosslink the thiolated-HA, coated with Alginate-Ca microspheres | Stable in AGF (pH 1.0) and ASF (pH 6.8), but degraded in ACF (pH 7.8) | Oral | iBMDM cells stimulated by LPS (1 µg/ml), 2.5 %DSS induced C57BL/6 mice | Pro-inflammatory cytokines ↓, typeⅡmacrophage differentiation↑, probiotics including Bifidobacterium and Lactobacillus↑, the detrimental communities are inhibited | [23] |
| pH- and receptor-responsive | GA | GA@Pec-FA-Zein NPs | Applying FA-Zein as the core and using pectin as the shell, exhibited by GA | Stable in AGF (pH 1.0) and ASF (pH 6.8), but degraded in ACF (pH 7.8) | Oral | 2.5 % DSS induced KM mice | Targeting macrophages↑, the bioavailability of GA↑, MPO, TNF-α↓ | [24] |
| Receptor-mediated responsive | BBR | BBR/MPN@YM | EGCG and Mn2+form MPN via the interaction, MPN and BBR encapsulated into YM | The interaction between macrophage dectin-1 receptors and β−1,3-d-glucan on the outer layer of YM | Oral | RAW264.7 cells induced by IL-4, 100 μM H2O2 induced L929 cells, 4 g/kg/d DSS induced C57BL/6 male mice | M1 → M2 macrophages, TNF-α, IL-1β, H2O2, and MDA↓ | [25] |
| Receptor-mediated responsive | C-dot nanozyme and CD98 CRISPR/Cas9 plasmid | C-dot nanozyme@ CD98 CRISPR/Cas9 plasmid @ZIF-8@M (CCZM) | CCZ fabricated through one pot method | Directed toward the inflamed colon under the control of the macrophage membrane | I.V. | RAW264.7 stimulated by LPS (100 ng/ml 24 h), 2 %DSS induced C57BL/6 mice | ROS eliminated by nanozymes and plasmids entering the cell nucleus and knocking down the CD98 gene | [26] |
| Time- and pH-mediated responsive | 5-ASA | 5-ASA@Eudragit S/L/ RS | 5-ASA coated by Eudragit®S and L with Eudragit® RS | pH 6.5 in vitro and inflammatory colon tissue in vivo | Oral | 2 ml acetic acid (2 %, v/v) enema induced Wistar rats | Improving colon/body mass ratio, reducing inflammatory infiltration | [27]] |
| ROS- mediated responsive | Lactobacillus | HA-LR | Lactobacillus was packaged in hydrogel during the gelation procedure of MeHA and a bonding agent | H2O2 (100 μM) in vitro , Inflammatory colon tissue in vivo | Oral | 100 μM H2O2 induced RAW264.7 cells, 5 % DSS induced Balb/c mice | ROS, TNF-α and IL-6 ↓, IL-10 ↑ | [28] |
| ROS-mediated responsive | Bud and antioxidant Tem | Bud-ATK-Tem | The nanoassemblies were created using B-ATK-T Janus-prodrug. The DSPE-PEG2k was used for producing a PEGylated surface. | Various concentrations of H2O2 (0.05–1 mM) in vitro and Inflammatory colon tissue in vivo | Oral | RAW264.7 treated with 200 μM H2O2, 3 %DSS induced C57BL/6 mice | PGE2, H2O2, TNF-α, MDA and IL-1β ↓ | [29] |
| Charge- and receptor-mediated responsive | CsA | CsA-loaded IT-NCs | Using polymeric nanodrug core emulsification-solvent evaporation method and layer-by layer biocoatings | Inflamed cationic mucosal tissue surfaces and CD44 receptor | Oral | TNBS-induced colitis, 2.5 % (w/v) DSS -induced acute colitis in mice | DAI score and additional observations on the phenomenology of colitis that pertain to inflammation and permeability ↓ | [30] |
| Charge-mediated responsive | TNF-α siRNA | siRNA-loaded ANPs, PNPs or CNPs | Prepared PEG5K-b-PLGA10K NPs with the assistance of a synthesized lipid featuring surface amine groups for subsequent charge tuning. | Charge in the inflamed mucosal tissue | Oral | 3 %DSS induced C57BL/6 mice | Silencing of the gene (TNF-α) and engendering more potent anti-inflammatory efficacy | [31] |
| Microorganism enzyme, time, and pH-mediate responsive | Cur | GAL-CPS bilayer microgels (Cur-loaded PS and bilayer microgels) | Polysaccharide GG and LMP were employed as the inner core, with the outer layer modified using SA and CS through polyelectrolyte interactions. | Degraded by colon enzymes, and bacteria | Oral | RAW264.7 treated with 200 μM H2O2, 3 %DSS induced C57BL/6 mice | Restoring the abundance and diversity of intestinal flora, improving macrophage infiltration in colonic tissue and inhibiting the level of TNF-α and IL-6 | [32] |
Silver ion (Ag+);α-lipoic acid (α-LA); artificial gastric fluid (AGF); artificial small intestine fluids (ASF); artificial colon fluid (ACF); glycyrrhizic acid (GA); intravenous injection (I.V.); Berberine (BBR); metal polyphenol network (MPN); yeast microcapsule (YM); inflammation-targeting system (IT-NC); Budesonide (Bud); tempol (Tem); Cyclosporine A (CsA); small interfering RNA (siRNA); aminated NPs (ANPs); plain NPs (PNPs); carboxylated NPs (CNPs); Curcumin (Cur); guar gum (GG); low-methoxyl pectin (LMP); sodium alginate (SA); chitosan (CS); porous starch (PS); inner gel core consists of GG and LMP -Cur loaded porous starch (GAL-CPS).
The GIT environment poses challenges for designing drug delivery devices. GIT is segmented into multiple sectors: the esophagus, stomach, small intestine, and large intestine. Various parts of the GIT have different characteristics, which must be considered while designing (Fig. 2A). These characteristics are as follows [19]: (1) Relatively stable physical conditions include swallowing function, lumen size, mucin and epithelial cell transition, and gastrointestinal wall thickness; (2) Relatively flexible main difference conditions in the GIT include changes in absorption area, pH, cell types and transporters, drug resident and transport time, adhesion and permeability of digestive tube wall, and bacterial diversity and population; (3) Physiological conditions differ between healthy individuals and patients and depend on their age group [20]. Moreover, the colon lacks villi, and its epithelium is encased in a double-layer mucus that contains water, electrolytes, lipids, and glycoproteins. As the colon is characterized by intraluminal fluid deficiency, which affects the transport, disintegration, and release of drugs, special attention should be paid to this aspect [21]. In conclusion, given the consideration of IBD treatment and the barriers to drug delivery mentioned above, it is of far-reaching significance to summarize the latest design strategies for CTDDs, including target effect response modes, skeleton types, and therapeutic mechanisms (Fig. 2B).
Fig. 2.
(A) General physiological conditions to be considered for the design of colon-targeted drug delivery systems. (B) The summary of the latest design strategies for CTDDs.
2. Colon-targeted delivery strategy
2.1. Target-action response mode of the delivery system
2.1.1. pH-dependent target-action response mode
pH-responsive nanodelivery systems have been developed for treating UC. The main design principle is that the linkers between the carrier and the drug and/or chemical groups in the nanostructured skeleton can be destabilized by pH changes, leading to the disintegration of the delivery skeleton. For example, Eudragit® EPO (EPO) and Eudragit® L100 (L100) are two pH-sensitive materials approved by the FDA, EPO dissolves under acidic conditions because of its tertiary amino group, and L100 dissolves at pH > 6 due to it contains a large number of carboxyl groups. Recently, Zhang et al. coated curcummin-loaded nanoparticles (CNs) with EPO and L100, to developed CNs@EPO@L100 core-shell nanoparticles. This pH-sensitive nano-system exhibited the precisely targeted therapy of UC (Fig. 3A) [33].
Fig. 3.
Representative studies of different response modes for CTDDs in IBD treatment. (A) pH-responsive CNs@EPO@L100 nanoparticles were prepared by coating CNs with EPO and L100. After oral administration, it degrades and releases drug primarily at the inflammatory sites in the colon. Reproduced from [33] Copyright 2023 Royal Society of Chemistry; (B) pH & microbial-response Dexa-GP/ES/Pu NCs nanocargoes could be degraded by branched-chain amylase only at the colonic site and bind preferentially to macrophage galactose-type lectin-C (MGL-2) surface receptors for highly localized drug release. Reproduced from [55] Copyright 2023 Elsevier; (C) Receptor-dependent EC/CS/HA/CS/Dex nanoparticle exerts anti-inflammatory effects by targeting CD44 receptor-mediated endocytosis, extending drug retention time. Reproduced from [65] Copyright 2023 Elsevier; (D) Charge-dependent CeO2@MMT(1:9) is stable in the stomach for oral delivery and targets the inflamed colon through electrostatic interactions. Reproduced from [69] Copyright 2020 Wiley-VCH GmbH; (E) ROS-responsive PSB@NP-FA were synthesized by a single microemulsion method and could be cleaved under ROS stimulation in colon. Reproduced from [71] Copyright 2023 The American Society of Gene and Cell Therapy; (F) Representative ROS-sensitive linkers used for targeted drug delivery to inflammatory regions in IBD treatment.
Although previous reports have shown variations in the pH range along the length of the GIT, it is considered almost similar. The minimum pH is observed in the stomach (fasting pH, 0.4–4.0; feeding pH, 2.0–4.5). In the small intestine's proximal region, the pH is between 6.15 and 7.35, whereas it can reach up to 7.5 in distal region. The pH of the colon is different from that of the upper digestive tract, and the pH range is 5.26–6.72 in the ascending colon [34]. Indigestible dietary carbohydrates are transformed into short-chain fatty acids (SCFAs) by colonic bacteria, leading to a decrease in the pH of the proximal colon [35]. Another study reported that the pH ranges from 1.5 to 3.5 in the stomach, whereas it is between 5.5 and 6.8 in the small intestine. The ascending colon has a pH of 6.4, which increases in the transverse colon and approaches neutrality in the descending colon. Importantly, pH variations in the colon affect the nature of the microbiota in the GIT [36]. Fatty acids are absorbed during transport through the transverse and descending colon, promoting carbonic acid secretion and making the environment more alkaline. The environmental pH can reach 6.6 in the distal colon, which might be further reduced during UC.
Although pH-dependent nanodelivery systems have been successfully developed, they mostly target the entire colon rather than specific UC sites. They are released throughout the colon after oral consumption, rendering insufficient accumulation in inflammatory colon lesions and causing adverse effects. More importantly, the disease state induces pH changes in the colon. For example, the colonic pH in patients with UC is approximately 3.0. Therefore, traditional single pH-responsive nanoparticles have certain limitations and cannot effectively treat IBD.
2.1.2. Time-dependent target-action response mode
In the time-dependent delivery system, the degradation time of the oral preparation is modulated according to the emptying time of the drug within the GIT past oral administration to degrade and disperse the medication in the colon section. Hence, the selection of coating materials and coating thickness are vital factors. However, the transport time is limited to drug absorption, and the difference is significant throughout the process [37]. Animal models or even non-human primates, have also been utilized to research drug delivery in the GIT. Accurate prediction is challenging owing to significant individual variations in GIT transit times, particularly in illness situations [38].
2.1.3. Colonic microbial-dependent target-action response mode
The colon contains more bacteria (1011–1013 CFU/g colon) than other parts of the GIT (103 CFU/g and 104–108 CFU/g in the stomach and small intestine, respectively). Certain enzymes, such as β-glucosidase, cellulase, azoreductase, and nitroreductase, are produced by these colonic microflora and are capable of breaking down various polymeric compounds, such as sodium alginate, CS, pectin, and dextran, which cannot be broken down in the stomach or small intestine [39]. By stimulating these microbes, several colonic microbe-activated drug delivery systems have been developed [40].
Pectin is a hydrophilic dietary fiber found within the cell exterior wall of fruits such as melons. It is made up of branching (1→4)-α-d-galacturonic acid (Gal-A) residues with various neutral sugars. Under the microscope, pectin chains can be split into two domains: the "smooth zone" and the "hairy zone". The former refers to the linear anionic backbone without side chains, while the latter refers to the region with nonionic side chains. Moreover, they fall into the following categories of polymeric forms: homogalacturonan, xylogalacturonan, and rhamnogalacturonan-I/II [41]. Pectin cannot be absorbed by the GIT and can only be degraded in the rectum and colon, possibly due to the colon's suitable intestinal flora and its optimal pH environment [24].
Guar gum (GG) is a nonionic polysaccharide abundant in guar or cluster beans. Over eighty percent of GG is produced in India [42]. Certain microbes, such as Clostridium butyricum in the large intestine, spontaneously secrete enzymes that break down the glycosidic bonds that are contained in GG. However, because of the high swelling characteristics of GG in aqueous solution, its potential as a transport carrier is limited. The properties of GG, which is widely used in biomedicine, can be improved by modification, grafting procedures, and forming networks [43]. GG and its derivatives, such as coated layers, matrix sheets, hydrogels, nanoparticles, and particles, can be used as prospective drug vectors [44].
With significant intrinsic qualities like adhesion, permeability, and antibacterial activity, CS is a common naturally arising cationic polysaccharide. It is a great oral medication delivery carrier substance that colonic bacterial enzymes can specifically hydrolyze [45]. To extend the duration of drug retention, it engages in electrostatic interactions with mucins that are negatively charged on the mucosal surface. Additionally, the amine group in CS can respond to fluctuations in environmental pH, which can cause pH-induced drug release from the carrier [46].
Hyaluronic acid (HA) is a prime option for IBD treatment because it interacts with CD44 on immune cells to control macrophage progression and produce anti-inflammatory effects [47]. However, its tendency to degrade in digestive juices during administration and uncontrolled systemic diffusion reduces its bioavailability, causing potential side effects and limiting its application in IBD treatment [48]. An approach to overcome this dilemma involves using a colon-targeting shell to avoid premature exposure to the drug and ensure its location-specific release.
Resistant starch (RS) can serve as a vector material for targeting the colon. RS are incapable of being rapidly degraded by enzymes involved in human digestion but can be swiftly metabolized by the bacteria in the colon, which makes them suitable for some controlled release systems [49]. However, starch derivatives are more laborious to enzymatic hydrolysis than natural starch. Research has indicated that starch derivatives obtained by appropriate physical or chemical modification, like cross-linked starch and copolymers, including starch-2-hydroxyethyl methacrylate [50], starch-methacrylic acid hydrogel [51], and starch-vinyl alcohol [52,53], can be used as delivery carrier materials for anti-inflammatory drugs and peptides. Starch acetate (SA) is a chemical derivative starch whose properties rely on the complete extent of substitution. Resistant starch acetate (RSA) with high digestive resistance is in the control of the degree of substitution. It can serve as a surficial coating material for potential oral CTDDs. Additionally, the plasticizer quantity and RSA coating film thickness can be readily adjusted to achieve the required colon-targeting and release qualities for RSA-coated microspheres. Further, this release system is appropriate for loading various active ingredients that differ in molecule weights and solubility [54]. Pullulan have been employed for colon targeting purposes, which can be degraded by branched-chain amylase produced by colonic microorganisms. Zeeshan et al. developed dexamethasone-loaded galactosylated-PLGA/Eudragit S100/pullulan nanocapsules (Dexa-GP/ES/Pu NCs) (Fig. 3B), which have dual irritation-sensitive coating sensitive to both colonic pH and microbiota, as well as a galactosylated-PLGA core (GP) at the bottom [55].
2.1.4. Receptor-mediated target-action response mode
Receptor-mediated target-action response mode based on ligand-receptor interactions might show potential as a treatment approach for IBD. Certain receptors and cellular adhesion molecules are found to be abundantly expressed onto the superficies of the colonic endothelial cells and/or immune cells during IBD, according to researchers' findings. By utilizing ligand-modified drug delivery systems that selectively target these above elements, drugs can be specifically delivered to the region of inflammation with enhanced efficacy [56]. High expression of receptors and cell adhesion molecules, including mannose receptor [57], intercellular adhesion molecule ICAM-1 [58], peptide transporter 1 [59], F4/80 [60], lactoferrin receptor [61], folic acid receptor [62], CD98 [63]and CD44 have been reported [64]. Previously, attempts have been made to modify the aforementioned ligands on delivery vectors to hamper their degradation in the upper digestive tract and to attain better cell targeting at local inflammatory sites in the colonic tissues. For example, Zhang et al. designed a HA/CS/Dex nanoparticle, coated with multilayers of CS, HA, and Eudragit® S100 (ECHCD MPs). These nanoparticles were ingested by macrophages via CD44 receptor-mediated endocytosis to regulate intestinal macrophage polarization and exerted colon-targeting anti-inflammatory effects (Fig. 3C) [65]. However, some highly expressed receptors linked to the pathological process of IBD and their respective ligands are poorly investigated. Therefore, for the purpose of developing, screening, and proving more specific receptors and ligands correlated with IBD, we need more sophisticated technology like molecular docking, molecular dynamics simulation, proteomics, and high-throughput screening.
2.1.5. Charge-dependent target-action response mode
During colonic mucosal inflammation, the mucus layer is depleted [66], resulting in the accumulation of positive charges in the damaged epithelium [67]. Therefore, an effective treatment strategy is to remove ROS by assembling negatively charged drugs or their carriers to target the colon site, where positively charged proteins are enriched due to inflammation, thereby reducing inflammation [68]. Based on this concept, an oral CeO2@MMT nanozyme drug was designed by combining CeO2 nanozyme, which possesses ROS-scavenging activity, with natural negative montmorillonite (MMT), which has the complementary advantages of each component and has demonstrated alleviated inflammation in a mouse colitis model (Fig. 3D). However, as these positively charged CeO2 NPs are not selective to the colonic inflammation area and the ultra-small size of these NPs promotes their rapid absorption by the GIT, the possibility of side effects is high. Combining CeO2 with MMT significantly inhibited the systemic absorption of CeO2 NPs, reducing its potential nanotoxicity and promoting MMT's ability to eliminate ROS. Therefore, CeO2@MMT achieved the effect of '1 + 1 > 2′, which led to substantial improvement in a DSS-induced mouse IBD model [69]. Briefly, some natural or synthetic anionic polysaccharides or negatively charged polymers are often used as surface coatings on nanocarriers. Future research on coating materials will further enhance both the targetability and adhesion capabilities of these delivery systems, thus enhancing the local delivery and retention of drugs.
2.1.6. ROS-responsive target-action response mode
The nosogenesis and growth of IBD are intrinsically connected to oxidative stress and irregularly high levels of local ROS that promote IBD development [70]. ROS-responsive nanoparticles administered orally may be used as an effective method for treating IBD. For example, Yan et al. developed a PSB@NP-FA, which are ROS responsive and folic acid (FA) functionalized nanoparticles loaded with pterostilbene (PSB). These nanoparticles could effectively release PSB in response to ROS, scavenge ROS, and protect cells from H2O2-induced oxidative damage (Fig. 3E) [71].
ROS-sensitive linkers are critical for designing ROS-responsive delivery systems. Fig. 3F summarizes the chemical structures of these linkers [28,[72], [73], [74]]. ROS-sensitive connectors include various connection modes that can be used between nanocarriers and drugs and two or more molecules of the same or different drugs. For example, Bud and Tem were combined by a ROS-sensitive aromatized thiopental to formulate a Janus-prodrug called B-ATK-T. Orally administered B-ATK-T appeared to have extraordinarily high drug loading capacity, and the release of two medicines was triggered by expansive ROS, accumulating drugs in the inflamed colon to treat colitis [29]. However, although this response mode is prevalent in treating inflammatory diseases, the real-time concentration and duration of ROS significantly influence the therapeutic effect. Reports that outline the ROS level in colon tissue during IBD, the concentration range required to achieve ROS response mode, and the degree of interference of redox substances in vivo are currently unavailable.
2.1.7. Multi-response mode delivery system
Precise and targeted therapy is extremely challenging because of intra- and interindividual differences in intestinal parameters, such as pH and transit time, which may increase the possibility of failure. Consequently, it's essential for integrating multiple response delivery systems to achieve more accurate positioning. For example, an exclusive coating technique (Phloral TM), which blends two separate release mechanisms; pH-triggered (Eudragit®S, ∼pH 7) and microbial-triggered (RS), provides an effective method for colon targeting [75]. This study demonstrated that the incorporation of RS in the coating layers could not impact pH-dependent drug release mechanism or the durability of the coating within the upper GIT. While encountering butanol, heat the starch to disrupt the crystalline structure of the starch granules for enhancing the digestibility of RS by bacterial enzymes. Limited exposure to high pH conditions and rapid colon transit in the distal small bowel fluid, often observed in patients with IBD, provides a revolutionary approach to colon-specific drug delivery. Furthermore, another technology, Opticore™, has successfully developed a coating technology by integrating an alkaline inside layer with an outermost layer with incorporated pH and enzymatic triggers. The coating system consists of an interior layer made of enteric polymers with partial neutralization and buffers, and an exterior layer made of Eudragit®S and RS [76].
2.2. Delivery system skeleton type
2.2.1. Prodrug
Prodrugs are derivatives of active molecules, of which modification approach links multiple drug molecules or between the molecule and nanoplatform by employing linkers [77]. Using simple and efficient synthetic means, prodrug design aims to solve problems, including poor solubility, poor permeability, instability, toxicity and non-targeting of the parent drug during drug delivery [78,79]. In Addition, prodrugs also suffer from the disadvantages of inconclusive toxicity, long-term retention and off-target efficiency [80]. Using prodrugs, such as azoreductase-responsive prodrug, is an effective colon-targeting strategy for related diseases. The azobenzene group in the molecular structure of these prodrugs can be transformed into the phenylamine group by azoreductase, leading to drug release in the colon. Saladazine and balsalazide are effective alternatives to 5-ASA systemic therapy [81].
Azobenzene is used as a linker to combine 4′-O-demethyl-4β-(4′′-aminoanilino)−4-desoxy-podophyllotoxin, a metabolite of the anti-colon cancer phase II drug GL331, with a near-infrared fluorophore. Simultaneously, combining modal imaging technology NIR/IMS is a novel method for real-time and accurate tracking of the prodrug release process and biodistribution in vivo [82]. Clinical data have shown that tofacitinib can significantly improve the remission of UC by inhibiting the JAK/STAT pathway, which is a common feature of multiple inflammatory factor responses [83]. To alleviate the systemic adverse reactions of tofacitinib, a 5-ASA-PABA-MAC, and 5-ASA-PABA-diamine colon-specific drug delivery system was constructed and used to synthesize facitinib azo prodrugs (Fig. 4A). Specifically, the compound displayed a 3.67-fold decrease in plasma AUC (tofacitinib, 0-∞) and a 9.61-fold increase in colonic AUC (tofacitinib, 0-12 h) compared with tofacitinib at mole-equivalent oral dose. The findings of in vivo experiments indicated that 5-ASA-PABABA-MAC and 5-ASA-PABA-diamine systems were effective in colon-specific administration and reduced the systemic adverse reactions of tofacitinib [84]. Furthermore, researchers have reported the use of triggerable precursor drug nanocoatings capable of on-demand activation of microbial and small-molecule therapeutic agents for combination therapy. ROS aromatic thioacetal linkers were used to prepare cationic CS-drug concatenates that could form nanocoatings on the surface of living bacteria via electrostatic interactions [85] (Fig. 4B-4D). Reaching the colonic site, cleavage of the thiochitosan linker leads to the release of therapeutic cinnamaldehyde. Simultaneously, charge reversal, achieved by the production of negatively charged thiochitosan, induced the disassembly of the nanocoatings, leading to the parallel release of live bacteria.
Fig. 4.
Representative cases of colon delivery of drugs by prodrug method. (A) Tofacitinib azo prodrugs that are specifically targeted to the colon were made using the 5-ASA-PABA-MAC and 5-ASA-PABA-diamine. Reproduced from [83] Copyright 2022 American Chemical Society. (B) Schematic diagram of the utilization of a triggerable prodrug nanocoating to enable lesion-targeted dual activation of LMTs and SMDs for combination therapy. (C) Synthetic route of ROS-responsive CS-TA-BG. (D) IVIS images of the intestinal tract harvested from PBS, EcN + metformin, EcN@CS, EcN@12C, or EcN@TA group. Reproduced from [85] Copyright 2023 American Chemical Society.
2.2.2. Liposomes
Liposomes are lipid-based spherical vesicles with a lipophilic bilayer surrounded by two hydrophilic layers [86], which comprise several components, such as lipids, sterols, polysaccharides, and surfactants. As they can accumulate at an inflammatory site, they are used to effectively treat IBD with reduced toxicity [87]. Determining the liposome type is crucial for selecting the synthesis method. Liposomes can be categorized as multilamellar vesicles with more than five bilayer membranes, oligolamellar vesicles with 2 to 5 bilayer membranes, and unilamellar vesicles (ULVs) with one bilayer membrane. ULVs could be subdivided into three categories: giant (>1 mm, GUVs), large (100 nm-1 mm, LUVs), and small (20 nm-100 nm in diameter, SUVs) [88].
Different preparation methods are used for different drugs. For example, solvent evaporation (also known as thin-film hydration) and solvent dispersion methods are suitable for delivering lipophilic drugs. Reverse-phase evaporation technology is the preferred means for packing hydrophilic drugs into liposomes. Further, there are various synthetic strategies, including the injection method, detergent removal, dehydration–rehydration, and pH jumping [89]. As shown in Fig. 5A, Ma et al. [90] come up with that the thin-film hydration approach was utilized to produce cationic liposomes (Lipo) employing Pluronic F127, 1,2-dioleoyl-3-trimethyl-ammonium-propane, and monoolein as raw materials. To derive C-dots@Lipo, the negative C-dots were electrostatically bound to the liposomes coated with a macrophage cell membrane. With a longer circulation duration and targeting functionality, C-dots@Lipo-M showed positive effects for colitis that were both preventative and therapeutic (Fig. 5B). However, liposome-based drugs are associated with several issues, including easy leakage and unsuitability for long-term storage. In addition to chemical modification, using solid lipid nanoparticles (LNPs), especially nanostructured lipid carriers, instead of traditional liposome preparations can significantly improve their stability and encapsulation efficiency.
Fig. 5.
Representative cases of colon-targeted delivery of drugs by liposome. (A) The synthesis route of C-dots@Lipo-M. (B) C-dots@Lipo-M productively attenuates UC by modifying inflammatory routes and altering the redox micro-environment. Reproduced from [77] Copyright 2022 Elsevier.
2.2.3. Lipid nanoparticle (LNPs)
LNPs, a specific kind of lipid vesicles, feature a lipid core that is homogeneous [91]. It has been commonly utilized for delivering small-molecule drugs and nucleic acids, providing good biocompatibility, enhanced drug stability, and controlled release, along with some drawbacks such as process complexity, storage stability and size variation [92,93]. LNPs have especially gained plenty of focus on their effectiveness in delivering COVID-19 mRNA vaccines [94]. LNPs can efficiently encapsulate and deliver molecules of various sizes to the desired regions in animal models. Liposomes, a precursor to LNPs, are incredibly adaptable nanocarriers that have successfully progressed from concept to clinical application due to their ability to transport small molecules, proteins, and nucleic acids [95]. The following generation of LNPs, including solid LNPs, nano structured lipid vectors, and cationic lipid-nucleic acid complexes, is characterized by more complicated internal designs and increased physical stabilities. As the accuracy and timing of local drug transport can be controlled across the body, LNPs are utilized for treating various genetic diseases [95]. While microinjection works well, its widespread use is limited by the need for surgery and specialized injection techniques. Therefore, researchers are focusing on developing oral LNPs. For example, IL-22, a cytokine belonging to the IL-10 family, is strongly associated with IBD susceptibility genes. It is a key regulator of epithelial homeostasis that promotes wound healing during intestinal inflammation. Junsik Sung et al. created LNPs using a basic formulation of specific lipids found in ginger previously reported [96]. They loaded the mRNA IL-22 into the nanoparticles using a cationic polymer called turbofectamine to achieve stabilization and condensation of mRNA. They assessed the protective effect of the generated particles on the target mRNA and revealed that oral delivery of IL-22/nLNPs led to increased expression of IL-22 in the mouse colonic mucosa and reduced the inflammatory levels without affecting the gut microbial composition [97].
2.2.4. Mesoporous silica
Mesoporous silica nanoparticles (MSNs) mainly include ordered-type MSNs (OMS) and hollow/rattle-type MSNs, which are promising candidates for biomedical applications [98]. Based on their unique vantage of high biocompatibility, distinct bioactivities, adjustable mesopore structure and properties, and versatile synthesis, furnishing multiple possibilities for transformative biomedical applications [99]. But unclear metabolism and the safety studies in vivo require attention. The size, shape, and structure of the pores of these MSNs can be intentionally created, and the procedure of synthesis can be easily regulated. Until now, most OMSs have been based on MCM-41, MCM-48, and SBA-15 type MSNs. Other types of MSNs, such as IBN and FDU-n series MSNs, are also ideal for biomedical applications [100]. Given the remarkably fast development of a wide range of applications, MSNs feature an interstitial hollow space and a mesoporous shell, resulting in low density and a large specific area. These characteristics make them excellent candidates for new-generation drug delivery systems because of their remarkably large loading capacity [101]. Due to their special structures, they are also suitable for multi-functionalization or co-delivery of several drug types. Currently, the primary development methods include the soft and hard template strategy, selective etching method, self-template method, self-assembly method, and a modified Stöber method [102].
A recent report proposed loading ROS-scavenging ceria nanoparticles onto MSNs and subsequently coated with negatively charged poly(acrylic acid) (PAA) on the surface (Fig. 6A). Fig. 6B shows that after oral delivery of inflamed colon-targeted antioxidant nanotherapeutics (ICANs), they sequentially reach the intestinal lumen, attach to epithelial cells, and finally translocate within the mucosal microenvironment, taking 6, 12 and 24 h respectively, according to IHC examination of sectioned colon tissue. The relative scavenging activity of the optimized nanoparticles against ROS was more than 60 % even after being exposed to artificial gastrointestinal fluid. A few in vitro experiments confirmed its adherence and internalization into inflamed epithelial cells and entering the lower layer cells of intestinal epithelium by means of transcytosis. Importantly, in vivo studies revealed that it demonstrated preferential adhesion to the inflammatory epithelium and was disseminated around the inflamed colon tissue, efficiently reducing the severity of the lesion. Simultaneously improving epithelial barrier function regeneration and reducing inflammatory cell infiltration and immunological responses are achieved by this redox balance modification. This strategy represents an uncomplicated yet efficient oral treatment for IBD [103].
Fig. 6.
(A) Representative cases of colon-targeted delivery of drugs by MSN. The ICANs were made of negatively charged poly(acrylic acid) (PAA) coated MSNs loaded with ceria nanoparticles (CeNPs), which scavenge reactive oxygen species (ROS); (B) IHC analyses of the mice's colons gathered at various intervals. Reproduced from [103] Copyright 2023 American Chemical Society; (C) Representative cases of colon-targeted delivery of drugs by YMs. The manufacturing of BBR/MPN@YM through BBR and EGCG Self-Assembly, along with the anti-inflammatory mechanism; (D) Biodistribution of BBR/MPN@YM. Reproduced from [25] Copyright 2022 American Chemical Society.
2.2.5. Yeast microcapsules (YMs)
YMs are biodegradable, naturally hollow, porous, uniform microspheres that can be used for the production of vaccines and biopharmaceuticals. They can also encapsulate and deliver active pharmaceutical ingredients, providing a promising platform for oral nanotherapy [104]. YMs, comprised of β−1,3-d-glucan, are elliptical and contain pores with a diameter of approximately 2–5 μm. Various therapeutic drugs can be encapsulated using YMs to attain restricted drug release in the upper GIT to shield it from breakdown within an acidic environment. After oral administration, intestinal immune cells can recognize β−1,3-d-glucan on the surface of YMs via several receptors (including complement receptor 3 and dectin-1 receptors) and transport it to Pan's plaque. In the Pan's plaque lymph nodes, it is rapidly and specifically recognized and phagocytized by macrophages and transported to the lesion site through macrophage-mediated transport. Therefore, YM can be used as a potential drug delivery carrier to be enriched in the gut immune system for treating UC [105]. For example, researchers have designed a safe macrophage-targeted oral by encapsulating anti-inflammatory drugs, such as berberine (BBR) and epigallocatechin gallate, in YMs to prepare BBR/MPN NPs. These anti-inflammatory NPs, which can polarize macrophages, were further encapsulated via chemical precipitation to form BBR/MPN@YM (Fig. 6C). The β−1,3-d-glucan on the superficies of these YMs can specifically bind to the dectin-1 receptor on macrophages to transport the drugs to the intestinal inflammatory site for internalization into macrophages, effectively avoiding gastric degradation. In vivo experiments have shown that BBR/MPN@YM has the ability to switch M1 into M2 macrophages, resulting in a targeted anti-inflammatory impact [25] (Fig. 6D). Therefore, YMs offer a novel approach to targeting macrophages in the clinical management of UC. The special advantage of yeast cells that distinguished from other delivery systems is the ability to transport both hydrophilic and hydrophobic molecules and to protect them from light, heat, oxygen, humidity, etc. Conversely, there are some issues, including time-consuming loading steps and complex development of a yeast-derived system.
2.2.6. Metal-organic frame (MOF)
MOF, a type of organic/inorganic hybrid material, has generated a lot of attention since its discovery [106]. MOFs have a high specific surface area and regular pore structure, which can improve the drug loading rate and achieve a good sustained release effect. Currently, drugs can be loaded onto MOFs by directly integrating the drug molecule into the MOFs or their pores [107]. Moreover, an assortment of interactions, including as hydrogen bonds, van der Waals forces, the π-π effect between aromatic rings, electrostatic contacts, coordination bonds, and covalent connections, can result in the chemical conjugation or physical encapsulation of pharmaceuticals within carriers. As a general rule, biomolecules are inserted into MOFs through diverse technologies [108]. Since the majority of MOFs are unsteady within physiological circumstances, the gradual decomposition of MOFs via surface modification is crucial to prevent the early release of drugs. Excessive ROS production and up-regulation of the membrane-spanning glycoprotein CD98 take on an essential function in the incidence and development of UC. Researchers have coencapsulated carbon nanodots and CD98 CRISPR/Cas9 plasmids to construct a biomimetic pH-responsive MOF carrier (ZIF-8) for site-specific treatment of UC. To improve the targeting ability, the CCZ and macrophage membrane were coextruded and disguised on the nanoparticles. After intravenous administration, the drug delivery system can accumulate in the UC inflammatory sites through the damaged vascular system. The mimmum pH (<7) conditions of the inflammatory site induce decomposition of ZIF-8, resulting in the releasing behavior of carbon nanodot SOD-like nanozymes and plasmids. The leaking nano-enzymes can effectively curb ROS and reshape the inflammatory microenvironment. Simultaneously, the CD98 CRISPR/Cas9 plasmid diminished expression of the CD98 gene at the genomic level, thereby reducing the inflammatory response. Therefore, combining carbon dot nanozymes and the CD98 CRISPR/Cas9 system is a potentially desirable strategy in effect for UC treatment [26].
The current problems of MOF include nucleation growth susceptible to fluctuations in reaction conditions, low yield and high cost of synthesis, reproducibility and stability, which will need to be overcome in the future.
2.2.7. Biomimetic nanoparticles
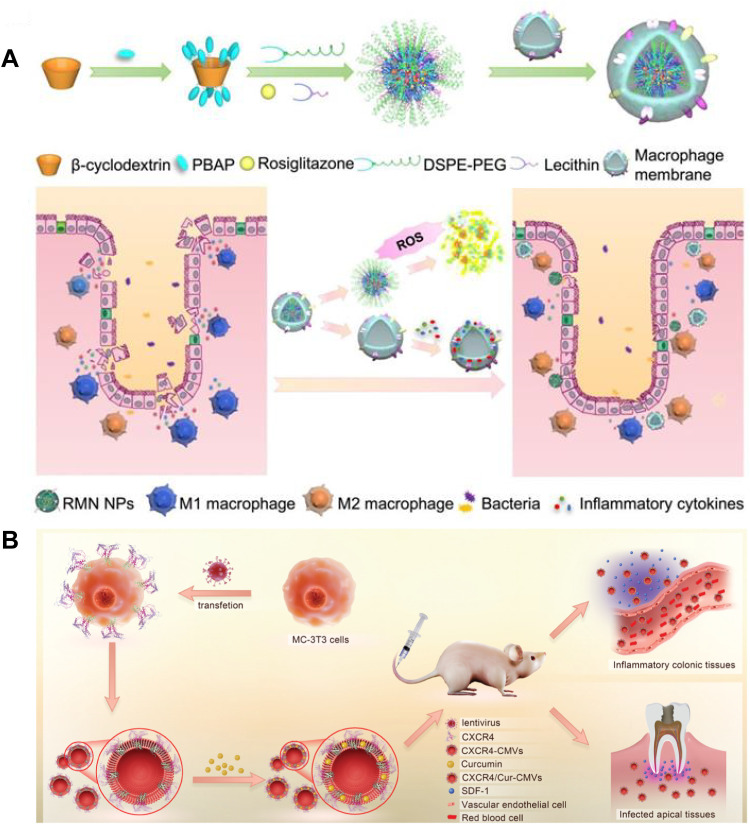
Biomimetic nanoparticles have been studied for treating IBD, which are composed of bionic functional parts and/or nanostructured material support parts [109], including cell membranes vesicles (CMVs) [110], extracellular vesicles (EVs) [111], programmable probiotics nanoparticles [112,113] and viruses particles that have been considered as promising candidates for CTDDs attributed to their biocompatibility and biodegradability. The nanoparticles can be imparted with specific characteristics, such as good targeting and low immunogenicity, by specially integrating them with biomimetic materials (cell membranes, lipoproteins, viruses or bacteria) to achieve better therapeutic effect [114]. Recently, exploration of carriers coated with cell membrane has been conducted to evade the immune system by disguising the drug delivery particles. ROS-responsive materials based on β-cyclodextrin were prepared using the characteristics of elevated ROS levels in UC, and rosiglitazone was loaded to obtain nanoparticles (Fig. 7A). Rosiglitazone is a PPARγ agonist that can induce macrophage M2 polarization, facilitating its therapeutic effect on UC. Initial research has shown that nanoparticles coated with macrophage membrane can target the inflammatory colon and possess an inflammatory homing effect, consuming substances that cause inflammation and hindering it. The drug-loaded rosiglitazone then reaches the inflammatory site and polarizes macrophages to M2, thereby even more handling the inflammatory surroundings. This synergistic effect significantly alleviated in vivo colon inflammation [114]. However, the coating film may be destroyed during the coating process, and its long-term stability and security still need further improvement.
Fig. 7.
(A) Representative cases of colon-targeted delivery of drugs by YMs. Fabrication process and targeted therapy of UC by RMN NPs. Reproduced from [94] Copyright 2020 Ivyspring International Publisher; (B) Representative cases of colon-targeted delivery of drugs by cell membrane vesicles. The illustrated diagram depicts the production, inflammatory targeting, and regulation of CXCR4-CMVs in vivo. Reproduced from [101] Copyright 2021 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Cell membrane vesicles, also known as CMVs, are vesicles that are nanosized and encased in a heterogeneous lipid bilayer membrane. In addition to facilitating communication between cells, they are in charge of carrying and delivering nucleic acids and proteins to a wide variety of recipient cells [115]. It is an effective drug delivery system composed of natural cell membranes with low immunogenicity and long circulation time [116]. CMVs are separated using ultracentrifugation, involving low-speed rotation to exfoliate cellular debris, and subsequent high-speed spins to collect CMVs. Sucrose gradients of densities can be employed to identify vesicle types on the basis of density and to extract vesicles from protein clusters. Cell membrane vesicles (CMVs) offer several benefits compared to existing drug delivery systems, including their capacity to surpass natural obstacles, inherent cell-specific targeting abilities, and durability in the circulatory system. However, ultracentrifugation and sucrose gradients have several limitations, such as time-consuming protocol, manipulator-dependent yield, and possible agglomeration and fragmentation of CMVs due to robust shear strength [117]. Therefore, ultracentrifugation is not conducive to the large-scale preparation of CMVs and their clinical applications. Therefore, novel isolation techniques, such as immunoaffinity isolation [118], polyethersulfone (PES) membrane filtration [119], and size-exclusion chromatography (SEC) [120], are currently being investigated.
CXCL12/CXCR4 binding is essential for chemotaxis, such as leukocyte transport and recruitment. Prior research has demonstrated a notable rise in CXCL12 levels in inflamed tissues. Wang et al. [121] and colleagues introduced CXCR4 recombinant lentivirus into MC-3T3 cells to produce MC-3T3 cell membranes enriched with CXCR4 (Fig. 7B). Then, they encapsulated curcumin into these CMVs via physical capture, obtaining CXCR4/cur-cmv with natural membrane surface properties and stable expression of the targeted protein CXCR4. This CMV shows good biocompatibility with enhanced targeting properties. Employing UC and periapical periodontitis as models of illness, CXCR4/Cur-cmv was shown to effectively target the inflammatory sites in vivo and significantly improve the symptoms degree of UC and periapical periodontitis, revealing that the CXCR4-rich membrane is a promising targeted delivery strategy for treating IBD. In short, biomimetic nanomaterials integrate the advantages of low immunogenicity of biomaterials with controllability of intelligent drug delivery systems, but remain a formidable problem with cumbersome preparation, poor reproducibility, and demanding storage conditions.
2.2.8. Hydrogels
Hydrogel is a type of water-swollen 3D polymer network and encounters adjustable physicochemical characteristics induced by various mechanisms through cross-linked polymer chains dispersed in aqueous media, including physical entanglement, ion interaction, and chemical crosslinking [122] (Fig. 8A). Physical gelation methods mostly rely on the intrinsic characteristics of the polymer, which limit their ability to fine-tune hydrogel properties. However, gelation can be easily achieved without modifying the polymer chain and can usually be easily reversed when necessary. Conversely, chemical methods can be used in a spatially and dynamically defined manner to allow for more controllable and precise crosslinking processes [123]. Hydrogels have been used in various biomedical fields, including cardiology, oncology, immunology, wound healing, and pain relief as a drug delivery system. The high water content (70 % - 99 %) of hydrogels simulates physical tissue conditions, enhancing their biocompatibility and capacity for encapsulating hydrophilic medications. Variations in the size, structure, and function of hydrogels determine how they can be used for drug delivery [124].
Fig. 8.
Cross-linking methods and representative cases of hydrogels. (A) Physical cross-linking methods. Reproduced from [122] Copyright 2021 Springer Nature; (B) Schematic illustration of the synthetic procedure and conservation effect of SeNG in a colitis model. Reproduced from [105] Copyright 2017 American Association for the Advancement of Science; (C) Schematic illustration of CS-CUR-NPs-Gel for oral macrophage-targeted drug delivery and pH/ROS dual-responsive drug release against UC. Reproduced from [125] Copyright 2022 Elsevier; (D) Schematic representation of the synthetic GAS hydrogel and the protective mechanism on the colitis epithelium. Reproduced from [126] Copyright 2023 American Chemical Society.
A functional material with ROS-scavenging activity and targeting properties has been developed using selenocysteine-coupling HA nanohydrogel (SeNG). SeNG first binds to CD44, which is exceedingly high expressing on the superficial of inflamed cells, through HA and mediates its specific enrichment in the colonic inflammatory sites (Fig. 8B). SeNG can reduce the ROS level of target cells by directly scavenging ROS and upregulating the Nrf2/HO-1 signaling pathway, thereby achieving efficient treatment of IBD [105]. Xu et al. developed a pH/ROS dual-sensitive chondroitin sulfate wrapped poly (β-amino ester)-SA-PAPE copolymer nanoparticle for UC treatment. After orally delivered, these hydrogel nanoparticles exhibited noticeable drug release in a ROS-rich environment, and could significantly alleviate inflammation in DSS-induced UC mice via TLR4-MAPK/NF-κB pathway [125] (Fig. 8C). In addition, the development of hydrogel-based nanomaterials targeting inflamed tissue and controlled to release anti-inflammatory drugs to modulate immune function may be an excellent way to treat IBD. Recently, Huang et al. designed a gallic acid/sodium alginate hybrid hydrogel (GAS), with multifunctional properties such as antiacid, mucoadhesive, sustained drug release, inhibited the expression of inflammatory cytokines, and regulated macrophage polarization; as expected, the GAS could significantly alleviate UC in mice [126] (Fig. 8D). Hydrogel delivery systems possess suitable release mechanisms, on-demand release kinetics, high drug loading rates, adjustable responsiveness to various stimuli, and specific thermodynamic compatibility properties. However, they also suffer from uncontrolled drug release rates and an as-yet-unknown long-term biocompatibility that needs to be addressed.
2.2.9. Micro/nanorobots
Micro/nanorobots are minimal smart devices capable of transforming the inner energy source or exterior physical stimulation into mechanical forces to create independent movement. They are mainly used for scheduled delivery of drugs because they can actively weave inside the human body using multifarious patterns of propulsion to reach lesion areas that are hard to reach by traditional drug delivery methods [127]. Presently, the primary power sources of the developed biomedical micro/nanorobots are active metals and H2O2, enzymes, light, electricity field, acoustic field, magnetic field, bacteria, and contractile and immune cells [128] (Fig. 9A). Although many methods have been used to transmit drugs to the colon in patients with moderate to severe IBD, it is difficult for drugs to penetrate the lower parts of the intestinal wall despite internal pressure of the intestinal cavity, abundant enzymes, microbial metabolites, and intestinal wall barriers. Nanorobots possessing autonomous power and active targeting capabilities may overcome this problem. For example, the twin-bioengine yeast micro/nanorobot (TBY-robot) effectively penetrates the mucus barrier. It significantly enhances the ability to retain in the intestines by utilizing a dual enzyme-driven mechanism that moves along the gradient of intestinal glucose. The TBY robot is transferred to the Peyers' plaque, where the enzyme-driven engine is switched in situ to macrophage bioengineering and then transferred to the inflammatory site using a chemokine gradient (Fig. 9B). TBY-robots can self-propel towards a glucose gradient, reach the intestinal wall within 2 min, and have a chemotactic speed of 16.74 μm/s, 2.4 times higher than in homogeneous glucose concentration (300 mM). (Fig. 9C-9E). EMS-based delivery increased drug accumulation at the lesion site by roughly 1000-fold, significantly reducing inflammation and improving disease pathology in mouse UC and GC models(Fig. 9F-9I). The adaptive TBY robot provides a safe and promising strategy for managing IBD and other inflammatory conditions [129].
Fig. 9.
The design strategy and representative cases of micro/nanorobots for precise drug delivery. (A) Different actuation energy methods include active metals and H2O2, enzymes, light, electricity field, acoustic field, magnetic field, bacteria, and contractile and immune cells. Reproduced from [128]. Copyright 2022 Elsevier; (B) Diagram illustrating the creation process of the TBY-robot and its use in delivering active targets and treating gastrointestinal inflammation; (C) Schematic illustration of intraintestinal barrier penetration of TBY-robot and transition to macrophage in a three-dimensional intestinal model; (D) Diagrammatic analysis of examination of TBY-robots' chemotactic motion using a microfluidic apparatus. The distance between the mouse intestinal wall and intestinal lumen center is represented by the length of the channel. There is a 0.2 to 300 mM glucose gradient between the intestinal lumen and intestinal wall. Time-lapse depicts the chemotactic movement of TBY-robots toward an area with a lot of glucose; (E) TBY-robots switched to the macrophage bioengine in Peyer's patches in situ. Nuclei (blue). Microfold cells (magenta). Macrophages (red). TBY-robots (green); (F-I) Representative H&E analysis of colons, bioluminescent images and colon length of are shown. Reproduced from [129]. Copyright 2023 American Association for the Advancement of Science. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Nevertheless, there are still many critical challenges about micro/nanorobots that necessitate immediate attention and further investigation. First, the develop of motion control techniques. Exploring motion control techniques, such as photoelectric control, ultrasonic control, magnetic field control, and chemical reaction control, should be concurrently promoted to facilitate real-time monitoring of the working conditions of micro/nanorobots. Second, the use and manufacture of new materials. Future research should focus on using and developing biocompatible nanomaterials for reducing costs and expanding the applications of micro/nanorobots in biomedical fields. Third, the problems of clinical testing and practical application. Some of the critical aspects that require attention include regulatory barriers, effective control mechanisms, and the implementation of large-scale systems in a safe and reliable manner.
3. Other complementary therapy options for UC
3.1. Transplantation of intestinal organoids
Intestinal organoid transplantation technology has revolutionized the biomedical field [130]. In a previous study, the 3D micro-organs were cultured in vitro using certain body cells that are structurally similar to the source tissue and organs [131]. Using the regenerative capacity of stem cells, researchers have used gut organoids to replace damaged intestinal tissue in regenerative medicine [130,132]. Stem cells have been reported to move only to damaged tissues and areas of inflammation with homing, promoting the regeneration of intestinal epithelium and angiogenesis, improving intestinal inflammation, facilitating the transformation of inflammatory infiltrating T cells and macrophages into an anti-inflammatory phenotype, and regulating the inflammatory response. To develop a system for transplanting intestinal organoids into the colon, researchers have used a DSS injury model to inject approximately 1000 organoids into the colon of mice with colitis through a flexible catheter. They closed the anal edge with glue to ensure that the cultured epithelial cells fully adhere to the damaged sites and fill the stem cell niche. The results revealed that the receptors on the epithelial lining of the inner intestinal walls were almost completely replaced by the donor cells [133]. The entire injection process requires only 10 min, making it a rapid and convenient treatment option for UC. Additionally, using cells from the patient's own receptor to prepare organoids can minimize the risk of transplant rejection. With their strong differentiation ability, stem cells have always been considered the ultimate means to cure many diseases completely. The clinical application and prospects are encouraging. Stem cell technology will be applied to treat various diseases to solve the key problems of disease treatment and enable customized precision medicine strategies.
3.2. Three dimensional printing (3DP)
For formulating pharmaceutical dosages, 3DP can provide on-demand medication by integrating intricate release patterns and geometries. Utilizing 3DP allows for a great level of flexibility and control in creating intricate customized dose forms with various release profiles, enhancing precision in individualized therapy [134]. Several 3DP methods have been explored for pharmaceutical applications, including binder injection, stereolithography [135], fused deposition modeling (FDM) [136], and semisolid extrusion.
For treating UC, 3DP technology is currently only applicable to solid preparations, mainly tablets and suppositories. FDM is the most commonly used 3DP technology owing to its versatility, simplicity, and low production cost. Moreover, by adjusting the infill density or dimensions, 3D-printed suppositories' dosage can be modulated. Kocabas et al. selected three kinds of formulations with 4 % (w/w) prednisolone sodium phosphate and diverse amounts of hydroxypropyl cellulose and mannitol [137] (Fig. 10A). They employed an FDM 3D printer to produce these compositions as suppositories. The excipients and drugs were mixed and placed in the extruder to melt and squeeze into filaments at high temperatures. Next, according to the required shape, filling density, and drug content, a 3D model was established using CAD software. Finally, the preparation was completed using layer-by-layer printing and deposition. In general, semisolid extrusion is another commonly used mild 3DP technology that is not related to the utilization of heat or UV. This method endorses the consolidation of heat- or UV-sensitive pharmaceutically active ingredients [138]. Ou et al.[139] developed a new type of 3D printed colon-targeted (CORR3CT) budesonide tablet with adjustable and controllable release to reduce off-target adverse reactions, which can plausibly supersede the use of enemas that are invasive and usually correlated with poor compliance. CraftMake™ was equipped with two printheads-used to print the outer shell and inner core-to print the CORR3CT tablets in a single print job (Fig. 10B-10E). The drug-release behavior of four formulations was investigated by optimizing the material composition of the shell and inner core. RC7000/R15M was selected for further study because the preparation can release budesonide rapidly and completely in the colon area (Fig. 10F).
Fig. 10.
Representative cases of colon-targeted delivery of drugs by 3D-printed technique. (A) Prednisolone phosphate suppositories made with 3D printing that have a customizable dose and release the drug quickly to treat IBD. Reproduced from [137] Copyright 2024 Elsevier. An outline of the 3D printing procedure for CORR3CT tablets. (B) Outside depict of CraftMake™ Printer. (C) Pictures of two-printer units. (D) Diagrammatic representation of the CORR3CT tablet 3D printing process. (E) and (F) Initial gastrointestinal simulated system screening of colon-targeting formulations (n = 3). Reproduced from [139] Copyright 2023 Elsevier.
3.3. Supplemental probiotics technology
Orally administered probiotics, especially Lactobacillus [140] or Bifidobacterium [141,142] as food supplements or microbial drugs, are safe and effective means to treat IBD patients due to their ability to restore the gut microenvironment. Numerous studies have revealed that gut microbiota and its metabolites exert multiple positive/ negative effects on inflammatory signaling pathways and colon tissue regeneration and repair. Because of the reduced biological activity of the digestive juices in the GIT after oral administration and the complex pathological environment at the lesion, probiotics are often challenging to colonize in the intestine; therefore, using a single probiotic is very limited [143]. Maintaining the biological activity of probiotics and improving the pathological environment of IBD to enhance the intestinal colonization of probiotics will effectively improve the efficacy of probiotics for treating IBD. To maintain the activity of bacteria and enhance the colonization of probiotics, it has been studied to encapsulate the prodrug molecules constructed by liposomes on the surface of probiotics. Balsalazide (Bal), the precursor drug for the treatment of UC, was assembled on the liposome 1-palmitoyl-sn‑glycero-3-phosphocholine (LPC) to form the prodrug nanomolecule LPC-Bal. LPC-Bal was modified on the surface of Lactobacillus rhamnosus GG (LGG) to construct a prodrug-encapsulated probiotic LBL, which significantly improved the pathological microenvironment, enhanced the intestinal colonization ability of probiotics and the therapeutic effect of UC. This research strategy has good potential for application in the development of probiotic therapy [144].
3.4. Delivering SCFAs
SCFAs are generated naturally in the colon as a result of microbial fermentation of both dietary and endogenous carbohydrates. They consist of 1–6 carbon-based anions, including acetic acid (C2), propionic acid (C3), and butyric acid (C4) [145]. SCFA mainly contributes to maintaining the intestinal barrier, facilitating cell proliferation, enabling a supplementary supply of epithelial barrier energy source, and exerting anti-inflammatory effects during IBD [146]. The main mechanisms that of SCFA signaling have been recognized, which involve GPCR activation and histone deacetylase inhibition [147]. Foxp3, a pivotal translational factor, is to be mainly expressed by regulatory T cells (Tregs), which are essential for controlling intestinal inflammation. In mice, SCFAs modulate the abundance and functions of Tregs in the colon and protect against colitis via GPR43 [148]. Moreover, GPR41 and GPR109A induce different pathways to regulate gut microbial ecology for treating IBD. However, even with the use of enterically coated or encapsulated, butyrate has a malodorous and prolonged odor and taste [149]. Orally administered butyrate, in the form of sodium salt, fails to be absorbed in the intestinal region, where it can provide therapeutic benefits. Instead, it is quickly metabolized to sustain its pharmacological impact. Specifically, a polymeric nanoscale system derived from polymeric micelles supported by block copolymers was developed to transport butyrate to specialized areas in the GIT. Butyrate is linked to the hydrophobic bulk through an ester bond in this structure and could be broken down by esterases located in the GIT for local release. The connected butyrate groups enhance hydrophobic properties in the hydrophobic segment. After being released, the leftover structure (an inactive polymer that dissolves in water) moves down the lower GIT until it is expelled. When the butyrate-incorporating lumps are at the central area of micelles, they can withstand the acidic conditions in the upper digestive tract, thus avoiding premature release before reaching the intestine. Both butyrate-prodrug micelles, NtL-ButM and Neg-ButM, have comparable structures but differ in their corona charges, with NtL-ButM being neutral and Neg-ButM being negative. As a result, they have different distributions in the lower GIT, with butyrate being released through enzymatic processes [150].
3.5. Colonic vaccine delivery
Oral vaccination triggers innate and adaptive elements, resulting in effective protection in both mucosal and systemic areas [151]. Induction of a protective or tolerogenic immune response requires the following: (1) efficient transportation of intact and functional antigens to the colon; (2) continued distribution of antigen to gut-linked lymphoid tissue and transportation over the mucosal barrier into the submucosa; and (3) subsequent immunomodulation with APCs [152,153]. C. difficile is an opportunistic intestinal pathogen that produces the toxins TcdA and TcdB, causing severe colitis and subsequently resulting in considerable morbidity and mortality [154]. In their third clinical trial phase, two intramuscular toxoid vaccines systematically induced toxin-neutralizing antibodies but failed to supply localized guard for the colon to prevent primary C. difficile infection (CDI). The authors investigated whether intestinal symbiotic nontoxic C. difficile (NTCD) can be furtherly used to evoke antibodies to restrain the adhesion of C. difficile to epithelial cells during the premature period of the disease by overexpressing the colonization factor CD0873 and TcdB domain in the NTCD strain T7. Hamsters were orally immunized with spores of the recombinant strains T7–0873 or T7TcdB, and their intestinal and systemic responses were analyzed. Inoculation with T7–0873 effectively stimulated the production of antibodies in the intestines and notably decreased the attachment of toxin-producing C. difficile to Caco-2 cells. However, this vaccine needs to be further developed to target NTCD [155]. Therefore, when treating IBD with colon-targeted delivery vaccines, attention should also be paid to the variations in the adhesion and abundance of flora that can easily cause intestinal infections.
4. Therapeutic mechanism of colon-targeted drug delivery
The mechanisms on which the CTDDs reach the region of inflammation and release the drug for the treatment of IBD revolve around four main scenarios (Fig. 11): (1) Modulation of immune-related pathways. By affecting the proliferation and differentiation of diverse categories of immunological cells and the profuse secretion of immune cytokines, the nanodrug can promote the systemic immunity to reach homeostasis and avoid further tissue damage caused by inflammation; (2) Regulating the multiplication and differentiation of stem cells. The nanodrug stimulates the stem cells positioned at the bases of the intestinal crypts through various signaling pathways, restores their stemness, and promotes the proliferate and differentiate of stem cells into other intestinal cell types, accelerating the repair of the tissue itself; (3) Modulating the constitution and metabolic profile of intestinal microorganisms. Nanomedicines can affect the formation and abundance of intestinal flora and interfere with the metabolism of endogenous substances by intestinal flora, such as SCFAs, bile acids and arachidonic acid. These endogenous substances play a prominent role in the relief of inflammation in IBD patients; (4) Regulating oxidative stress homeostasis. Nanomedicines can balance oxidants and antioxidants by promoting peroxidase activity, delivering antioxidants, or adjusting oxidative stress-related pathways to alleviate inflammatory tissue damage.
Fig. 11.
The mechanism of nanomedicines for IBD treatment.
4.1. Regulating immune-related pathways
The effective site of the CTDDs depends on the drug, and the mechanism of treatment is mainly to regulate immune-related pathways, mainly involving pathways such as MAPK, NF-κB, and the JAK-STAT pathway. The essential substances influencing these pathways are unclear, but the overall interactions can be subdivided into four categories: (1) Between the drug and the local target directly after delivery to the colon; (2) Between the product of microbial or enzyme metabolism and the local target after the drug reaches the colon; (3) Between the drug and intestinal microorganisms to alter the metabolites produced after reaching the colon; and (4) The drug only removes the damage factors around the lesion instead of acting on the host tissue. All these modes of action affect the local composition and content of inflammatory factors by regulating the immune cell typing (e.g., M1/M2 macrophages and Treg/Th17 T cells) or secretion function of immune cells. This further alleviates the evidences of IBD by recovering intestinal permeability, restoring mucus secretion, and removing inflammatory substances.
A statistic has shown that cytokines can influence intestinal permeability under numerous conditions. The cytokine levels in UC depend on the disease stage and patient characteristics, which may lead to differences in treatment response [156] (Fig. 12A). However, the regulation of cytokines on the intestinal barrier is performed in a multi-immune cell cooperative manner. Statistically, immune-related inflammatory factors have been shown to significantly affect intestinal barrier permeability during IBD. For example, IFN-α, IL-1β, IL-4, IL-13, IL-18 and TNF-α increase cell permeability, while IL-1α, IFN-γ, IL-10, IL-15, IL-2β and TGF-β reduce it. Moreover, the effects of IL-17, IL-23 and IL-33 are double-regulated under different conditions [157]. Colon-targeted delivery of drugs to regulate immune-related pathways has been described as a treatment for IBD. For example, Li et al. [158] designed orally administered microspheres with olsalazine encapsulated in multilayer pectin/CS/alginate composites treating UC. The multilayer microspheres could downregulate NF-κB and TNF-α and regulate the inflammatory factors IL-6 and IL-1β to restore the intestinal barrier and alleviate colitis.
Fig. 12.
(A) Immune cells and cytokines that contribute to the pathology of UC. Reproduced from [156] Copyright 2022 Elsevier. (B) Microenvironment of the ISCs niche that promotes epithelial regeneration. (C) Potential mechanisms for recruiting immune cells following injury. Reproduced from [162] Copyright 2020 Elsevier.
4.2. Regulating intestinal cell proliferation and differentiation-related pathways
Promoting the proliferation and differentiation of intestinal stem cells (ISCs) is also a key mechanism. Currently, a few studies on nanomedicine have investigated this mechanism, and it is considered a critical issue affecting the reconstruction of the entire intestinal injury tissue. Regeneration of epithelial cells is crucial for maintaining barriers and organ function after intestinal damage. As shown in Fig. 12B, after intestinal inflammatory infiltration injury, various cells provide factors to form niches that promote intestinal epithelial cell regeneration. DAMPs have the ability to activate immune cells like macrophages during cell death. Epithelial cells can transmit signals to macrophages through IL-25 as well as to ILC3s through IL-7. Moreover, bacterial infection could sensitize these immune cells. These ISC niches provide Wnt [159], Notch [160], and epidermal growth factor signals [161] that support Lgr5 + crypt basal columnar ISCs for regular epithelial cell maintenance. The matrix in the epithelial layer is not entirely internally regulated but requires regulation by nonepithelial components. The niche microenvironment of ISCs promotes epithelial cell regeneration, which is essential for regulating the regeneration response [162]. The cellular and noncellular elements that support regeneration following intestinal epithelial damage are depicted in Fig. 12C. The microenvironment furnishes the epithelium with factors that upregulate Wnt and YAP pathways. These components are mainly derived from mesenchymal cells, immune cells, bacteria, extracellular matrix, enteric nervous system, and nutritional state.
Studies have shown that IL-22 endures the ability to activate phosphorylation of STAT3 in Lgr5+ ISCs, and that STAT3 is essential for the creation of organoids and for IL-22-mediated regeneration. IL-22 connects the immune response to the regeneration of epithelial cells by directly affecting ISCs. ILCs that have been purified stimulate the growth of organoids in a way that relies on IL-22. IL-22 boosts epithelial regeneration mediated by ISCs, encouraging cell cycle advancement, epithelial proliferation, and the restoration of the ISC pool. IL-22 prompts the phosphorylation of STAT3 in Lgr5 + ISCs. While IL-22 may not be the sole regulator in ISCs, STAT3 is crucial for the formation of organoids and the regeneration of epithelial cells dependent on IL-22. On the opposite side, the regeneration triggered by IL-22 does not depend on Paneth cells. These data indicate that IL-22 could necessarily take part in regulating the compartment of injury-induced ISCs by activating IL-22 production and increasing crypt IL-22R expression [163]. Therefore, along with alterations to the matrix and epithelial components needed for maintaining normal epithelium, IL-22 is activated after tissue damage to restore the epithelium, showing the immunological involvement of the ISC niche.
4.3. Regulating the composition of gut microbiota
Through Toll-like receptors found on immunological and epithelial cells, the intestinal lumen bacteria can regulate intestinal healing by identifying commensal bacteria. Studies have shown that TLR4 knockout mice cannot recognize symbiotic bacteria and initiate intestinal regeneration pathways [164]. IBD is often associated with intestinal flora disorders. Reversing the disorder to achieve homeostasis of the intestinal flora is a critical therapeutic mechanism. Based on several studies, microorganisms with imbalanced homeostasis play opposing roles in IBD, exerting protective and aggravating effects [165].
Some intestinal microorganisms, including Bacteroides, Clostridium, Bifidobacterium, Lactobacillus, and Faecalibacterium genera, are beneficial for treating IBD. Bacteroides and Clostridium species evoke the amplification of Treg cells and reduce intestinal inflammation. Further, even the most noticeable bacterial species, including Bifidobacterium, Lactobacillus, and Faecalibacterium, might safeguard the circulatory system against inflammation in the mucosal lining through many pathways, such as reducing rates of proinflammatory cytokines or boosting the plane of the anti-inflammation cytokine IL-10.
Contrastingly, some other steady-state imbalanced microorganisms play a vital part in aggravating the disease. Escherichia coli, for instance, is closely associated with treating IBD with mesalamine, along with the reduction of E. coli/Shigella during treatment. Fusobacterium is another microorganism mainly colonized in the colon and associated with colonic erosion damage. A study showed that the abundance of Fusobacterium in the colonic mucosa was extremely more massive in IBD patients than in the controls. The invasive ability of human Fusobacterium isolates was positively correlated with the state of the IBD host, suggesting that it was invasive. In addition, it has been demonstrated that rectal enema of human Fusobacterium isolates causes erosion of the intestinal mucosa in rats and that IBD is closely linked with an upsurge of sulfate-reducing bacteria, including Desulfovibrio.
4.4. Regulating the level of oxidative stress
Oxidative stress is strongly associated with inflammatory diseases [166]. Clinical studies have revealed that excessive ROS, reactive nitrogen species (RNS), and proinflammatory cytokine levels are closely linked with IBD [167]. Molecular oxygen (O2) is the primary precursor for ROS, which includes free radicals, reactive molecules, and ions derived from O2, such as H2O2, O2•−, 1O2, and hydroxyl •OH. Continuous exposure of the inflamed intestinal mucosa exhibits cytotoxicity towards cellular DNA [168], lipids, and proteins, resulting in progressively oxidizing damage to gastrointestinal function, including poor absorption of nutrition [169], elevated intestinal permeability and disrupted gut motility [167]. Moreover, ROS steady-state imbalance can induce lysosomal impairment and revitalize apoptosis or necrosis pathways.
Some CTDDs are treated by delivering natural antioxidants, including vitamins, polyphenols, stilbenes, flavonoids, metals (zinc, CeO2, Mn3O4), and other naturally occurring substances, which can potentially scavenge ROS [170]. Certain intracellular antioxidants in organisms, such as SOD, GSH, CAT, NRF2 and glutathione peroxidase (GPx), help eliminate extras ROS to uphold cellular redox homeostasis. Therefore, the currently developed redox CTDDs for IBD mainly include enzyme-loaded, metal-loaded, polyphenol-loaded, nitroxide radical-loaded, and natural pigment-loaded nanodrugs. For example, Zhang et al. [171] synthesized oral zero-valent molybdenum nanodots (ZVMNs) to treat IBD by scavenging ROS. ZVMNs have been shown to downregulate the ROS levels and alleviate colitis in a mouse model without noticeable side effects. Moreover, RNA sequencing showed that ZVMNs protect colon tissues against oxidative stress by blocking the NF-κB signal pathway and decreasing the generation of proinflammatory molecules. Moreover, the degradability and metabolic excretion of nanocarriers should also be considered. Ruoyao Li et al. [172] prepared 2D atomic crystals of tannic acid-coated hafnium disulfide (HfS2@TA) nanosheets by liquid phase exfoliation, which can be used as high-performance anti-inflammatory nanoreagents for targeted therapy of IBD. Utilizing the S2-/S6+ valence state transition and large specific surface area for advantages, the yielded HfS2@TA nanosheets can effectively eliminate ROS/RNS and downregulate proinflammatory factors but can also be excreted through the kidney and hepatoenteric system. Surprisingly, HfS2@TA maintains superior targeting ability to the inflammatory colon even in hostile gastrointestinal environments, mainly accounting for the electrostatic interaction between negatively charged TA and positively charged inflammatory epithelium. Encouragingly, when administered orally or intravenously, HfS2@TA rapidly inhibits inflammation and repairs the intestinal mucosal barrier.
5. Conclusion
This review presents the emerging research landscape and multiple strategies for CTDDs for managing symptoms of IBD within the past 5 years. Despite the lack of effective treatment protocols, targeted nanodelivery systems have become a promising solution. However, several factors must be considered and verified, from the experimental development of the delivery system to clinical application. First, greater attention should be paid to patient population classification and the pertinence and suitability of nanopreparation development. The data from clinical trials available on the Clinicaltrials.gov website showed that the number of projects for children (< 17), adults (18–64), and older individuals (> 65) is 795, 3131 and 2687, respectively, which are within the statistical limits. This suggests that we must consider the physiological differences between different age groups while developing colon-targeted nanodelivery systems. Optimizing applicable dosage forms for different populations may be a better alternative approach. Second, the safety and toxicity of these CTDDs need to be investigated in detail. The guidelines and systematic regulatory documents should be developed and continuously improved. Due to the shortage of research data and details on acute and long-term toxicity after the administration of drug delivery carriers, it is difficult to provide practical support for the clinical application of drugs. Although studies have shown that certain delivery carriers have different extent of toxicity, further research is warranted to determine the dosage and scope of their applications. For example, carbon nanotubes (CNTs) and engineered nanomaterials have enormous beneficial applications and have previously been the research hotspot of drug delivery system development. However, studies have revealed that CNT exposure causes brain toxicity [173], cardiovascular toxicity [174], and pulmonary fibrosis [175]. The nanomaterial's residual metal catalyst content, stiffness, aggregated state, superficial charge, and other physicochemical characteristics dictate the intensity of the fibrogenic reaction. CNT-induced fibrosis pathways include oxidative stress, macrophages' immunological responses, secretory cytokines and growth factor release, epithelial cell damage, proliferation of pulmonary myofibroblasts, and subsequent accumulation of extracellular matrix [176]. Because of the safety concerns and lack of practical strategies for controlling toxicity to ensure safe medication, research on the use of CNTs as delivery carriers has decreased significantly in recent years. In summary, the exploitation of CTDDs is still in its initial stages. Further research should be conducted to thoroughly assess the toxicity of these nanocarriers to ensure safety and avoid unnecessary expenses. Third, the in vivo distribution, metabolism, and nondrug-related biological effects of nanomedicines require additional attention. Because of the complexity of the colonic microenvironment, selecting a disease model that can closely simulate human diseases and the patient's conditions, such as the content and composition of intestinal microorganisms, types and contents of local endogenous and exogenous substances, types and activities of enzymes, and levels of pH and redox molecules, are critical.
Additionally, challenges and future directions are worthy of in-depth discussion for CTDDs. Firstly, the impact of artificial intelligent (AI) technology may revolutionize the design and development of CTDDs in the future. AI in this area mainly involve machine learning (ML) and deep learning (DL) [177]. The integration of continuously updated AI into CTDDs design may be enhanced high-efficiently in many aspects, including active molecule discovery [178], delivery system screening [179], structure-activity relationships forecasts [180], dosage form optimization [181], quality control, pharmacokinetic simulation [182,183], efficacy and toxicity prediction [[184], [185], [186]], etc. It usually requires the creation of databases and training sets, the construction of models and algorithms for evaluation and tuning, and the forecasting and assessment of actual metrics [187]. In recent years, especially based on the physicochemical properties, AI has demonstrated bumper potential for predicting and assessing the toxicity of nanomaterials [188]. Secondly, microenvironments impose higher demands on delivery systems but also provide some novel opportunities to design and validate a variety of advanced nanocarriers. Some key pathogenic bacteria in the IBD patients may be promising targets. In response to the novel coronavirus (COVID-19) that has swept the world in the past few years, vaccine research and development has been the layout focus and center of attention, and high expectations have been placed on it. Among them, two mRNA nanovaccines, BNT162b [189] (trade name Comirnaty) and mRNA-1273 [190] (trade name Spikevax), are well known for their high protection efficiency and development speed. In addition to the development of mRNA vaccines for humans, the world's first mRNA vaccine for bacteria has also been developed [191]. Since easily degradable mRNAs must be delivered to cells in order to encode proteins and currently rely mainly on LNP delivery, CTDDs may serve as one of the important platforms for future mRNA vaccines to treat IBD. Third, CTDDs in IBD diagnostics and drug monitoring will probably also be an important area of research. Nanobiosensors [192], for example, in detecting disease markers and monitoring gut health, will probably help inform and underpin new research on CTDDs. In short, nanotechnology will play an increasingly important role in the prevention, treatment and diagnosis of IBD. Interdisciplinary cooperation will be the key to achieving this goal. It is believed that nanomedicine will have more breakthroughs in the treatment of IBD.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was supported by CACMS Innovation Fund (CI2021B016, CI2021A04801), National Natural Science Foundation of China (82192913, 82174073); Qihuang Scholar Program; CACMS Foundation (ZZ13-035-10); China Postdoctoral Science Foundation (2023M733913).
References
- 1.Doherty G., Katsanos K.H., Burisch J., Allez M., Papamichael K., Stallmach A., et al. European Crohn's and Colitis Organisation topical review on treatment withdrawal ['exit strategies'] in inflammatory bowel disease. J Crohns Colitis. 2018;12:17–31. doi: 10.1093/ecco-jcc/jjx101. [DOI] [PubMed] [Google Scholar]
- 2.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Stillhart C., Vučićević K., Augustijns P., Basit A.W., Batchelor H., Flanagan T.R., et al. Impact of gastrointestinal physiology on drug absorption in special populations–an UNGAP review. Eur J Pharm Sci. 2020;147 doi: 10.1016/j.ejps.2020.105280. [DOI] [PubMed] [Google Scholar]
- 5.Burger D., Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–1837. doi: 10.1053/j.gastro.2011.02.045. e2. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya A., Osterman M.T. Biologic Therapy for ulcerative colitis. Gastroenterol Clin North Am. 2020;49:717–729. doi: 10.1016/j.gtc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Loftus E.V., Panés J., Lacerda A.P., Peyrin-Biroulet L., D'Haens G., Panaccione R., et al. Upadacitinib induction and maintenance therapy for Crohn's disease. N Engl J Med. 2023;388:1966–1980. doi: 10.1056/NEJMoa2212728. [DOI] [PubMed] [Google Scholar]
- 8.Neurath M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat Rev Immunol. 2024 doi: 10.1038/s41577-024-01008-6. [DOI] [PubMed] [Google Scholar]
- 9.Schirmer M., Garner A., Vlamakis H., Xavier R.J. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos G.P., Papadakis K.A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T., Siegmund B., Le Berre C., Wei S.C., Ferrante M., Shen B., et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. doi: 10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y., Kamada N., Moon J.J. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv Drug Deliv Rev. 2021;179 doi: 10.1016/j.addr.2021.114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Di B., Xu L.L. Recent advances in the treatment of IBD: targets, mechanisms and related therapies. Cytokine Growth Factor Rev. 2023;71–72:1–12. doi: 10.1016/j.cytogfr.2023.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante M., Pouillon L., Mañosa M., Savarino E., Allez M., Kapizioni C., et al. Results of the eighth scientific workshop of ECCO: prevention and treatment of postoperative recurrence in patients with crohn's disease undergoing an ileocolonic resection with ileocolonic anastomosis. J Crohns Colitis. 2023;17:1707–1722. doi: 10.1093/ecco-jcc/jjad053. [DOI] [PubMed] [Google Scholar]
- 15.Weingarden A.R., Vaughn B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Haens G., Dubinsky M., Kobayashi T., Irving P.M., Howaldt S., Pokrotnieks J., et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2023;388:2444–2455. doi: 10.1056/NEJMoa2207940. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S., Zhang J., Qiu M., Hou Y., Li X., Zhong G., et al. Mucoadhesive and thermosensitive Bletilla striata polysaccharide/chitosan hydrogel loaded nanoparticles for rectal drug delivery in ulcerative colitis. Int J Biol Macromol. 2024;254 doi: 10.1016/j.ijbiomac.2023.127761. [DOI] [PubMed] [Google Scholar]
- 18.Jung Y., Kim Y.M. What should be considered on design of a colon-specific prodrug? Expert Opin Drug Deliv. 2010;7:245–258. doi: 10.1517/17425240903490401. [DOI] [PubMed] [Google Scholar]
- 19.Chu J.N., Traverso G. Foundations of gastrointestinal-based drug delivery and future developments. Nat Rev Gastroenterol Hepatol. 2022;19:219–238. doi: 10.1038/s41575-021-00539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duricova D., Burisch J., Jess T., Gower-Rousseau C., Lakatos P.L. ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8:1351–1361. doi: 10.1016/j.crohns.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Awad A., Madla C.M., McCoubrey L.E., Ferraro F., Gavins F.K.H., Buanz A., et al. Clinical translation of advanced colonic drug delivery technologies. Adv Drug Deliv Rev. 2022;181 doi: 10.1016/j.addr.2021.114076. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Zhu B.W., Sun R., Li X., Wu D., Hu J.N. Colon-targeting angelica sinensis polysaccharide nanoparticles with dual responsiveness for alleviation of ulcerative colitis. ACS Appl Mater Interfaces. 2023;15:26298–26315. doi: 10.1021/acsami.3c02128. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Cai Z., Wang F., Hong L., Deng L., Zhong J., et al. Colon-targeted adhesive hydrogel microsphere for regulation of gut immunity and flora. Adv Sci. 2021;8 doi: 10.1002/advs.202101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu A., Chen C., Lu J., Sun J., Xiao M., Yue X., et al. Preparation of oral core-shell zein nanoparticles to improve the bioavailability of glycyrrhizic acid for the treatment of ulcerative colitis. Biomacromolecules. 2022;23:210–225. doi: 10.1021/acs.biomac.1c01233. [DOI] [PubMed] [Google Scholar]
- 25.Feng X., Xie Q., Xu H., Zhang T., Li X., Tian Y., et al. Yeast microcapsule mediated natural products delivery for treating ulcerative colitis through anti-inflammatory and regulation of macrophage polarization. ACS Appl Mater Interfaces. 2022;14:31085–31098. doi: 10.1021/acsami.2c05642. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y., Gao W., Zhang Y., Yang M., Yan X., Zhang Y., et al. Biomimetic MOF nanoparticles delivery of c-dot nanozyme and CRISPR/Cas9 system for site-specific treatment of ulcerative colitis. ACS Appl Mater Interfaces. 2022;14:6358–6369. doi: 10.1021/acsami.1c21700. [DOI] [PubMed] [Google Scholar]
- 27.Optimization study of combined enteric and time-dependent polymethacrylates as a coating for colon targeted delivery of 5-ASA pellets in rats with ulcerative colitis. Eur J Pharm Sci. 2022;168 doi: 10.1016/j.ejps.2021.106072. [DOI] [PubMed] [Google Scholar]
- 28.Huang L., Wang J., Kong L., Wang X., Li Q., Zhang L., et al. ROS-responsive hyaluronic acid hydrogel for targeted delivery of probiotics to relieve colitis. Int J Biol Macromol. 2022;222:1476–1486. doi: 10.1016/j.ijbiomac.2022.09.247. [DOI] [PubMed] [Google Scholar]
- 29.Li S., Xie A., Li H., Zou X., Zhang Q. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J Controll Release. 2019;316:66–78. doi: 10.1016/j.jconrel.2019.10.054. [DOI] [PubMed] [Google Scholar]
- 30.Kotla N.G., Singh R., Baby B.V., Rasala S., Rasool J., Hynes S.O., et al. Inflammation-specific targeted carriers for local drug delivery to inflammatory bowel disease. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2022.121364. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal S., Du X., Wang J., Li H., Yuan Y., Wang J. Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis. Nano Res. 2018;11:2872–2884. [Google Scholar]
- 32.Wen Z., Kang L., Fu H., Zhu S., Ye X., Yang X., et al. Oral delivery of porous starch-loaded bilayer microgels for controlled drug delivery and treatment of ulcerative colitis. Carbohydr Polym. 2023;314 doi: 10.1016/j.carbpol.2023.120887. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G., Han W., Zhao P., Wang Z., Li M., Sui X., et al. Programmed pH-responsive core-shell nanoparticles for precisely targeted therapy of ulcerative colitis. Nanoscale. 2023;15:1937–1946. doi: 10.1039/d2nr04968f. [DOI] [PubMed] [Google Scholar]
- 34.Chen E.P., Mahar Doan K.M., Portelli S., Coatney R., Vaden V., Shi W. Gastric pH and gastric residence time in fasted and fed conscious cynomolgus monkeys using the Bravo pH system. Pharm Res. 2008;25:123–134. doi: 10.1007/s11095-007-9358-5. [DOI] [PubMed] [Google Scholar]
- 35.Dressman J.B., Amidon G.L., Reppas C., Shah V.P. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22. doi: 10.1023/a:1011984216775. [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Fishman M.L., Kost J., Hicks K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24:3333–3343. doi: 10.1016/s0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 37.Worsøe J., Fynne L., Gregersen T., Schlageter V., Christensen L.A., Dahlerup J.F., et al. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011;11:145. doi: 10.1186/1471-230X-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nandhra G.K., Mark E.B., Di Tanna G.L., Haase A.-M., Poulsen J., Christodoulides S., et al. Normative values for region-specific colonic and gastrointestinal transit times in 111 healthy volunteers using the 3D-Transit electromagnet tracking system: influence of age, gender, and body mass index. Neurogastroenterol Motil. 2020;32:e13734. doi: 10.1111/nmo.13734. [DOI] [PubMed] [Google Scholar]
- 39.Chen S., Song Y., Wang C., Tao S., Yu F., Lou H., et al. Chitosan-modified lipid nanodrug delivery system for the targeted and responsive treatment of ulcerative colitis. Carbohydr Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115613. [DOI] [PubMed] [Google Scholar]
- 40.Sousa T., Paterson R., Moore V., Carlsson A., Abrahamsson B., Basit A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Li D.Q., Li J., Dong H.L., Li X., Zhang J.Q., Ramaswamy S., et al. Pectin in biomedical and drug delivery applications: a review. Int J Biol Macromol. 2021;185:49–65. doi: 10.1016/j.ijbiomac.2021.06.088. [DOI] [PubMed] [Google Scholar]
- 42.Garg S.S., Gupta J. Guar gum-based nanoformulations: implications for improving drug delivery. Int J Biol Macromol. 2023;229:476–485. doi: 10.1016/j.ijbiomac.2022.12.271. [DOI] [PubMed] [Google Scholar]
- 43.Chandrika K.P., Singh A., Rathore A., Kumar A. Novel cross linked guar gum-g-poly(acrylate) porous superabsorbent hydrogels: characterization and swelling behaviour in different environments. Carbohydr Polym. 2016;149:175–185. doi: 10.1016/j.carbpol.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 44.Prabaharan M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol. 2011;49:117–124. doi: 10.1016/j.ijbiomac.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Harugade A., Sherje A.P., Pethe A. Chitosan: a review on properties, biological activities and recent progress in biomedical applications. React Funct Polym. 2023;191 [Google Scholar]
- 46.Oshi M.A., Naeem M., Bae J., Kim J., Lee J., Hasan N., et al. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr Polym. 2018;198:434–442. doi: 10.1016/j.carbpol.2018.06.107. [DOI] [PubMed] [Google Scholar]
- 47.Litwiniuk M., Krejner A., Speyrer M.S., Gauto A.R., Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28:78–88. [PubMed] [Google Scholar]
- 48.Zheng X., Wang B., Tang X., Mao B., Zhang Q., Zhang T., et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: a review. Carbohydr Polym. 2023;299 doi: 10.1016/j.carbpol.2022.120153. [DOI] [PubMed] [Google Scholar]
- 49.Bello-Perez L.A., Flores-Silva P.C., Agama-Acevedo E., Tovar J. Starch digestibility: past, present, and future. J Sci Food Agric. 2020;100:5009–5016. doi: 10.1002/jsfa.8955. [DOI] [PubMed] [Google Scholar]
- 50.Wöhl-Bruhn S., Bertz A., Harling S., Menzel H., Bunjes H. Hydroxyethyl starch-based polymers for the controlled release of biomacromolecules from hydrogel microspheres. Eur J Pharm Biopharm. 2012;81:573–581. doi: 10.1016/j.ejpb.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Cui C., Jia Y., Sun Q., Yu M., Ji N., Dai L., et al. Recent advances in the preparation, characterization, and food application of starch-based hydrogels. Carbohydr Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119624. [DOI] [PubMed] [Google Scholar]
- 52.Lee C.S., Hwang H.S. Starch-based hydrogels as a drug delivery system in biomedical applications. Gels. 2023;9:951. doi: 10.3390/gels9120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anwar H., Ahmad M., Minhas M.U., Rehmani S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, Optimization and in-vitro characterization. Carbohydr Polym. 2017;166:183–194. doi: 10.1016/j.carbpol.2017.02.080. [DOI] [PubMed] [Google Scholar]
- 54.Pu H., Chen L., Li X., Xie F., Yu L., Li L. An oral colon-targeting controlled release system based on resistant starch acetate: synthetization, characterization, and preparation of film-coating pellets. J Agric Food Chem. 2011;59:5738–5745. doi: 10.1021/jf2005468. [DOI] [PubMed] [Google Scholar]
- 55.Zeeshan M., Ain Q.U., Weigmann B., Story D., Smith B.R., Ali H. Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis. Asian J Pharm Sci. 2023;18 doi: 10.1016/j.ajps.2023.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu P., Gao C., Chen H., Vong C.T., Wu X., Tang X., et al. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: opportunities and emerging strategies. Acta Pharm Sin B. 2021;11:2798–2818. doi: 10.1016/j.apsb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno Y., Toshino M., Mohammed A.F.A., Fujiwara Y., Komohara Y., Onodera R., et al. Mannose-methyl-β-cyclodextrin suppresses tumor growth by targeting both colon cancer cells and tumor-associated macrophages. Carbohydr Polym. 2023;305 doi: 10.1016/j.carbpol.2023.120551. [DOI] [PubMed] [Google Scholar]
- 58.Mane V., Muro S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int J Nanomed. 2012;7:4223–4237. doi: 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y., Sun M., Wang D., Li G., Huang J., Tan S., et al. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater Sci. 2019;7:4299–4309. doi: 10.1039/c9bm00925f. [DOI] [PubMed] [Google Scholar]
- 60.Laroui H., Viennois E., Xiao B., Canup B.S.B., Geem D., Denning T.L., et al. Fab'-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release. 2014;186:41–53. doi: 10.1016/j.jconrel.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Yang Y., Zhang J., Wang R., Cheng B., Kalambhe D., et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases. Acta Pharm Sin B. 2020;10:1966–1976. doi: 10.1016/j.apsb.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Low P.S., Henne W.A., Doorneweerd D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 63.Xiao B., Yang Y., Viennois E., Zhang Y., Ayyadurai S., Baker M., et al. Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. J Mater Chem B. 2014;2:1499–1508. doi: 10.1039/C3TB21564D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X., Gu H., Zhang H., Xian J., Li J., Fu C., et al. Oral core-shell nanoparticles embedded in hydrogel microspheres for the efficient site-specific delivery of magnolol and enhanced antiulcerative colitis therapy. ACS Appl Mater Interfaces. 2021;13:33948–33961. doi: 10.1021/acsami.1c09804. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Ma R., You C., Leng X., Wang D., Deng S., et al. Hyaluronic acid modified oral drug delivery system with mucoadhesiveness and macrophage-targeting for colitis treatment. Carbohydr Polym. 2023;313 doi: 10.1016/j.carbpol.2023.120884. [DOI] [PubMed] [Google Scholar]
- 66.Gustafsson J.K., Johansson M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2022;19:785–803. doi: 10.1038/s41575-022-00675-x. [DOI] [PubMed] [Google Scholar]
- 67.Yang C., Merlin D. Nanoparticle-mediated drug delivery systems for the treatment of ibd: current perspectives. Int J Nanomed. 2019;14:8875–8889. doi: 10.2147/IJN.S210315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coco R., Plapied L., Pourcelle V., Jérôme C., Brayden D.J., Schneider Y.J., et al. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm. 2013;440:3–12. doi: 10.1016/j.ijpharm.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 69.Zhao S., Li Y., Liu Q., Li S., Cheng Y., Cheng C., et al. An orally administered ceo2@montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv Funct Mater. 2020;30 [Google Scholar]
- 70.Dudzińska E., Gryzinska M., Ognik K., Gil-Kulik P., Kocki J. Oxidative stress and effect of treatment on the oxidation product decomposition processes in ibd. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7918261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan X., Meng L., Zhang X., Deng Z., Gao B., Zhang Y., et al. Reactive oxygen species-responsive nanocarrier ameliorates murine colitis by intervening colonic innate and adaptive immune responses. Mol Ther. 2023;31:1383–1401. doi: 10.1016/j.ymthe.2023.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi C., Dawulieti J., Shi F., Yang C., Qin Q., Shi T., et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci Adv. 2022;8:eabj2372. doi: 10.1126/sciadv.abj2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan Y., Zhang C.J., Xu S., Liu B. A self-reporting AIE probe with a built-in singlet oxygen sensor for targeted photodynamic ablation of cancer cells. Chem Sci. 2016;7:1862–1866. doi: 10.1039/c5sc03583j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., Li H., Deng Y., Sun H., Ke X., Ci T. Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment. Acta Biomater. 2017;51:374–392. doi: 10.1016/j.actbio.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Varum F., Freire A.C., Fadda H.M., Bravo R., Basit A.W. A dual pH and microbiota-triggered coating (Phloral™) for fail-safe colonic drug release. Int J Pharm. 2020;583 doi: 10.1016/j.ijpharm.2020.119379. [DOI] [PubMed] [Google Scholar]
- 76.Varum F., Freire A.C., Bravo R., Basit A.W. OPTICORE™, an innovative and accurate colonic targeting technology. Int J Pharm. 2020;583 doi: 10.1016/j.ijpharm.2020.119372. [DOI] [PubMed] [Google Scholar]
- 77.Fralish Z., Chen A., Khan S., Zhou P., Reker D. The landscape of small-molecule prodrugs. Nat Rev Drug Discov. 2024;23:365–380. doi: 10.1038/s41573-024-00914-7. [DOI] [PubMed] [Google Scholar]
- 78.Liu H., Ji M., Xiao P., Gou J., Yin T., He H., et al. Glucocorticoids-based prodrug design: current strategies and research progress. Asian J Pharm Sci. 2024;19 doi: 10.1016/j.ajps.2024.100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding Y., Hu X., Piao Y., Huang R., Xie L., Yan X., et al. Lipid prodrug nanoassemblies via dynamic covalent boronates. ACS Nano. 2023;17:6601–6614. doi: 10.1021/acsnano.2c12233. [DOI] [PubMed] [Google Scholar]
- 80.Torres-Herrero B., Armenia I., Alleva M., Asín L., Correa S., Ortiz C., et al. Remote Activation of Enzyme Nanohybrids for Cancer Prodrug Therapy Controlled by Magnetic Heating. ACS Nano. 2023;17:12358–12373. doi: 10.1021/acsnano.3c01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tursi A. Balsalazide in treating colonic diseases. Expert Opin Drug Metab Toxicol. 2009;5:1555–1563. doi: 10.1517/17425250903228842. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X.B., Ha W., Gao K., Shi Y.P. Precisely traceable drug delivery of azoreductase-responsive prodrug for colon targeting via multimodal imaging. Anal Chem. 2020;92:9039–9047. doi: 10.1021/acs.analchem.0c01220. [DOI] [PubMed] [Google Scholar]
- 83.Danese S., Grisham M., Hodge J., Telliez J.B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155–G162. doi: 10.1152/ajpgi.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao J., Zhang B., Mao Q., Ping K., Zhang P., Lin F., et al. Discovery of a colon-targeted azo prodrug of tofacitinib through the establishment of colon-specific delivery systems constructed by 5-asa-paba-mac and 5-asa-paba-diamine for the treatment of ulcerative colitis. J Med Chem. 2022;65:4926–4948. doi: 10.1021/acs.jmedchem.1c02166. [DOI] [PubMed] [Google Scholar]
- 85.Kuang X., Liu Y., Luo H., Li Q., Wu F., Fan C., et al. Triggerable prodrug nanocoating enables on-demand activation of microbial and small-molecular therapeutics for combination treatment. J Am Chem Soc. 2023;145:26932–26946. doi: 10.1021/jacs.3c10015. [DOI] [PubMed] [Google Scholar]
- 86.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154-155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Zylberberg C., Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23:3319–3329. doi: 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- 88.Alavi M., Karimi N., Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharm Bull. 2017;7:3–9. doi: 10.15171/apb.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 90.Ma Y., Zhao J., Deng Z., Gao B., Xu C., Yan X., et al. Macrophage-biomimetic liposomes delivery of carbon dots nanozymes ameliorate ulcerative colitis by modulating inflammation pathways and remodeling the redox microenvironment. Chem Eng J. 2023;477 [Google Scholar]
- 91.Sun D., Lu Z.R. Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharm Res. 2023;40:27–46. doi: 10.1007/s11095-022-03460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim B., Hosn R.R., Remba T., Yun D., Li N., Abraham W., et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J Controll Release. 2023;353:241–253. doi: 10.1016/j.jconrel.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kafetzis K.N., Papalamprou N., McNulty E., Thong K.X., Sato Y., Mironov A., et al. The effect of cryoprotectants and storage conditions on the transfection efficiency, stability, and safety of lipid-based nanoparticles for mrna and dna delivery. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of lipid nanoparticles for rna delivery. Acc Chem Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 95.Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles-from liposomes to mrna vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M., Wang X., Han M.K., Collins J.F., Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sung J., Alghoul Z., Long D., Yang C., Merlin D. Oral delivery of IL-22 mRNA-loaded lipid nanoparticles targeting the injured intestinal mucosa: a novel therapeutic solution to treat ulcerative colitis. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121707. [DOI] [PubMed] [Google Scholar]
- 98.Dou G., Tian R., Liu X., Yuan P., Ye Q., Liu J., et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci Adv. 2020;6:eaba2987. doi: 10.1126/sciadv.aba2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen L., Zhang S., Duan Y., Song X., Chang M., Feng W., et al. Silicon-containing nanomedicine and biomaterials: materials chemistry, multi-dimensional design, and biomedical application. Chem Soc Rev. 2024;53:1167–1315. doi: 10.1039/d1cs01022k. [DOI] [PubMed] [Google Scholar]
- 100.Tang F., Li L., Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 101.Wu S.H., Mou C.Y., Lin H.P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42:3862–3875. doi: 10.1039/c3cs35405a. [DOI] [PubMed] [Google Scholar]
- 102.Zhang H., Xu H., Wu M., Zhong Y., Wang D., Jiao Z. A soft-hard template approach towards hollow mesoporous silica nanoparticles with rough surfaces for controlled drug delivery and protein adsorption. J Mater Chem B. 2015;3:6480–6489. doi: 10.1039/c5tb00634a. [DOI] [PubMed] [Google Scholar]
- 103.Min D.K., Kim Y.E., Kim M.K., Choi S.W., Park N., Kim J. Orally administrated inflamed colon-targeted nanotherapeutics for inflammatory bowel disease treatment by oxidative stress level modulation in colitis. ACS Nano. 2023;17:24404–24416. doi: 10.1021/acsnano.3c11089. [DOI] [PubMed] [Google Scholar]
- 104.Sabu C., Mufeedha P., Pramod K. Yeast-inspired drug delivery: biotechnology meets bioengineering and synthetic biology. Expert Opin Drug Deliv. 2019;16:27–41. doi: 10.1080/17425247.2019.1551874. [DOI] [PubMed] [Google Scholar]
- 105.Coradello G., Tirelli N. Yeast cells in microencapsulation. General features and controlling factors of the encapsulation process. Molecules. 2021;26:3123. doi: 10.3390/molecules26113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu M.X., Yang Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017;29 doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 107.Lawson H.D., Walton S.P., Chan C. Metal-organic frameworks for drug delivery: a design perspective. ACS Appl Mater Interfaces. 2021;13:7004–7020. doi: 10.1021/acsami.1c01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Begum S., Hassan Z., Bräse S., Wöll C., Tsotsalas M. Metal-organic framework-templated biomaterials: recent progress in synthesis, functionalization, and applications. Acc Chem Res. 2019;52:1598–1610. doi: 10.1021/acs.accounts.9b00039. [DOI] [PubMed] [Google Scholar]
- 109.Sheikhpour M., Barani L., Kasaeian A. Biomimetics in drug delivery systems: a critical review. J Control Release. 2017;253:97–109. doi: 10.1016/j.jconrel.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 110.Gupta D., Wiklander O.P.B., Görgens A., Conceição M., Corso G., Liang X., et al. Amelioration of systemic inflammation via the display of two different decoy protein receptors on extracellular vesicles. Nat Biomed Eng. 2021;5:1084–1098. doi: 10.1038/s41551-021-00792-z. [DOI] [PubMed] [Google Scholar]
- 111.Liu J., Ren H., Zhang C., Li J., Qiu Q., Zhang N., et al. Orally-delivered, cytokine-engineered extracellular vesicles for targeted treatment of inflammatory bowel disease. Small. 2023;19 doi: 10.1002/smll.202304023. [DOI] [PubMed] [Google Scholar]
- 112.Zhou J., Li M., Chen Q., Li X., Chen L., Dong Z., et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13:3432. doi: 10.1038/s41467-022-31171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liang D., Liu C., Li Y., Wu C., Chen Y., Tan M., et al. Engineering fucoxanthin-loaded probiotics' membrane vesicles for the dietary intervention of colitis. Biomaterials. 2023;297 doi: 10.1016/j.biomaterials.2023.122107. [DOI] [PubMed] [Google Scholar]
- 114.Sun T., Kwong C.H.T., Gao C., Wei J., Yue L., Zhang J., et al. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10:10106–10119. doi: 10.7150/thno.48448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barile L., Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Meng W., He C., Hao Y., Wang L., Li L., Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 118.Sharma P., Ludwig S., Muller L., Hong C.S., Kirkwood J.M., Ferrone S., et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7 doi: 10.1080/20013078.2018.1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grant R., Ansa-Addo E., Stratton D., Antwi-Baffour S., Jorfi S., Kholia S., et al. A filtration-based protocol to isolate human plasma membrane-derived vesicles and exosomes from blood plasma. J Immunol Methods. 2011;371:143–151. doi: 10.1016/j.jim.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 120.Sidhom K., Obi P.O., Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21:6466. doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang D., Jiang S., Zhang F., Ma S., Heng B.C., Wang Y., et al. Cell membrane vesicles with enriched cxcr4 display enhances their targeted delivery as drug carriers to inflammatory sites. Adv Sci. 2021;8 doi: 10.1002/advs.202101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao H., Duan L., Zhang Y., Cao J., Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6:426. doi: 10.1038/s41392-021-00830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu H., Luo R., Dong L., Pu X., Chen Q., Ye N., et al. pH/ROS dual-sensitive and chondroitin sulfate wrapped poly (β-amino ester)-SA-PAPE copolymer nanoparticles for macrophage-targeted oral therapy for ulcerative colitis. Nanomedicine. 2022;39 doi: 10.1016/j.nano.2021.102461. [DOI] [PubMed] [Google Scholar]
- 126.Huang H.B., Gong W., Hou Y.Y., He W.Y., Wang R., Wang X.C., et al. Mucoadhesive hydrogel with anti-gastric acid and sustained-release functions for amelioration of dss-induced ulcerative colitis. J Agric Food Chem. 2023;71:4016–4028. doi: 10.1021/acs.jafc.2c07777. [DOI] [PubMed] [Google Scholar]
- 127.Li J., Esteban-Fernández de Ávila B., Gao W., Zhang L., Wang J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Robot. 2017;2:eaam6431. doi: 10.1126/scirobotics.aam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu Y., Bian Q., Wang R., Gao J. Micro/nanorobots for precise drug delivery via targeted transport and triggered release: a review. Int J Pharm. 2022;616 doi: 10.1016/j.ijpharm.2022.121551. [DOI] [PubMed] [Google Scholar]
- 129.Zhang B., Pan H., Chen Z., Yin T., Zheng M., Cai L. Twin-bioengine self-adaptive micro/nanorobots using enzyme actuation and macrophage relay for gastrointestinal inflammation therapy. Sci Adv. 2023;9:eadc8978. doi: 10.1126/sciadv.adc8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hou Q., Huang J., Ayansola H., Masatoshi H., Zhang B. Intestinal stem cells and immune cell relationships: potential therapeutic targets for inflammatory bowel diseases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hirota A., AlMusawi S., Nateri A.S., Ordóñez-Morán P., Imajo M. Biomaterials for intestinal organoid technology and personalized disease modeling. Acta Biomater. 2021;132:272–287. doi: 10.1016/j.actbio.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Edgar L., Pu T., Porter B., Aziz J.M., La Pointe C., Asthana A., et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107:793–800. doi: 10.1002/bjs.11686. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe S., Kobayashi S., Ogasawara N., Okamoto R., Nakamura T., Watanabe M., et al. Transplantation of intestinal organoids into a mouse model of colitis. Nat Protoc. 2022;17:649–671. doi: 10.1038/s41596-021-00658-3. [DOI] [PubMed] [Google Scholar]
- 134.Charbe N.B., McCarron P.A., Lane M.E., Tambuwala M.M. Application of three-dimensional printing for colon targeted drug delivery systems. Int J Pharm Investig. 2017;7:47–59. doi: 10.4103/jphi.JPHI_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karakurt I., Aydoğdu A., Çıkrıkcı S., Orozco J., Lin L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: a controlled release study. Int J Pharm. 2020;584 doi: 10.1016/j.ijpharm.2020.119428. [DOI] [PubMed] [Google Scholar]
- 136.Parulski C., Jennotte O., Lechanteur A., Evrard B. Challenges of fused deposition modeling 3D printing in pharmaceutical applications: where are we now? Adv Drug Deliv Rev. 2021;175 doi: 10.1016/j.addr.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 137.Kocabas L.I., Ayyoubi S., Tajqurishi M., Quodbach J., Vermonden T., Kok R.J. 3D-printed prednisolone phosphate suppositories with tunable dose and rapid release for the treatment of inflammatory bowel disease. Int J Pharm. 2024;649 doi: 10.1016/j.ijpharm.2023.123639. [DOI] [PubMed] [Google Scholar]
- 138.Goh W.J., Tan S.X., Pastorin G., Ho P.C.L., Hu J., Lim S.H. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. Int J Pharm. 2021;598 doi: 10.1016/j.ijpharm.2021.120360. [DOI] [PubMed] [Google Scholar]
- 139.Ou Y.H., Goh W.J., Lim S.H. Form & formulation approaches for COntRollable release in 3D printed Colonic Targeting (CORR3CT) budesonide tablet. Int J Pharm. 2023;635 doi: 10.1016/j.ijpharm.2023.122680. [DOI] [PubMed] [Google Scholar]
- 140.Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kurilshikov A., Medina-Gomez C., Bacigalupe R., Radjabzadeh D., Wang J., Demirkan A., et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156–165. doi: 10.1038/s41588-020-00763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Engevik M.A., Luk B., Chang-Graham A.L., Hall A., Herrmann B., Ruan W., et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. MBio. 2019;10 doi: 10.1128/mBio.01087-19. e01087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.J., et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang K., Zhu L., Zhong Y., Xu L., Lang C., Chen J., et al. Prodrug integrated envelope on probiotics to enhance target therapy for ulcerative colitis. Adv Sci. 2023;10 doi: 10.1002/advs.202205422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gill P.A., Van Zelm M.C., Muir J.G., Gibson P.R. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- 146.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 148.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., et al. The microbial metabolites, short chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu H., Wang J., He T., Becker S., Zhang G., Li D., et al. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang R., Cao S., Bashir M.E.H., Hesser L.A., Su Y., Hong S.M.C., et al. Treatment of peanut allergy and colitis in mice via the intestinal release of butyrate from polymeric micelles. Nat Biomed Eng. 2023;7:38–55. doi: 10.1038/s41551-022-00972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pasetti M.F., Simon J.K., Sztein M.B., Levine M.M. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–148. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van der Weken H., Cox E., Devriendt B. Advances in oral subunit vaccine design. Vaccines. 2020;9:1. doi: 10.3390/vaccines9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kunisawa J., Kurashima Y., Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 154.Chandrasekaran R., Lacy D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol Rev. 2017;41:723–750. doi: 10.1093/femsre/fux048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hughes J., Aston C., Kelly M.L., Griffin R. Towards development of a non-toxigenic clostridioides difficile oral spore vaccine against toxigenic C. difficile. Pharmaceutics. 2022;14:1086. doi: 10.3390/pharmaceutics14051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakase H., Sato N., Mizuno N., Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022;21 doi: 10.1016/j.autrev.2021.103017. [DOI] [PubMed] [Google Scholar]
- 157.Meyer F., Wendling D., Demougeot C., Prati C., Verhoeven F. Cytokines and intestinal epithelial permeability: a systematic review. Autoimmun Rev. 2023;22 doi: 10.1016/j.autrev.2023.103331. [DOI] [PubMed] [Google Scholar]
- 158.Li J., Pu Y., Li S., He B., Chen J. Orally administrated olsalazine-loaded multilayer pectin/chitosan/alginate composite microspheres for ulcerative colitis treatment. Biomacromolecules. 2023;24:2250–2263. doi: 10.1021/acs.biomac.3c00146. [DOI] [PubMed] [Google Scholar]
- 159.Zhou M., Xu W., Wang J., Yan J., Shi Y., Zhang C., et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–360. doi: 10.1016/j.ebiom.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pope J.L., Bhat A.A., Sharma A., Ahmad R., Krishnan M., Washington M.K., et al. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–634. doi: 10.1136/gutjnl-2012-304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCole D.F., Rogler G., Varki N., Barrett K.E. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 162.Hageman J.H., Heinz M.C., Kretzschmar K., van der Vaart J., Clevers H., Snippert H.J.G. Intestinal regeneration: regulation by the microenvironment. Dev Cell. 2020;54:435–446. doi: 10.1016/j.devcel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 163.Lindemans C.A., Calafiore M., Mertelsmann A.M., O'Connor M.H., Dudakov J.A., Jenq R.R., et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Neal M.D., Sodhi C.P., Jia H., Dyer M., Egan C.E., Yazji I., et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287:37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jeon Y.D., Lee J.H., Lee Y.M., Kim D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109847. [DOI] [PubMed] [Google Scholar]
- 167.Bourgonje A.R., Feelisch M., Faber K.N., Pasch A., Dijkstra G., van Goor H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 168.Shadfar S., Parakh S., Jamali M.S., Atkin J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl Neurodegener. 2023;12:18. doi: 10.1186/s40035-023-00350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Russell L.A., Balart M.T., Serrano P., Armstrong D., Pinto-Sanchez M.I. The complexities of approaching nutrition in inflammatory bowel disease: current recommendations and future directions. Nutr Rev. 2022;80:215–229. doi: 10.1093/nutrit/nuab015. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y., Shui M., Yuan Q., Vong C.T., Yang Z., Chen Z., et al. Wielding the double-edged sword: redox drug delivery systems for inflammatory bowel disease. J Controll Release. 2023;358:510–540. doi: 10.1016/j.jconrel.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 171.Zhang C., Wang H., Yang X., Fu Z., Ji X., Shi Y., et al. Oral zero-valent-molybdenum nanodots for inflammatory bowel disease therapy. Sci Adv. 2022;8:eabp9882. doi: 10.1126/sciadv.abp9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li R., Fan Y., Liu L., Ma H., Gong D., Miao Z., et al. Ultrathin hafnium disulfide atomic crystals with ros-scavenging and colon-targeting capabilities for inflammatory bowel disease treatment. ACS Nano. 2022;16:15026–15041. doi: 10.1021/acsnano.2c06151. [DOI] [PubMed] [Google Scholar]
- 173.Samiei F., Shirazi F.H., Naserzadeh P., Dousti F., Seydi E., Pourahmad J. Toxicity of multi-wall carbon nanotubes inhalation on the brain of rats. Environ Sci Pollut Res Int. 2020;27:12096–12111. doi: 10.1007/s11356-020-07740-5. [DOI] [PubMed] [Google Scholar]
- 174.Lam C.-W., James J.T., McCluskey R., Arepalli S., Hunter R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 175.Gaté L., Knudsen K.B., Seidel C., Berthing T., Chézeau L., Jacobsen N.R., et al. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: comparison between intratracheal instillation and inhalation exposure. Toxicol Appl Pharmacol. 2019;375:17–31. doi: 10.1016/j.taap.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 176.Duke K.S., Bonner J.C. Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10:e1498. doi: 10.1002/wnan.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lu M., Yin J., Zhu Q., Lin G., Mou M., Liu F., et al. Artificial intelligence in pharmaceutical sciences. Engineering. 2023;27:37–69. [Google Scholar]
- 178.Mullowney M.W., Duncan K.R., Elsayed S.S., Garg N., van der Hooft J.J.J., Martin N.I., et al. Artificial intelligence for natural product drug discovery. Nat Rev Drug Discov. 2023;22:895–916. doi: 10.1038/s41573-023-00774-7. [DOI] [PubMed] [Google Scholar]
- 179.He S., Leanse L.G., Feng Y. Artificial intelligence and machine learning assisted drug delivery for effective treatment of infectious diseases. Adv Drug Deliv Rev. 2021;178 doi: 10.1016/j.addr.2021.113922. [DOI] [PubMed] [Google Scholar]
- 180.Staszak M., Staszak K., Wieszczycka K., Bajek A., Roszkowski K., Tylkowski B. Machine learning in drug design: use of artificial intelligence to explore the chemical structure–biological activity relationship. WIREs Comput Mol Sci. 2022;12:e1568. [Google Scholar]
- 181.Hsueh H.T., Chou R.T., Rai U., Liyanage W., Kim Y.C., Appell M.B., et al. Machine learning-driven multifunctional peptide engineering for sustained ocular drug delivery. Nat Commun. 2023;14:1–19. doi: 10.1038/s41467-023-38056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J Controll Release. 2023;361:53–63. doi: 10.1016/j.jconrel.2023.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tran T.T.V., Tayara H., Chong K.T. Artificial intelligence in drug metabolism and excretion prediction: recent advances, challenges, and future perspectives. Pharmaceutics. 2023;15:1260. doi: 10.3390/pharmaceutics15041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao X., Wei J., Song T., Wang Z., Yang D., Zhang X., et al. Computational insights into carbon dots: evolution of structural models and structure–activity relationships. Chem Eng J. 2024;481 [Google Scholar]
- 185.Yan X., Yue T., Winkler D.A., Yin Y., Zhu H., Jiang G., et al. Converting nanotoxicity data to information using artificial intelligence and simulation. Chem Rev. 2023;123:8575–8637. doi: 10.1021/acs.chemrev.3c00070. [DOI] [PubMed] [Google Scholar]
- 186.Zhang F., Wang Z., Peijnenburg W.J.G.M., Vijver M.G. Machine learning-driven QSAR models for predicting the mixture toxicity of nanoparticles. Environ Int. 2023;177 doi: 10.1016/j.envint.2023.108025. [DOI] [PubMed] [Google Scholar]
- 187.The significance of artificial intelligence in drug delivery system design. Adv Drug Deliv Rev. 2019;151-152:169–190. doi: 10.1016/j.addr.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 188.Huang Y., Guo X., Wu Y., Chen X., Feng L., Xie N., et al. Nanotechnology's frontier in combatting infectious and inflammatory diseases: prevention and treatment. Sig Transduct Target Ther. 2024;9:1–50. doi: 10.1038/s41392-024-01745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 190.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mrna-1273 sars-cov-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kon E., Levy Y., Elia U., Cohen H., Hazan-Halevy I., Aftalion M., et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci Adv. 2023;9:eadg1036. doi: 10.1126/sciadv.adg1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.De la Paz E, Maganti N.H., Trifonov A., Jeerapan I., Mahato K., Yin L., et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat Commun. 2022;13:7405. doi: 10.1038/s41467-022-35074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]