Abstract
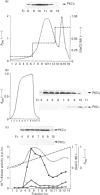
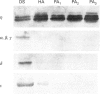
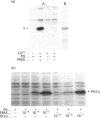
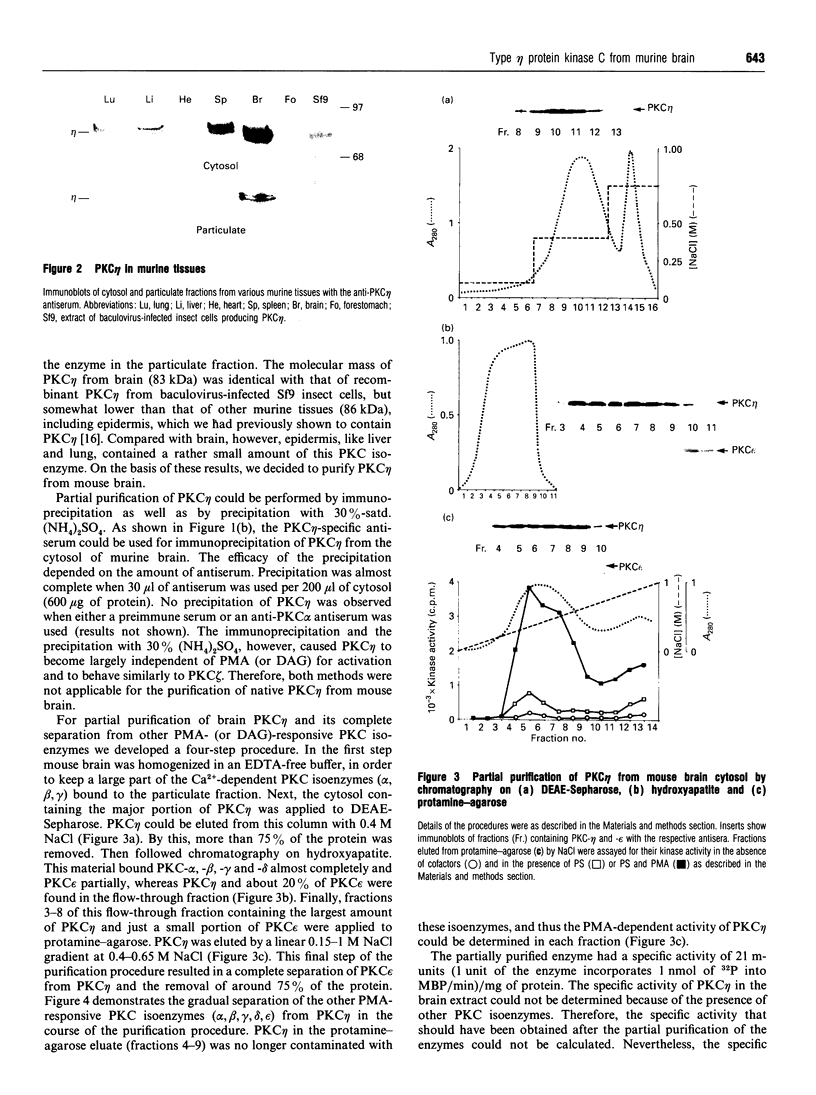
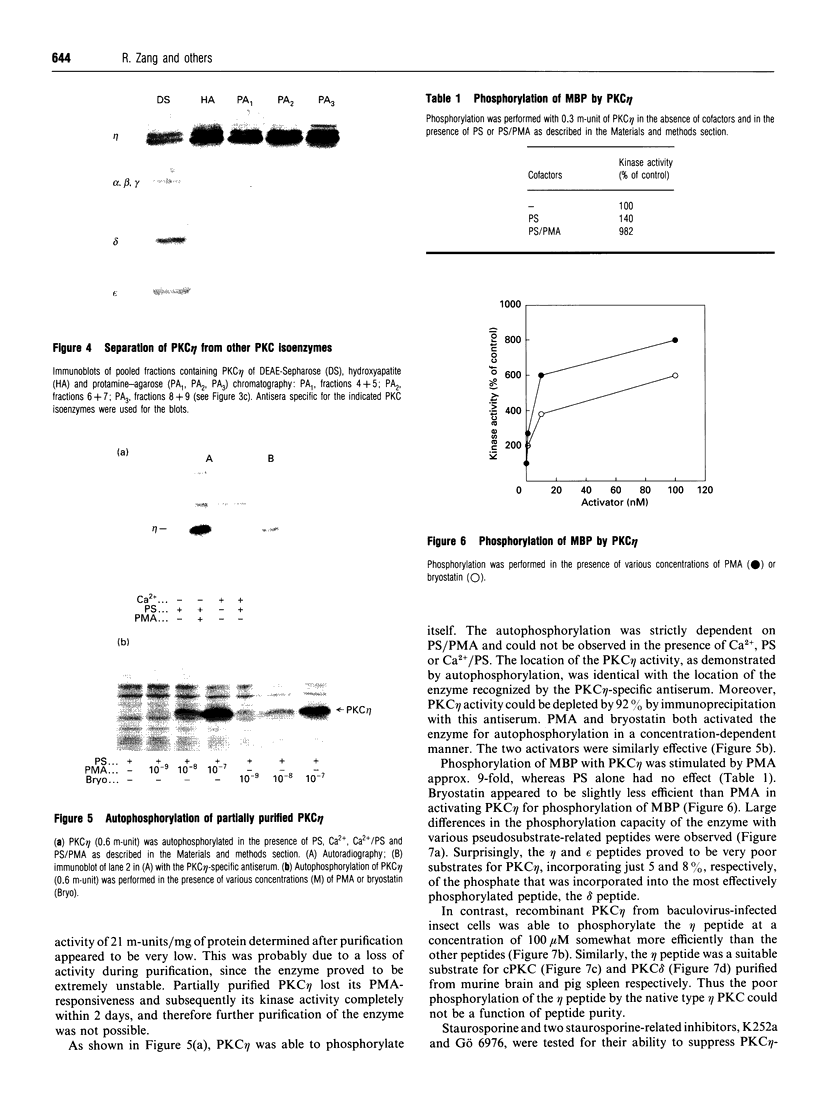
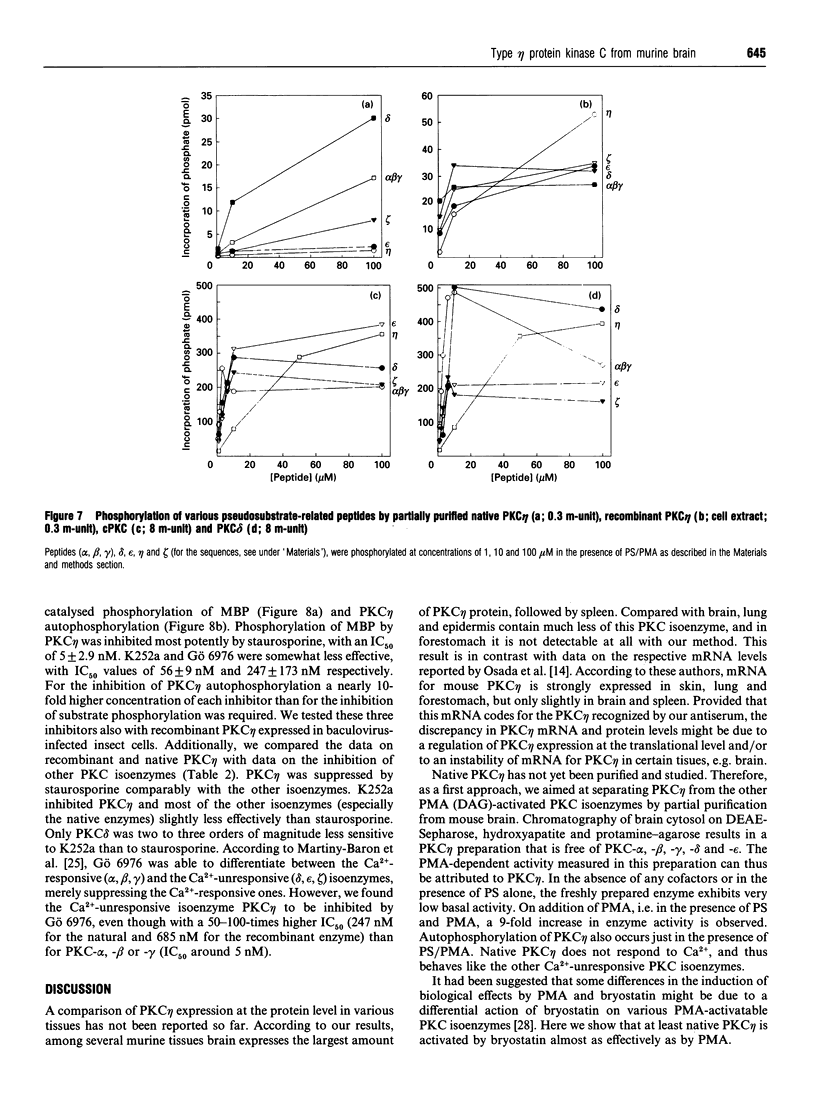
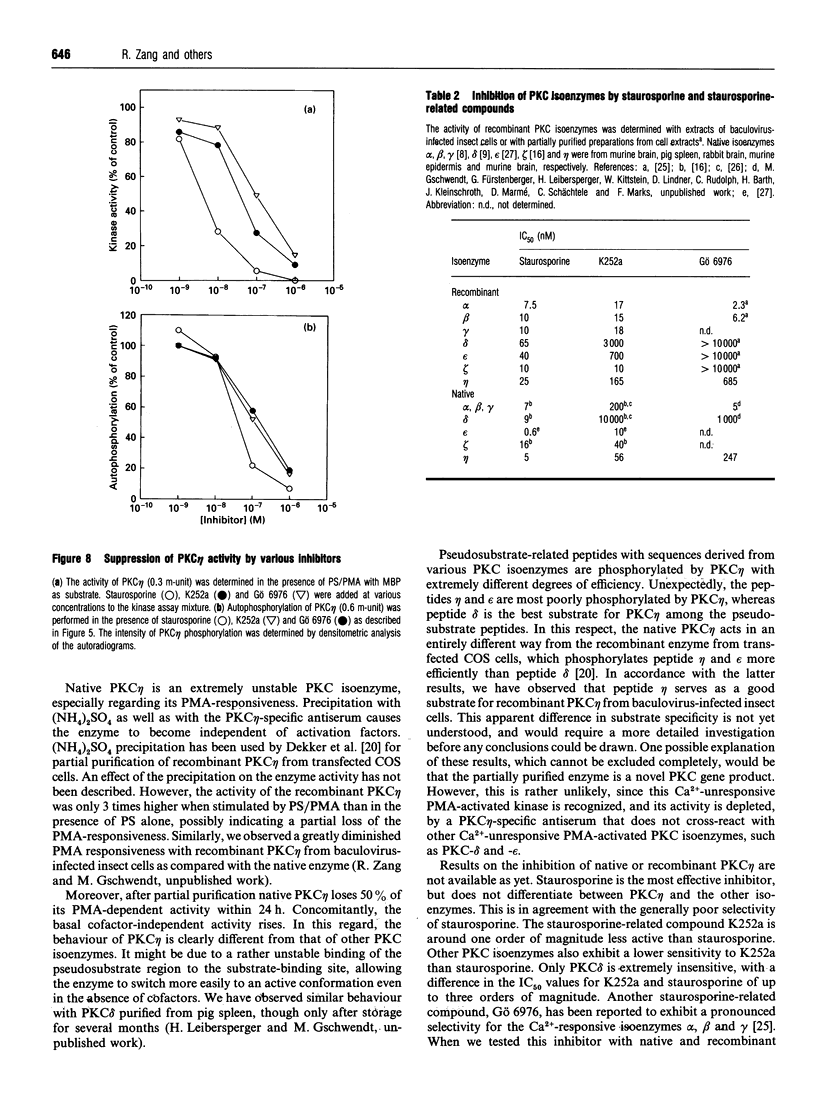
Various murine tissues were tested, by using a protein kinase C-eta-specific antiserum, for the expression of type eta protein kinase C. Brain was found to be the richest source of a type eta isoenzyme. Native protein kinase C-eta was partially purified from the cytosol of murine brain by chromatography on DEAE-Sepharose, hydroxyapatite and protamine-agarose. This procedure resulted in a separation of protein kinase C-eta from the other phorbol 12-myristate 13-acetate (PMA)-responsive isoenzymes (alpha, beta, gamma, delta, epsilon) and allowed, for the first time, characterization of the native enzyme. The protein kinase C of type eta from mouse brain is a phospholipid-dependent Ca(2+)-unresponsive protein kinase. Both PMA and bryostatin activate the kinase for phosphorylation of a substrate as well as for autophosphorylation. Various pseudo-substrate-related peptides are suitable as substrates for the eta-type kinase, peptide delta being the best and peptides eta and epsilon the poorest substrates. The enzyme is inhibited by staurosporine and staurosporine-related compounds, such as K252a and Gö 6976. However, protein kinase C-eta, like protein kinase C-delta, is around two orders of magnitude less sensitive towards Gö 6976 than are the Ca(2+)-responsive isoenzymes (alpha, beta, gamma). The eta-type protein kinase C exhibits an extreme tendency to lose its PMA-responsiveness. Consequently, purification of the enzyme to homogeneity has not yet been successful.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993 Sep;59(3):257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., McIntyre P., Parker P. J. Mutagenesis of the regulatory domain of rat protein kinase C-eta. A molecular basis for restricted histone kinase activity. J Biol Chem. 1993 Sep 15;268(26):19498–19504. [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J., McIntyre P. Biochemical properties of rat protein kinase C-eta expressed in COS cells. FEBS Lett. 1992 Nov 9;312(2-3):195–199. doi: 10.1016/0014-5793(92)80934-9. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W., Horn F., Leibersperger H., Marks F. A phorbol ester and phospholipid-activated, calcium-unresponsive protein kinase in mouse epidermis: characterization and separation from protein kinase C. J Cell Biochem. 1989 Jul;40(3):295–307. doi: 10.1002/jcb.240400306. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W., Lindner D., Marks F. Differential inhibition by staurosporine of phorbol ester, bryostatin and okadaic acid effects on mouse skin. Cancer Lett. 1992 Sep 30;66(2):139–146. doi: 10.1016/0304-3835(92)90226-l. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Leibersperger H., Kittstein W., Marks F. Protein kinase C zeta and eta in murine epidermis. TPA induces down-regulation of PKC eta but not PKC zeta. FEBS Lett. 1992 Jul 28;307(2):151–155. doi: 10.1016/0014-5793(92)80756-7. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Leibersperger H., Marks F. Differentiative action of K252a on protein kinase C and a calcium-unresponsive, phorbol ester/phospholipid-activated protein kinase. Biochem Biophys Res Commun. 1989 Nov 15;164(3):974–982. doi: 10.1016/0006-291x(89)91765-8. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Leibersperger H., Rincke G., Marks F. Immunological demonstration of epsilon PKC. Murine tissue distribution, ontogeny, cellular localization and translocation. FEBS Lett. 1991 Sep 23;290(1-2):115–118. doi: 10.1016/0014-5793(91)81239-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Blumberg P. M., Pettit G. R., Herald C. L., Shores R., Yuspa S. H. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987 Sep;8(9):1343–1346. doi: 10.1093/carcin/8.9.1343. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Koide H., Ogita K., Kikkawa U., Nishizuka Y. Isolation and characterization of the epsilon subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1149–1153. doi: 10.1073/pnas.89.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H., Kohno Y., Osada S., Ohno S., Ohkawara A., Kuroki T. Differentiation-associated localization of nPKC eta, a Ca(++)-independent protein kinase C, in normal human skin and skin diseases. J Invest Dermatol. 1993 Dec;101(6):858–863. doi: 10.1111/1523-1747.ep12371707. [DOI] [PubMed] [Google Scholar]
- Leibersperger H., Gschwendt M., Marks F. Purification and characterization of a calcium-unresponsive, phorbol ester/phospholipid-activated protein kinase from porcine spleen. J Biol Chem. 1990 Sep 25;265(27):16108–16115. [PubMed] [Google Scholar]
- Liyanage M., Frith D., Livneh E., Stabel S. Protein kinase C group B members PKC-delta, -epsilon, -zeta and PKC-L(eta). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992 May 1;283(Pt 3):781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- Mizuno K., Saido T. C., Ohno S., Tamaoki T., Suzuki K. Staurosporine-related compounds, K252a and UCN-01, inhibit both cPKC and nPKC. FEBS Lett. 1993 Sep 13;330(2):114–116. doi: 10.1016/0014-5793(93)80254-r. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ogita K., Miyamoto S., Yamaguchi K., Koide H., Fujisawa N., Kikkawa U., Sahara S., Fukami Y., Nishizuka Y. Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1592–1596. doi: 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S., Hashimoto Y., Nomura S., Kohno Y., Chida K., Tajima O., Kubo K., Akimoto K., Koizumi H., Kitamura Y. Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993 Mar;4(3):167–175. [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990 Dec 25;265(36):22434–22440. [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T. C., Mizuno K., Konno Y., Osada S., Ohno S., Suzuki K. Purification and characterization of protein kinase C epsilon from rabbit brain. Biochemistry. 1992 Jan 21;31(2):482–490. doi: 10.1021/bi00117a026. [DOI] [PubMed] [Google Scholar]
- Stabel S., Schaap D., Parker P. J. Expression of protein kinase C isotypes using baculovirus vectors. Methods Enzymol. 1991;200:670–673. doi: 10.1016/0076-6879(91)00179-z. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]