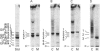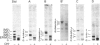Abstract
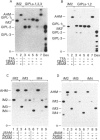
Glycoinositol-phospholipids (GIPLs) are the major glycolipid class and prominant surface antigens of leishmanial parasites. The GIPLs from four serologically distinct Old World strains of Leishmania were characterized to determine inter- and intra-specific differences in these glycolipids. These studies showed that: (1) the major GIPLs of Leishmania topica (LRC-L36) and Leishmania aethiopica (LRC-L495) belong to the alpha-mannose-terminating GIPL series (iM2, iM3 and iM4) that are structurally related to the glycosyl-phosphatidylinositol anchors of both the surface proteins and the abundant lipophosphoglycan (LPG). In contrast, the GIPLs from two Leishmania major strains (LRC-L456 and LRC-L580) belong to the alpha-galactose-terminating GIPL series (GIPL-1, -2 and -3) that are more structurally related to the LPG anchor; (2) the GIPL profiles of the L. major strains differed in that a significant proportion of the GIPL-2 and -3 species (approximately 40% and 80%, respectively) in LRC-L580 are substituted with a glucose-1-PO4 residue, while this type of substitution was not detected in LRC-L456; and (3) all the GIPLs contained either an alkylacyl- or a lysoalkyl-phosphatidylinositol lipid moiety. However, the alkyl chain compositions of different GIPLs within the same strain was variable. In L. major, the major GIPL species contained alkylacylglycerols with predominantly C18:0 and C24:0 alkyl chains, whereas the glucose-1-PO4-substituted GIPLs contained exclusively lysoalkylglycerols with C24:0 alkyl chains. In L. tropica, the major GIPL, iM2, contained predominantly C24:0 alkyl chains whereas the structurally related iM3 and iM4 GIPLs in this strain contained predominantly C18:0 alkyl chains. In L. aethiopica all the GIPLs (iM2, iM3, iM4) contained C18:0 alkyl chains. These data suggest that the synthesis of the GIPLs may occur in more than one subcellular compartment. The possibility that species-specific differences in the predominantly surface glycan structures may modulate the interaction of the parasite with the insect and mammalian hosts is discussed.
Full text
PDF



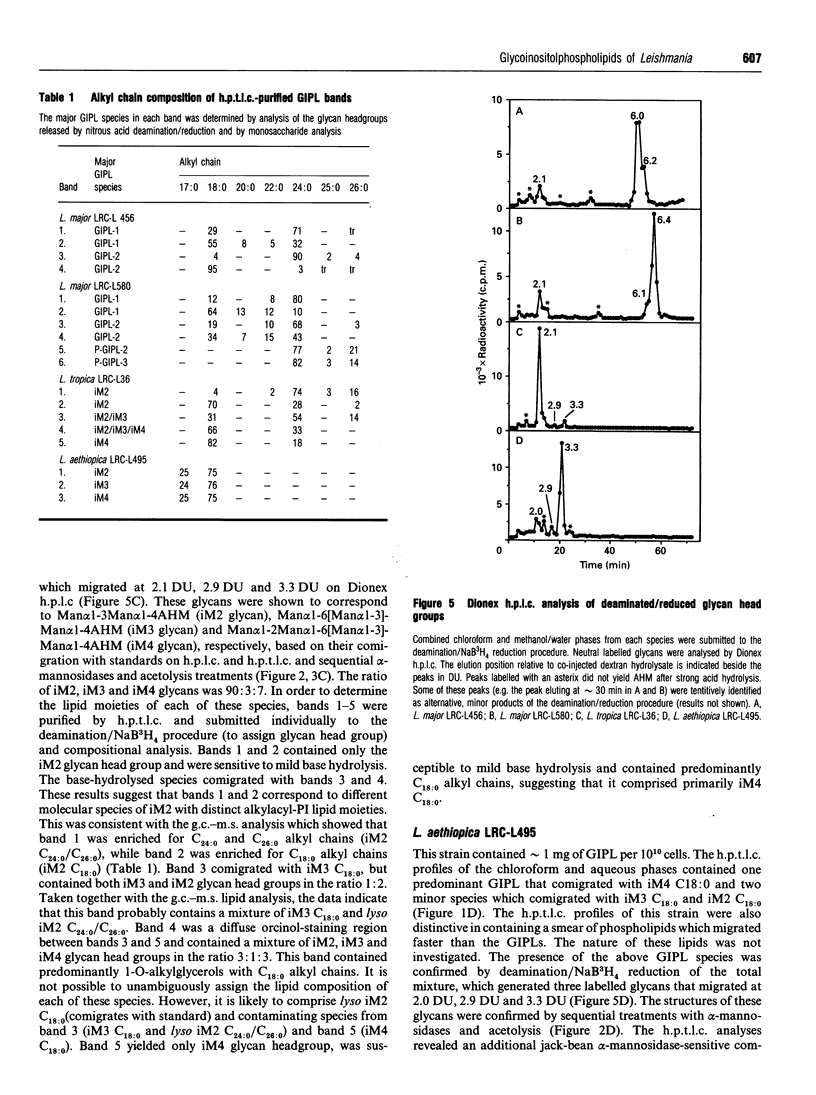


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford R. W., Desjeux P., Deraadt P. Estimation of population at risk of infection and number of cases of Leishmaniasis. Parasitol Today. 1992 Mar;8(3):104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Bahr V., Stierhof Y. D., Ilg T., Demar M., Quinten M., Overath P. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993 Mar;58(1):107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi F., Solfanelli S., Binaghi R. A. Trichinella spiralis: modifications of the cuticle of the newborn larva during passage through the lung. Exp Parasitol. 1992 Aug;75(1):1–9. doi: 10.1016/0014-4894(92)90116-r. [DOI] [PubMed] [Google Scholar]
- Green P. J., Feizi T., Stoll M. S., Thiel S., Prescott A., McConville M. J. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol Biochem Parasitol. 1994 Aug;66(2):319–328. doi: 10.1016/0166-6851(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Ilg T., Harbecke D., Wiese M., Overath P. Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur J Biochem. 1993 Oct 15;217(2):603–615. doi: 10.1111/j.1432-1033.1993.tb18283.x. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., Sarfstein R. Species-specific antibodies to Leishmania tropica (minor) recognize somatic antigens and exometabolites. J Immunol. 1987 Aug 15;139(4):1310–1319. [PubMed] [Google Scholar]
- Masterson W. J., Ferguson M. A. Phenylmethanesulphonyl fluoride inhibits GPI anchor biosynthesis in the African trypanosome. EMBO J. 1991 Aug;10(8):2041–2045. doi: 10.1002/j.1460-2075.1991.tb07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A. The glycoinositolphospholipid profiles of two Leishmania major strains that differ in lipophosphoglycan expression. Mol Biochem Parasitol. 1990 Jan 1;38(1):57–67. doi: 10.1016/0166-6851(90)90205-z. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- McConville M. J., Collidge T. A., Ferguson M. A., Schneider P. The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J Biol Chem. 1993 Jul 25;268(21):15595–15604. [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Homans S. W. Identification of the defect in lipophosphoglycan biosynthesis in a non-pathogenic strain of Leishmania major. J Biol Chem. 1992 Mar 25;267(9):5855–5861. [PubMed] [Google Scholar]
- McConville M. J., Homans S. W., Thomas-Oates J. E., Dell A., Bacic A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem. 1990 May 5;265(13):7385–7394. [PubMed] [Google Scholar]
- McConville M. J., Turco S. J., Ferguson M. A., Sacks D. L. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J. 1992 Oct;11(10):3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Acosta E., Beverley S. M., Russell D. G. Evolution and expression of the Leishmania surface proteinase (gp63) gene locus. Infect Agents Dis. 1993 Feb;2(1):25–34. [PubMed] [Google Scholar]
- Mensa-Wilmot K., LeBowitz J. H., Chang K. P., al-Qahtani A., McGwire B. S., Tucker S., Morris J. C. A glycosylphosphatidylinositol (GPI)-negative phenotype produced in Leishmania major by GPI phospholipase C from Trypanosoma brucei: topography of two GPI pathways. J Cell Biol. 1994 Mar;124(6):935–947. doi: 10.1083/jcb.124.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta P. F., Saraiva E. M., Sacks D. L. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp Parasitol. 1991 Feb;72(2):191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- Pimenta P. F., Turco S. J., McConville M. J., Lawyer P. G., Perkins P. V., Sacks D. L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992 Jun 26;256(5065):1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- Rao P., Pattabiraman T. N. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem. 1989 Aug 15;181(1):18–22. doi: 10.1016/0003-2697(89)90387-4. [DOI] [PubMed] [Google Scholar]
- Schlein Y., Schnur L. F., Jacobson R. L. Released glycoconjugate of indigenous Leishmania major enhances survival of a foreign L. major in Phlebotomus papatasi. Trans R Soc Trop Med Hyg. 1990 May-Jun;84(3):353–355. doi: 10.1016/0035-9203(90)90315-6. [DOI] [PubMed] [Google Scholar]
- Schneider P., Rosat J. P., Ransijn A., Ferguson M. A., McConville M. J. Characterization of glycoinositol phospholipids in the amastigote stage of the protozoan parasite Leishmania major. Biochem J. 1993 Oct 15;295(Pt 2):555–564. doi: 10.1042/bj2950555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur L. F., Sarfstein R., Jaffe C. L. Monoclonal antibodies against leishmanial membranes react with specific excreted factors (EF). Ann Trop Med Parasitol. 1990 Oct;84(5):447–456. doi: 10.1080/00034983.1990.11812494. [DOI] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Sevlever D., Påhlsson P., Rosen G., Nilsson B., Londner M. V. Structural analysis of a glycosylphosphatidylinositol glycolipid of Leishmania donovani. Glycoconj J. 1991 Aug;8(4):321–329. doi: 10.1007/BF00731344. [DOI] [PubMed] [Google Scholar]
- Straus A. H., Levery S. B., Jasiulionis M. G., Salyan M. E., Steele S. J., Travassos L. R., Hakomori S., Takahashi H. K. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993 Jun 25;268(18):13723–13730. [PubMed] [Google Scholar]
- Thomas J. R., McConville M. J., Thomas-Oates J. E., Homans S. W., Ferguson M. A., Gorin P. A., Greis K. D., Turco S. J. Refined structure of the lipophosphoglycan of Leishmania donovani. J Biol Chem. 1992 Apr 5;267(10):6829–6833. [PubMed] [Google Scholar]
- Turco S. J., Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986 Jun 15;136(12):4681–4688. [PubMed] [Google Scholar]