Abstract
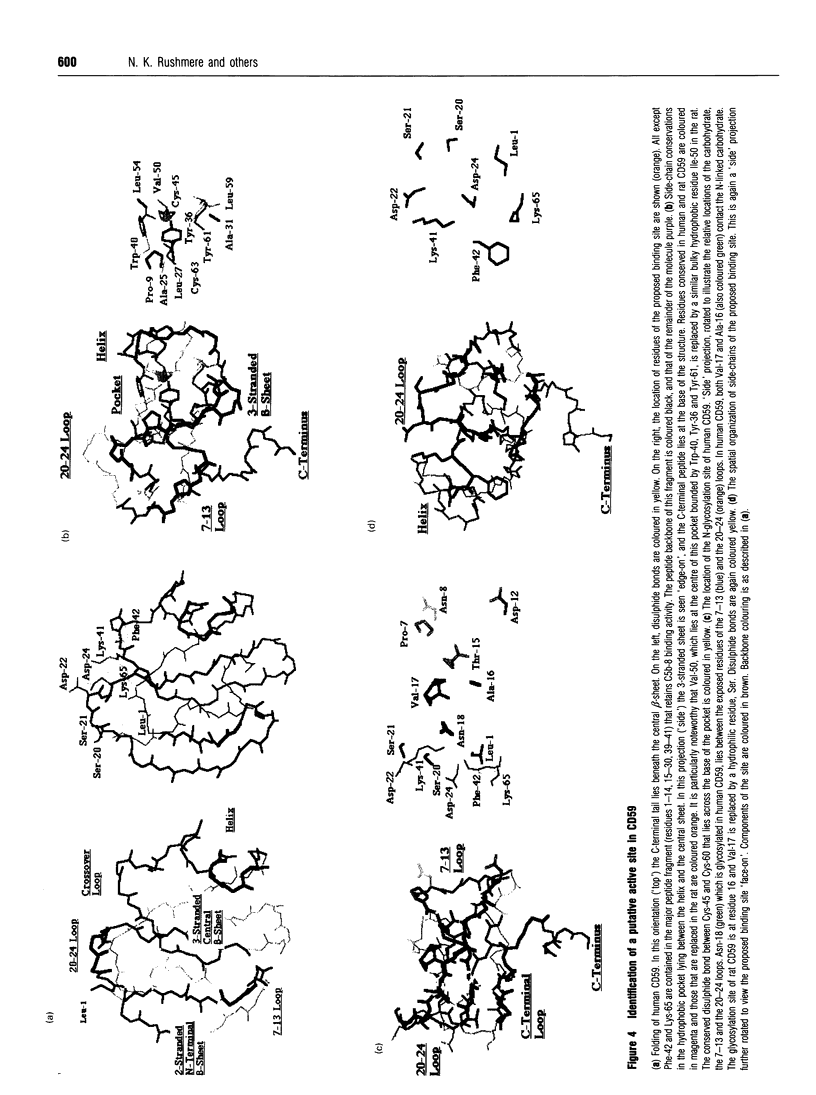
We have previously described the purification and partial characterization of the rat analogue of the human complement regulatory molecule CD59 [Hughes, Piddlesden, Williams, Harrison and Morgan (1992) Biochem. J. 284, 169-176]. We present here the molecular cloning and full sequence analysis of this molecule. A PCR-based approach utilizing primers designed from the amino-terminal protein sequence was used to isolate a full-length cDNA clone from a rat kidney cDNA library. This clone encoded a 92 bp 5'-flanking sequence, a 66 bp signal peptide and a 315 bp coding region containing putative glycosylation and GPI-anchor signals. The 3' untranslated flanking region was approximately 1.1 kbp long and included the poly-A tail and a CATA repeating sequence. The coding region was 58% identical with the human cDNA at the nucleotide level and 44% identical at the amino acid level. Despite this relatively low overall sequence conservation, several highly conserved stretches were apparent, particularly in the N-terminal portion of the molecule, in the cysteine-rich region immediately preceding the site of glycolipid attachment and in the C-terminal peptide removed during glycolipid attachment. An N-glycosylation site was identified at Asn-16 and a putative glycosylphosphatidylinositol anchor addition site at Asn-79, indicating that the mature processed protein was two residues longer than human CD59. Comparison of the sequences of rat and human CD59, together with consideration of the published three-dimensional structure of human CD59 and functional data, implicates specific regions of the protein in interactions with C-8 and/or C-9.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Nicholas J., Cameron K. R., Newman C., Fleckenstein B., Honess R. W. Herpesvirus saimiri has a gene specifying a homologue of the cellular membrane glycoprotein CD59. Virology. 1992 Sep;190(1):527–530. doi: 10.1016/0042-6822(92)91247-r. [DOI] [PubMed] [Google Scholar]
- Bickmore W. A., Longbottom D., Oghene K., Fletcher J. M., van Heyningen V. Colocalization of the human CD59 gene to 11p13 with the MIC11 cell surface antigen. Genomics. 1993 Jul;17(1):129–135. doi: 10.1006/geno.1993.1293. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. J., O'hUigin C., Malek T. R. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J Immunol. 1993 Jun 15;150(12):5379–5390. [PubMed] [Google Scholar]
- Fletcher C. M., Harrison R. A., Lachmann P. J., Neuhaus D. Sequence-specific 1H-NMR assignments and folding topology of human CD59. Protein Sci. 1993 Dec;2(12):2015–2027. doi: 10.1002/pro.5560021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C. M., Harrison R. A., Lachmann P. J., Neuhaus D. Structure of a soluble, glycosylated form of the human complement regulatory protein CD59. Structure. 1994 Mar 15;2(3):185–199. doi: 10.1016/s0969-2126(00)00020-4. [DOI] [PubMed] [Google Scholar]
- Forsberg U. H., Bazil V., Stefanová I., Schröder J. Gene for human CD59 (likely Ly-6 homologue) is located on the short arm of chromosome 11. Immunogenetics. 1989;30(3):188–193. doi: 10.1007/BF02421205. [DOI] [PubMed] [Google Scholar]
- Funabashi K., Okada N., Matsuo S., Yamamoto T., Morgan B. P., Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 1994 Mar;81(3):444–451. [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber L. D., Kodukula K., Udenfriend S. Phosphatidylinositol glycan (PI-G) anchored membrane proteins. Amino acid requirements adjacent to the site of cleavage and PI-G attachment in the COOH-terminal signal peptide. J Biol Chem. 1992 Jun 15;267(17):12168–12173. [PubMed] [Google Scholar]
- Göbel U., Maas R., Clad A. Quantitative electroelution of oligonucleotides and large DNA fragments from gels and purification by electrodialysis. J Biochem Biophys Methods. 1987 Aug;14(5):245–260. doi: 10.1016/0165-022x(87)90050-9. [DOI] [PubMed] [Google Scholar]
- Holguin M. H., Fredrick L. R., Bernshaw N. J., Wilcox L. A., Parker C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Jul;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. R., Meri S., Davies M., Williams J. D., Morgan B. P. Immunolocalization and characterization of the rat analogue of human CD59 in kidney and glomerular cells. Immunology. 1993 Nov;80(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Hughes T. R., Piddlesden S. J., Williams J. D., Harrison R. A., Morgan B. P. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992 May 15;284(Pt 1):169–176. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N., Singer L., Wetsel R. A., Colten H. R. Cis- and trans-acting elements required for constitutive and cytokine-regulated expression of the mouse complement C3 gene. Biochem J. 1992 May 1;283(Pt 3):705–712. doi: 10.1042/bj2830705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. D., Lindwall G., Maher S. E., Bothwell A. L. Characterization of promoter elements of an interferon-inducible Ly-6E/A differentiation antigen, which is expressed on activated T cells and hematopoietic stem cells. Mol Cell Biol. 1990 Oct;10(10):5150–5159. doi: 10.1128/mcb.10.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Hanioka N., Matsunaga E., Gonzalez F. J. The rat clofibrate-inducible CYP4A gene subfamily. I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA. 1989 Sep;8(7):503–516. doi: 10.1089/dna.1.1989.8.503. [DOI] [PubMed] [Google Scholar]
- Matsuo S., Nishikage H., Yoshida F., Nomura A., Piddlesden S. J., Morgan B. P. Role of CD59 in experimental glomerulonephritis in rats. Kidney Int. 1994 Jul;46(1):191–200. doi: 10.1038/ki.1994.259. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15(4):369–396. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya H., Stewart B. H., Rollins S. A., Zhao J., Bothwell A. L., Sims P. J. Contribution of the N-linked carbohydrate of erythrocyte antigen CD59 to its complement-inhibitory activity. J Biol Chem. 1992 Apr 25;267(12):8404–8410. [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Piddlesden S. J., Morgan B. P. Killing of rat glial cells by complement: deficiency of the rat analogue of CD59 is the cause of oligodendrocyte susceptibility to lysis. J Neuroimmunol. 1993 Nov-Dec;48(2):169–175. doi: 10.1016/0165-5728(93)90189-6. [DOI] [PubMed] [Google Scholar]
- Pierce R. A., Alatawi A., Deak S. B., Boyd C. D. Elements of the rat tropoelastin gene associated with alternative splicing. Genomics. 1992 Apr;12(4):651–658. doi: 10.1016/0888-7543(92)90289-5. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Sawada R., Ohashi K., Anaguchi H., Okazaki H., Hattori M., Minato N., Naruto M. Isolation and expression of the full-length cDNA encoding CD59 antigen of human lymphocytes. DNA Cell Biol. 1990 Apr;9(3):213–220. doi: 10.1089/dna.1990.9.213. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Oda E., Noda K., Tobe T., Miura N. H., Tomita M. Determination of carboxyl-terminal residue and disulfide bonds of MACIF (CD59), a glycosyl-phosphatidylinositol-anchored membrane protein. J Biochem. 1993 Oct;114(4):473–477. doi: 10.1093/oxfordjournals.jbchem.a124202. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Ohshima Y. Three types of rat U1 small nuclear RNA genes with different flanking sequences are induced to express in vivo. Eur J Biochem. 1988 May 16;174(1):125–132. doi: 10.1111/j.1432-1033.1988.tb14071.x. [DOI] [PubMed] [Google Scholar]
- van den Berg C. W., Harrison R. A., Morgan B. P. The sheep analogue of human CD59: purification and characterization of its complement inhibitory activity. Immunology. 1993 Mar;78(3):349–357. [PMC free article] [PubMed] [Google Scholar]
- van den Berg C. W., Morgan B. P. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994 Apr 15;152(8):4095–4101. [PubMed] [Google Scholar]
- van den Berg C. W., Williams O. M., Morgan B. P. Presence of a dysfunctional form of CD59 on a CD59+ subclone of the U937 cell line. Immunology. 1994 Apr;81(4):637–642. [PMC free article] [PubMed] [Google Scholar]