Abstract
Background
Honey improves probiotic survival in vitro. However, if this effect translates to humans has not been investigated.
Objectives
We aimed to determine effects of honey plus yogurt containing the probiotic Bifidobacterium animalis subsp. lactis DN-173 010/CNCM I-2494 (B. animalis) on intestinal transit time, probiotic enrichment, digestive health, mood, and cognition in adults.
Methods
Sixty-six healthy adults (34 female; 33.6 ± 9.8 y; 24.6 ± 3.0 kg/m2) in a crossover trial were randomly assigned to 2-wk yogurt conditions in a counterbalanced order with ≥4-wk washout: 1) Honey (HON): yogurt plus honey and 2) Negative Control (NC): heat-treated yogurt plus sugar. Of the participants, n = 62 completed the trial, and n = 37 (17 female; 32.0 ± 8.3 y; 25.0 ± 2.9 kg/m2) elected to enroll in a third condition (a nonrandomized study extension) after ≥4-wk washout with a reference Positive Control (PC): yogurt plus sugar. At baseline and end of each of the 3 conditions, intestinal transit time was measured with dye capsules; probiotic abundance with fecal DNA 16S sequencing; digestive health with symptom/function records, Bristol stool consistency, Gastrointestinal Tolerability, and Gastrointestinal Quality of Life Index; mood with Positive and Negative Affect Schedule-Short Form, Depression Anxiety Stress Scales-42, Patient-Reported Outcomes Measurement Information System questionnaires, and an emotional image task; and cognition with a spatial reconstruction task. Data were analyzed using linear mixed-effects models (LMMs) with significance at P ≤ 0.05. Baseline and end data were included in the LMM, with fixed effects being treatment, time, treatment by time interaction, and baseline covariate, and the random effect being the participant.
Results
B. animalis was enriched in HON (d = 3.54; P = 0.0002) compared to controls with linear discriminant analysis effect size. Intestinal transit time, gastrointestinal health, mood, and cognition did not differ between conditions (LMM: Ps > 0.05).
Conclusions
Yogurt + honey enriched B. animalis but did not reduce intestinal transit time or have other functional gastrointestinal, mood, or cognitive effects in adults.
This trial was registered at www.clinicaltrials.gov as NCT04187950 and NCT04901390.
Keywords: regularity, mood, cognition, anxiety, depression, stress
Introduction
Many people report unsatisfactory bowel function. There is a 10% to 25% prevalence of irritable bowel syndrome (IBS), including altered bowel habits and abdominal pain, in the United States [1]. Moreover, constipation is a concern in older adults, with a global prevalence of 19% [2]. Therefore, dietary approaches are needed to address undesirable bowel function.
Consumption of the probiotic Bifidobacterium animalis subsp. lactis DN-173 010/CNCM I-2494 (B. animalis) has been shown to reduce intestinal transit time and improve gastrointestinal symptoms in adults aged 18 to 75 y [[3], [4], [5], [6], [7]]. These studies provided 125 to 375 g (1 serving = 125 g) daily of a commercial dairy product (BIO, Danone) fermented by yogurt cultures and containing 5 × 107 to 2.6 × 108 colony forming units (CFU)/g of B. animalis. One to 3 fermented milk servings daily reduced intestinal transit times in the studies. Furthermore, in a meta-analysis of 11 clinical trials, probiotics were related to decreased intestinal transit time compared to controls; this reduction was predicted by constipation, increased age, and being female, with B. animalis and another Bifidobacterium strain exhibiting medium to large treatment effects [8].
Probiotics can affect cognitive function and mood. In healthy females, consumption of fermented milk with B. animalis influenced brain regions that regulate emotion and sensation processing [9]. Consumption of another fermented milk product improved relational memory in healthy adults [10]. In a cross-sectional analysis of healthy adults, higher dairy intake was related to lower participant stress and greater fecal Bifidobacterium spp., while greater Lactobacillus spp. abundance was related to superior participant mood states [11]. Also, a systematic review and meta-analysis reported that probiotics improved depression and anxiety in humans [12].
Honey has been shown to improve probiotic microorganism survival in vitro [[13], [14], [15], [16]]. We reported that B. animalis survival was superior in yogurt with added clover honey compared to other honey varietals and controls after the intestinal phase of in vitro digestion [17].
As yogurt with honey is a common food pairing, and honey supports probiotic survival in vitro, we aimed to determine if this functional food combination would enhance probiotic abundance and improve functional outcomes in vivo. Our primary aim was to determine the effects of consuming yogurt plus clover honey on intestinal transit time; secondary aims included assessing probiotic abundance, digestive health, mood states, and cognitive function in healthy adults. We hypothesized that yogurt plus honey would enhance probiotic abundance, thereby reducing intestinal transit time and improving digestive health measures, mood, and cognition.
Methods
Participants
Healthy participants were recruited from central Illinois from June 2021 to August 2022 through e-mail, print, and word-of-mouth advertising. Inclusion criteria included participants 1) be 22 to 64 y; 2) have BMI 18.5 to 29.9 kg/m2; 3) have normal or corrected-to-normal vision; 4) be able to drop off fecal samples ≤30 min of the bowel movement; and 5) have 3 to 6 bowel movements per week.
Exclusion criteria included 1) current pregnancy, lactation, or postmenopausal; 2) tobacco use; 3) honey allergy or intolerance; 4) dairy allergy or lactose intolerance; 5) food dye allergy/intolerance; 6) prior physician-diagnosed gastrointestinal disease (chronic constipation, diarrhea, Crohn’s disease, celiac disease, ulcerative colitis, IBS, diverticulosis, stomach or duodenal ulcers, hepatitis, gastroesophageal reflux disease); 7) current use or use of antibiotics in the past 3 mo; 8) current use of any of the following medications: laxatives, antidiarrhea medications, narcotics, enemas, antispasmodics, anticonvulsants, prescription proton pump inhibitors, prokinetic agents, histamine-2 Rc antagonists; 9) BMI >29.9 kg/m2; 10) prior malabsorptive bariatric surgery (i.e., gastric bypass, sleeve gastrectomy) or restrictive bariatric surgery (i.e., adjustable gastric band) within the past 5 y; and 11) concurrent enrollment in another dietary, exercise, or medication study.
All participants provided written informed consent before study initiation. Study procedures were administered in accordance with the Declaration of Helsinki and were approved by the University of Illinois Institutional Review Board.
Experimental design and treatments
The study had a randomized, controlled, single-blind, crossover design with 2 2-wk intervention periods separated by a ≥4-wk washout period (NCT04187950). Beginning during a 2-wk lead-in period and throughout the study, including washout periods, participants were instructed to refrain from consuming all supplemental and dietary probiotics, fermented dairy products, and fermented foods.
Yogurt beverages were consumed twice daily during each intervention period: 1) Negative Control (NC): 170 g heat-treated yogurt plus 15 g sugar; and 2) Honey (HON): 170 g yogurt plus 21 g clover honey. Clover honey (National Honey Board), from a single batch produced and packaged in 454-g (1-lb) containers for retail sale in North America, was shipped directly from the supplier to our laboratory and simultaneously to the analytic laboratory for composition testing. Within 24 h of receipt, samples were stored at −20°C in airtight 454-g containers. Product remained frozen until prepared for use within 10 d of removal from the freezer. General industry practices were used by the producer to yield honey free of foreign organic matter (heated to <85°C, filtered to 16 microns, and cooled to 51°C for packaging). Honey was tested by the producer to ensure authenticity. Based on information from the supplier, the clover honey was primarily from North and South Dakota during 2020. Additional honey information and characteristics are displayed in Supplemental Table 1.
The base product was a low-fat vanilla yogurt with B. animalis labeled to contain billions of live and active cultures (Activia, Danone). Although the specific probiotic amount was proprietary, refrigerated live and active culture yogurt must have a total viable count at the time of manufacture of ≥108 CFU/g according to International Dairy Foods Association criteria [18]. An in vitro digestion experiment in our laboratory confirmed that the yogurt had ≥108 CFU/g of B. animalis [17]. The NC and HON yogurt beverages were isocaloric and of similar consistency. Nutritional information is reported in Supplemental Table 2. Although HON developed a natural beverage consistency, the NC was homogenized to achieve a beverage consistency. Additionally, the NC was heat-treated to inactivate the probiotics in the yogurt using a tunnel pasteurizer set at 49°C for the low cycle, 63°C for the high cycle, and 24°C for the cool-down cycle. Our previous in vitro work demonstrated that heating this yogurt to 63°C for 30 min yielded no detectable bifidobacteria [17].
Fecal microbiota
Participants were asked to provide fecal samples within 30 min of the bowel movement before and after each 2-wk beverage consumption period. Fecal samples for microbial DNA analysis were aliquoted into cryovials, flash-frozen in liquid nitrogen, and stored at –80°C. Fecal microbial DNA was extracted utilizing the PowerLyzer PowerSoil DNA Isolation Kit (MO BIO Laboratories, Inc.). Following fecal DNA extraction, the V4 region of the 16S rRNA gene was amplified. Primers used included V4_515F (5'-GTGYCAGCMGCCGCGGTAA) and V4_806R (5'-GGACTACNVGGGTWTCTAAT). High-throughput sequencing was performed at the W.M. Keck Center for Biotechnology, University of Illinois Urbana-Champaign on an Illumina NovaSeq to generate 2 × 250 nt reads. The average number of reads per sample was 888,747. Following sequencing, FASTX-Toolkit, DADA2 [19], and QIIME2 [20] were used to process the high-quality sequence data. FASTX trimmer trimmed primer sequences, and reads were formatted using the paired-end Earth Microbiome Project protocol (https://earthmicrobiome.org/protocols-and-standards/16s). After demultiplexing, DADA2 function “denoise-paired” was utilized, and sequences were truncated to retain quality scores ≤20 [21]. Taxonomy was assigned with SILVA 138 [22]. Probiotic enrichment was assessed using relative abundance percent sequences of B. animalis to the species level.
Intestinal transit time
Size 3 gelatin capsules were filled with 40 to 60 mg food-grade Brilliant Blue FCF dye powder (FD&C Blue No. 1 Granular; DyStar) [23]. Participants used an intestinal transit log to record times of capsule consumption and dye passing in bowel movements. Participants were instructed to consume each capsule following a bowel movement. Intestinal transit time logs were completed during the 4 d prior to each intervention period and during the last 4 d of each 2-wk intervention period. To allow for variation in intestinal transit time, yogurt consumption was delayed or extended if >4 d were needed for tracking the capsules. Three capsules were consumed consecutively, and average intestinal transit times were calculated.
Digestive health
Digestive health was measured using 7-d records, questionnaires, and stool processing in the laboratory before and after each intervention period. Seven-day records (Supplemental Figure 1) included ratings from 1 (absent) to 4 (severe) of burping, cramping/pain, distension/bloating, flatulence/gas, nausea, reflux (heartburn), and rumblings [24]. In addition, all bowel movements were reported and rated for consistency and ease of passage for 7 d. The Bristol stool scale was used to rate consistency from 1 (separate hard lumps, like nuts) to 7 (watery, no solid pieces, entirely liquid). Ease of passage was rated from 1 (very easy) to 5 (very difficult). In the laboratory, pH and Bristol stool consistency were measured from samples collected before and after each intervention period.
The Gastrointestinal Tolerability questionnaire was used to assess digestive health through 6 questions relating to nausea, bloating, rumblings, gas/flatulence, abdominal pain, and diarrhea [25]. Each question pertained to symptom experience in the past 7 d. Lastly, the Gastrointestinal Quality of Life Index (GIQLI) assessed gastrointestinal health through 36 questions about daily life and gastrointestinal symptoms experienced in the past 2 wk [26], with minor adjustments for use with a healthy population (Supplemental Figure 2). Specifically, text stating gastrointestinal “illness” was adjusted to “gastrointestinal discomfort.” For example, item 9 was changed from “Because of your illness, to what extent have you restricted the kinds of food you eat?” to “Because of gastrointestinal discomfort, to what extent have you restricted the kinds of food you eat?”
Mood states and cognitive function
Mood was measured using validated questionnaires and a computer task. The Positive and Negative Affect Schedule-Short Form (PANAS-SF) assessed general mood using a 20-item scale that included words associated with positive (10 words) or negative (10 words) emotions [27]. For each word, participants rated the extent they experienced this emotion over the past week from 1 (very slightly or not at all) to 5 (extremely). The Depression Anxiety Stress Scales-42 (DASS-42) was used to assess mental health over the past week and included subscales with 42 questions on negative emotional states [28]. Each item was rated 0 (did not apply to me at all) to 3 (applied to me very much, or most of the time). Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires were used to measure mental and general health [29]. Specific PROMIS surveys utilized included PROMIS SF v1.0 - Anger 8a, PROMIS SF v1.0 - Anxiety 8a, PROMIS SF v1.0 - Fatigue 8a, PROMIS SF v1.0 - Positive Affect 15a, PROMIS SF v1.0 - Self-Efficacy Manage Emotions 8a, PROMIS SF v1.0 - Fatigue 13a (FACIT-Fatigue), PROMIS SF v1.1 - Global Health, and PROMIS SF v2.0 - Cognitive Function 8a. PROMIS surveys were scored using the recommended HealthMeasures Scoring Service [29].
Additionally, participants completed a task that involved viewing neutral, positive, and negative images on a computer screen, modeled after a similar task [30], and completed image ratings using the Self-Assessment Manikin (SAM) (Supplemental Figure 3) [31]. There were 3 blocks of 18 images, for a total of 54 images. Participants viewed 3 blocks of images before and after each consumption period. The images were obtained from the International Affective Picture System database [32]. Following display of each image, participants used the SAM to rate the images using 3 scales: 1) valence: 1 (negative) to 9 (positive); 2) arousal: 1 (calm) to 9 (excited); and 3) dominance: 1 (controlled) to 9 (in control) [32].
Cognitive function was measured using a spatial reconstruction task (Supplemental Figure 4) [10]. Participants were asked to study an array of 6 objects placed at different locations on a computer display. Following a brief interval, the objects would appear at the top of the display, and participants were asked to re-create the spatial layout of objects as viewed in the study phase. The task required participants to successfully encode and retrieve information on the position of the objects in relation to one other, necessitating recruitment of the hippocampal memory system. Reconstruction was self-paced, and the task included 20 trials (4 blocks of 5 trials). Participants were assessed in performance of object-location binding, which is the number of objects correctly placed within a predefined radius around the original location, with a higher score indicating better performance.
Statistical analyses
Using an anticipated small-to-moderate effect size (f = 0.15–0.20) based on previously published work studying the same probiotic, which reported reductions in intestinal transit time in adults [[3], [4], [5], [6], [7]], the alternative hypothesis proposed was directional (i.e., 1-sided, α of 0.10) and aimed for 80% power. Thus, the minimum sample size necessary to test differences between the HON and NC interventions was 46 participants. To allow for study attrition, our original enrollment target was 60 participants.
Data were analyzed in RStudio 2023.03.0 [33] with linear mixed-effects models (LMMs) using the package lme4. Treatment, time, treatment by time interaction, and baseline covariate were fixed effects, and participant was the random effect in the LMMs. Mean replacement was employed for missing item responses in questionnaires because missing item responses were rare and random. For example, the summed score of the answered items would be multiplied by the total number of items divided by the number of answered items. Additionally, for the primary outcome, intestinal transit time, winsorization was utilized to replace extreme values that were >3 SDs from the condition means. Significance was accepted at P ≤ 0.05.
Intention-to-treat analyses were conducted for the primary outcome, intestinal transit time, using participants from the NC and HON conditions, including dropouts. Missing data were replaced with participant baseline values. For participants who did not provide baseline or any intestinal transit time data, missing values were replaced with the mean intestinal transit time from the first baseline period. Probable random study drop-out (travel: n = 1, moving: n = 2, COVID-19 infection: n = 1) and use of healthy participants justified baseline and mean (when baseline not available) replacement techniques for handling missing data [34,35].
Microbiota differential abundance analysis was conducted using linear discriminant analysis effect size (LEfSe) via Galaxy (Huttenhower Laboratory, Harvard School of Public Health, MA) [36]. Diversity analyses were conducted using RStudio and the phyloseq package [37]. Following creation of a phyloseq object, alpha diversity was determined with pairwise Wilcoxon rank-sum tests, and beta diversity was assessed with permutational multivariate analysis of variance using the adonis2 function in the R vegan package. Songbird was used to conduct multinomial regression on compositional data and create differentials that provided log-fold taxa changes in reference to specified metadata variables [38]. Using differential output from Songbird, the tool Quantitative Rank/Ratio Observations (Qurro) was used to visualize taxa rankings and sample log-ratio plots [39]. Log-ratios and variables of interest were exported, and t tests or Pearson correlations were performed in R to verify Qurro visual observations. Lastly, Spearman correlations and regression analyses (forward stepwise regression, linear models, LMMs) were conducted to explore relationships between intestinal transit time and microbial taxa.
Extension study
Upon completion of the randomized, controlled trial, which included the NC and HON conditions, participants were eligible to participate in a nonrandomized study extension (NCT04901390). The study extension procedures were administered in accordance with the Declaration of Helsinki and were approved by the University of Illinois Institutional Review Board. Those electing to continue into the extension study underwent an additional ≥4-wk washout before starting the 2-wk consumption period of the Positive Control (PC; 170 g yogurt plus 15 g sugar) yogurt beverage twice daily. The rationale for this study extension was to provide a reference condition to assess the effects of the probiotic within the standard yogurt formulation, without honey, similar to our in vitro work [17]. Sugar was added to the yogurt to make the product isocaloric to the NC and HON yogurt beverages; however, sugar was not a novel ingredient, as it was already present in the yogurt. The PC was homogenized to achieve a beverage consistency similar to the NC and HON beverages. Participants in the extension study underwent identical testing procedures to the primary study at baseline and end of the condition, including fecal microbiota, intestinal transit time, digestive health, mood states, and cognitive function, which are detailed above. The statistical analyses for the extension study were conducted using the same approaches as the primary phase. Statistical analyses were conducted to analyze all available data from participants in all 3 conditions (NC, HON, PC), as well as in a subsample of the 36 participants that completed all 3 conditions.
Results
Primary study: Honey (HON) and Negative Control (NC) comparisons
Participant characteristics
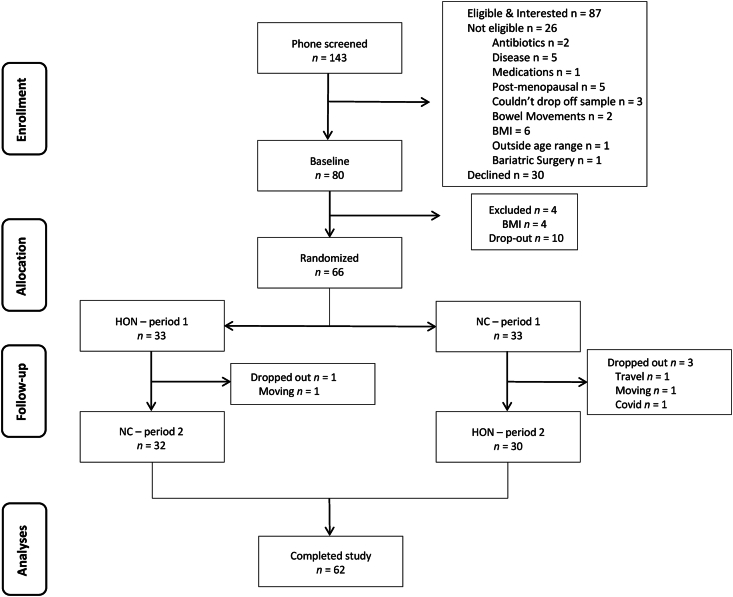
Figure 1 displays recruitment, enrollment, and retention. Sixty-six participants were randomly allocated to receive either NC or HON for the first 2-wk consumption period. Due to the COVID-19 pandemic, 6 additional study participants were enrolled beyond our original enrollment target (60) to ensure a minimum of 46 participants completed the study. During the first study intervention period, 4 participants dropped out due to moving (n = 2), travel (n = 1), and COVID-19 infection (n = 1). No participants dropped out during the second intervention period. Sixty-two participants completed the study. Retention from randomization to completion was 94%. Study participants were nearly equally split by sex (52% female) and were predominantly White (56%) and Asian (33%). Baseline participant characteristics are displayed in Table 1.
FIGURE 1.
CONSORT diagram of study enrollment and retention. BMI, body mass index; HON, Honey.
TABLE 1.
Participant characteristics at baseline
| Characteristic | All (n = 66) |
|---|---|
| Age, y | 33.5 ± 9.8 |
| BMI, kg/m2 | 24.6 ± 3.1 |
| Normal (18.5–24.9), n (%) | 36 (55) |
| Overweight (25.0–29.9), n (%) | 30 (45) |
| Waist circumference, cm | 91.0 ± 9.8 |
| Race, n (%) | |
| Asian | 22 (33) |
| African American | 3 (5) |
| White | 37 (56) |
| Mixed or other | 4 (6) |
| Education, n (%) | |
| High school graduate | 3 (5) |
| 1–3 y college | 3 (5) |
| College/university graduate | 26 (39) |
| Master’s degree | 23 (35) |
| PhD/equivalent | 11 (17) |
| Income, n (%) | |
| <$10,000 | 5 (8) |
| $10,000–$20,000 | 5 (8) |
| $21,000–$30,000 | 16 (24) |
| $31,000–$40,000 | 8 (12) |
| $41,000–$50,000 | 4 (6) |
| $51,000–$60,000 | 4 (6) |
| $61,000–$70,000 | 5 (8) |
| $71,000–$80,000 | 4 (6) |
| $81,000–$90,000 | 6 (9) |
| $91,000–$100,000 | 3 (5) |
| >$100,000 | 6 (9) |
Abbreviations: BMI, body mass index; SEM, standard error of the mean.
Values are means ± SEM or n (%).
Probiotic enrichment
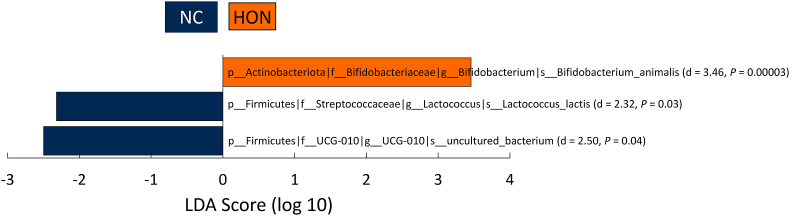
Using LEfSe at the species level at end, B. animalis was enriched with HON (d = 3.46; P < 0.0001) compared to NC (Figure 2). Using LMM, yogurt consumption (the time effect at end), regardless of condition, predicted higher abundance of B. animalis (β: 0.36; P = 0.001). Notably, the treatment by time interaction of HON at end predicted higher B. animalis (β: 0.58; P < 0.001). Means of B. animalis (Supplemental Figure 5) and other species present in the yogurt, as well as LMM fixed effects, are displayed in Supplemental Table 3.
FIGURE 2.
LEfSe differential abundance at the species level. This tool detected differential abundances of species between the conditions at end. LDA, linear discriminant analysis; LEfSe, linear discriminant analysis effect size; HON, Honey; NC, Negative Control.
Intestinal transit time
Baseline intestinal transit times before the first consumption period are displayed in Supplemental Figure 6, with a mean of 26.5 ± 8.82 h and a range of 10.3 to 49.1 h. No treatment, time, or treatment by time interaction effects were found to predict intestinal transit time using LMMs (note: winsorization was utilized to replace 2 extreme values that were >3 SDs from the condition means). Table 2 displays end timepoint intestinal transit time means and LMM fixed effects.
TABLE 2.
Intestinal transit time measured in hours by blue dye capsules after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Transit time, h | 27.9 ± 1.79 (52) | 25.8 ± 1.19 (50) | β: 0.02 CI: −2.17, 2.21 P = 0.99 |
β: 0.51 CI: −1.73, 2.76 P = 0.66 |
β: −0.27 CI: −3.50, 2.96 P = 0.87 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMM and are presented as mean ± SEM (n).
The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment × time).
In the intention-to-treat analyses (total: n = 66; completed: n = 62, dropouts: n = 4), no effects predicted intestinal transit time with LMM. Number of participants with value(s) replaced and replacement methods for intention-to-treat analyses are displayed in Supplemental Table 4.
Digestive health
No treatment, time, or interaction effects were found to predict Gastrointestinal Tolerability or GIQLI scores (Table 3). Additionally, there were no treatment, time, or interaction effects found to be predictors of gastrointestinal symptoms (Table 4), function (Table 5), stool consistency, or pH (Table 6).
TABLE 3.
Gastrointestinal health measured by the tolerability and GIQLI questionnaires after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Tolerability1 | 0.70 ± 0.16 (54) | 0.87 ± 0.22 (54) | β: 0.01 CI: −0.38, 0.40 P = 0.96 |
β: 0.07 CI: −0.34, 0.48 P = 0.72 |
β: 0.358 CI: −0.22, 0.93 P = 0.23 |
| GIQLI2 | 129 ± 1.33 (54) | 127 ± 1.72 (54) | β: −0.60 CI: −2.68, 1.49 P = 0.57 |
β: 0.626 CI: −1.52, 2.79 P = 0.57 |
β: 0.636 CI: −2.39, 3.65 P = 0.68 |
Abbreviations: CI, confidence interval; GIQLI, Gastrointestinal Quality of Life Index; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Tolerability score: 0 (no symptoms/no more than usual) to 12 (much more than usual for every symptom).
GIQLI score: 0 (worst quality of life) to 144 (best quality of life).
TABLE 4.
Gastrointestinal symptoms measured by a 7-d record after 2-wk consumption of NC and HON conditions
| Symptom1 | NC |
HON |
LMM fixed effects |
||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Burping, rating | 1.27 ± 0.07 (57) | 1.27 ± 0.06 (56) | β: −0.002 CI: −0.10, 0.09 P = 0.97 |
β: 0.06 CI: −0.04, 0.16 P = 0.24 |
β: −0.02 CI:6, 0.12 P = 0.80 |
| Cramping/pain, rating | 1.08 ± 0.03 (57) | 1.12 ± 0.04 (56) | β: 0.004 CI: −0.06, 0.07 P = 0.91 |
β: −0.01 CI: −0.07, 0.06 P = 0.84 |
β: −0.002 CI: −0.09, 0.09 P = 0.97 |
| Distension/bloating, rating | 1.16 ± 0.05 (57) | 1.20 ± 0.05 (56) | β: 0.01 CI: −0.07, 0.10 P = 0.74 |
β: 0.01 CI: −0.08, 0.09 P = 0.84 |
β: −0.05 CI: −0.17, 0.07 P: 0.46 |
| Flatulence/gas, rating | 1.55 ± 0.07 (57) | 1.53 ± 0.06 (56) | β: 0.01 CI: −0.09, 0.12 P = 0.80 |
β: 0.04 CI: −0.07, 0.15 P = 0.48 |
β: −0.13 CI: −0.28, 0.03 P: 0.11 |
| Nausea, rating | 1.06 ± 0.02 (57) | 1.06 ± 0.02 (56) | β: −0.002 CI: −0.04, 0.04 P = 0.90 |
β: 0.03 CI: −0.004, 0.07 P = 0.09 |
β: −0.005 CI: −0.06, 0.05 P = 0.86 |
| Reflux (heartburn), rating | 1.06 ± 0.02 (57) | 1.05 ± 0.02 (56) | β: −0.01 CI: −0.05, 0.03 P = 0.68 |
β: 0.003 CI: −0.04, 0.05 P = 0.91 |
β: 0.02 CI: −0.04, 0.09 P = 0.51 |
| Rumblings, rating | 1.15 ± 0.05 (57) | 1.15 ± 0.04 (56) | β: −0.001 CI: −0.08, 0.08 P = 0.98 |
β: 0.003 CI: −0.08, 0.09 P = 0.95 |
β: −0.02 CI: −0.13, 0.10 P = 0.80 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Symptom rating scale: 1 = absent, 2 = mild, 3 = moderate, 4 = severe.
TABLE 5.
Gastrointestinal function measured by a 7-d record after 2-wk consumption of NC and HON conditions
| Function | NC |
HON |
LMM fixed effects |
||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Frequency, stool/d | 1.20 ± 0.09 (58) | 1.21 ± 0.08 (56) | β: 0.001 CI: −0.09, 0.09 P = 0.99 |
β: −0.02 CI: −0.11, 0.08 P = 0.73 |
β: −0.01 CI: −0.15, 0.12 P = 0.84 |
| Consistency, rating1 | 3.71 ± 0.13 (57) | 3.68 ± 0.12 (56) | β: 0.02 CI: −0.17, 0.21 P = 0.87 |
β: −0.13 CI: −0.32, 0.07 P = 0.20 |
β: −0.02 CI: −0.30, 0.25 P = 0.86 |
| Ease of passage, rating2 | 2.01 ± 0.10 (57) | 2.05 ± 0.09 (56) | β: 0.01 CI: −0.12, 0.13 P = 0.93 |
β: 0.05 CI: −0.08, 0.18 P = 0.44 |
β: −0.09 CI: −0.26, 0.09 P = 0.34 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Consistency rating scale (Bristol stool scale): 1 (separate hard lumps, like nuts), 2 (sausage-shaped but lumpy), 3 (like a sausage but with cracks on surface), 4 (like a sausage or snake, smooth and soft), 5 (soft blobs with clear-cut edges), 6 (fluffy pieces with ragged edges, mushy), 7 (watery, no solid pieces, entirely liquid).
Ease of passage rating scale: 1 (very easy), 2 (easy), 3 (neither easy nor difficult), 4 (difficult), 5 (very difficult).
TABLE 6.
Stool characteristics measured in the laboratory after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Consistency, rating1 | 3.82 ± 0.20 (61) | 4.08 ± 0.22 (61) | β: 0.05 CI: −0.34, 0.43 P = 0.81 |
β: −0.34 CI: −0.73, 0.04 P = 0.09 |
β: 0.03 CI: −0.51, 0.58 P = 0.91 |
| pH | 6.73 ± 0.07 (57) | 6.72 ± 0.07 (59) | β: −0.01 CI: −0.15, 0.13 P = 0.91 |
β: 0.05 CI: −0.09, 0.19 P = 0.49 |
β: 0.01 CI: −0.19, 0.21 P = 0.91 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Consistency rating scale (Bristol stool scale): 1 (separate hard lumps, like nuts) to 7 (watery, no solid pieces, entirely liquid).
Mood states and cognitive function
PANAS-SF analyses indicated the time effect at end predicted lower negative affect (better mood) (β: −1.73; P = 0.02) (Table 7). There were no treatment, time, or interaction effects found to determine DASS-42 mood states (Table 8). Cognitive function analyses revealed the interaction of HON at end predicted lower cognitive function (β: −2.97; P = 0.03). In contrast, the time effect at end predicted higher self-efficacy (β: 2.42; P = 0.004). No other treatment, time, or interaction effects were found to predict PROMIS questionnaire scores. (Table 9). No LMM effects were found to predict emotional response (Table 10). In contrast to the PROMIS cognitive questionnaire, no LMM effects predicted cognitive function as measured by the reconstruction task (Table 11).
TABLE 7.
Mood states measured by the PANAS-SF questionnaire after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Positive affect1 | 34.1 ± 0.96 (54) | 33.6 ± 1.03 (54) | β: −0.004 CI: −1.39, 1.37 P = 1.00 |
β: 0.02 CI: −1.41, 1.46 P = 0.98 |
β: −0.61 CI: −2.63, 1.40 P = 0.55 |
| Negative affect2 | 16.6 ± 0.90 (54) | 17.4 ± 0.91 (54) | β: 0.06 CI: −1.32, 1.44 P = 0.93 |
β: −1.73 CI: −3.17, −0.30 P = 0.02 |
β: 1.26 CI: −0.75, 3.28 P = 0.22 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; PANAS-SF, Positive and Negative Affect Scales – Short Form; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Positive affect score: 10 (low positive affect) to 50 (high positive affect).
Negative affect score: 10 (low negative affect) to 50 (high negative affect).
TABLE 8.
Mood states measured by the DASS-42 questionnaire after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM Fixed Effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Depression1 | 4.64 ± 0.86 (53) | 4.47 ± 0.85 (54) | β: 0.07 CI: −0.95, 1.08 P = 0.90 |
β: −0.13 CI: −1.19, 0.92 P = 0.80 |
β: −0.36 CI: −1.85, 1.12 P = 0.63 |
| Anxiety2 | 2.96 ± 0.60 (53) | 2.43 ± 0.51 (54) | β: −0.02 CI: −0.64, 0.60 P = 0.94 |
β: −0.62 CI: −1.27, 0.03 P = 0.06 |
β: 0.24 CI: −0.67, 1.14 P = 0.61 |
| Stress3 | 6.45 ± 0.99 (53) | 5.56 ± 0.83 (54) | β: 0.02 CI: −1.01, 1.05 P = 0.97 |
β: −0.81 CI: −1.89, 0.26 P = 0.14 |
β: −0.33 CI: −1.83, 1.17 P = 0.67 |
Abbreviations: CI, confidence interval; DASS-42, Depression Anxiety Stress Scales – 42; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Depression score: 0–9 (normal), 10–13 (mild), 14–20 (moderate), 21–27 (severe), 28+ (extremely severe).
Anxiety score: 0–7 (normal), 8–9 (mild), 10–14 (moderate), 15–19 (severe), 20+ (extremely severe).
Stress score: 0–14 (normal), 15–18 (mild), 19–25 (moderate), 26–33 (severe), 34+ (extremely severe).
TABLE 9.
Mood states measured by the HealthMeasures PROMIS questionnaire after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Anxiety1 | 49.1 ± 1.26 (53) | 49.6 ± 1.01 (54) | β: 0.04 CI: −1.65, 1.73 P = 0.96 |
β: −1.60 CI: −3.36, 0.16 P = 0.08 |
β: 1.529 CI: −0.94, 4.00 P = 0.23 |
| Fatigue1 | 47.2 ± 1.42 (53) | 47.1 ± 1.33 (54) | β: 0.17 CI: −1.61, 1.94 P = 0.85 |
β: −1.00 CI: −2.85, 0.84 P = 0.29 |
β: −0.80 CI: −3.38, 1.79 P = 0.55 |
| FACIT1 | 50.2 ± 1.08 (53) | 50.0 ± 1.01 (54) | β: −0.02 CI: −1.33, 1.30 P = 0.98 |
β: −0.84 CI: −2.21, 0.53 P = 0.23 |
β: 0.21 CI: −1.70, 2.14 P = 0.83 |
| Positive affect1 | 48.6 ± 1.34 (53) | 45.8 ± 1.16 (54) | β: −0.08 CI: −1.91, 1.75 P = 0.93 |
β: 1.34 CI: −0.56, 3.25 P = 0.17 |
β: −1.73 CI: −4.40, 0.94 P = 0.21 |
| Self-efficacy1 | 50.7 ± 1.17 (53) | 49.8 ± 1.02 (54) | β: 0.08 CI: −1.46, 1.62 P = 0.92 |
β: 2.418 CI: 0.82, 4.02 P = 0.004 |
β: −1.33 CI: −3.58, 0.92 P = 0.25 |
| Cognitive function1 | 50.2 ± 1.11 (53) | 49.0 ± 1.31 (54) | β: 0.19 CI: −1.63, 2.01 P = 0.84 |
β: 1.40 CI: −0.48, 3.29 P = 0.15 |
β: −2.97 CI: −5.61, −0.33 P = 0.03 |
| Global health – physical1 | 50.1 ± 0.77 (53) | 48.9 ± 0.73 (54) | β: −0.19 CI: −1.17, 0.79 P = 0.71 |
β: 0.63 CI: −0.39, 1.64 P = 0.23 |
β: −0.36 CI: −1.78, 1.06 P = 0.62 |
| Global health – mental1 | 48.7 ± 0.96 (53) | 47.3 ± 0.95 (54) | β: −0.15 CI: −1.50, 1.20 P = 0.83 |
β: 0.92 CI: −0.47, 2.32 P = 0.20 |
β: −0.02 CI: −1.97, 1.93 P = 0.98 |
| General health2 | 3.94 ± 0.12 (53) | 3.85 ± 0.10 (54) | β: −0.04 CI: −0.20, 0.13 P = 0.67 |
β: 0.07 CI: −0.10, 0.24 P = 0.42 |
β: 0.08 CI: −0.15, 0.32 P = 0.49 |
| Social health2 | 3.92 ± 0.12 (53) | 3.89 ± 0.12 (54) | β: −0.05 CI: −0.28, 0.17 P = 0.64 |
β: 0.09 CI: −0.14, 0.33 P = 0.44 |
β: 0.18 CI: −0.15, 0.51 P = 0.28 |
| Anger1 | 46.5 ± 1.12 (53) | 45.5 ± 1.14 (54) | β: 0.07 CI: −1.72, 1.86 P = 0.94 |
β: −1.05 CI: −2.92, 0.81 P = 0.27 |
β: −1.04 CI: −3.65, 1.58 P = 0.44 |
Abbreviations: CI, confidence interval; FACIT, PROMIS SF v1.0-Fatigue 13a (FACIT-Fatigue); HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; PROMIS, Patient-Reported Outcomes Measurement Information System; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
Survey scores: HealthMeasures PROMIS surveys use a T-score metric in which 50 is the mean of a relevant reference population and 10 is the standard deviation of that population.
Survey scores: 1 (poor) to 5 (excellent). These 2 items are from the PROMIS Global Health survey. HealthMeasures instructs the raw score to be used for these 2 items only.
TABLE 10.
Mood states measured by the emotional image task after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effect P-values |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Valence | |||||
| Neutral | 5.41 ± 0.09 (62) | 5.31 ± 0.10 (62) | β: −0.01 CI: −0.11, 0.09 P = 0.85 |
β: −0.01 CI: −0.11, 0.09 P = 0.85 |
β: 0.006 CI: −0.13, 0.15 P = 0.92 |
| Positive | 7.67 ± 0.13 (62) | 7.74 ± 0.13 (62) | β: 0.02 CI: −0.15, 0.20 P = 0.79 |
β: 0.0049 CI: −0.17, 0.18 P = 0.96 |
β: −0.148 CI: −0.40, 0.10 P = 0.25 |
| Negative | 2.21 ± 0.10 (62) | 2.20 ± 0.12 (62) | β: −0.03 CI: −0.21, 0.15 P = 0.78 |
β: 0.04 CI: −0.14, 0.22 P = 0.69 |
β: 0.12 CI: −0.14, 0.37 P = 0.38 |
| Arousal | |||||
| Neutral | 3.75 ± 0.18 (62) | 3.64 ± 0.19 (62) | β: −0.004 CI: −0.19, 0.18 P = 0.97 |
β: 0.156 CI: −0.03, 0.35 P = 0.11 |
β: −0.05 CI: −0.32, 0.22 P = 0.72 |
| Positive | 5.15 ± 0.24 (62) | 5.17 ± 0.26 (62) | β: 0.01 CI: −0.25, 0.27 P = 0.96 |
β: −0.11 CI: −0.38, 0.15 P = 0.40 |
β: −0.02 CI: −0.39, 0.35 P = 0.91 |
| Negative | 6.09 ± 0.18 (62) | 6.04 ± 0.20 (61) | β: −0.01 CI: −0.21, 0.19 P = 0.91 |
β: −0.12 CI: −0.33, 0.08 P = 0.23 |
β: 0.179 CI: −0.11, 0.46 P = 0.22 |
| Dominance | |||||
| Neutral | 6.56 ± 0.17 (62) | 6.52 ± 0.19 (62) | β: 0.03 CI: −0.22, 0.28 P = 0.83 |
β: 0.17 CI: −0.08, 0.43 P = 0.19 |
β: −0.18 CI: −0.54, 0.18 P = 0.33 |
| Positive | 6.96 ± 0.17 (62) | 6.99 ± 0.19 (61) | β: 0.03 CI: −0.21, 0.28 P = 0.79 |
β: 0.14 CI: −0.11, 0.39 P = 0.27 |
β: −0.27 CI: −0.62, 0.08 P = 0.13 |
| Negative | 3.24 ± 0.15 (62) | 3.32 ± 0.18 (62) | β: −0.01 CI: −0.23, 0.22 P = 0.95 |
β: 0.06 CI: −0.16, 0.29 P = 0.60 |
β: 0.17 CI: −0.15, 0.49 P = 0.30 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (treatment, time, interaction).
TABLE 11.
Cognitive function measured by the spatial reconstruction task after 2-wk consumption of NC and HON conditions
| NC |
HON |
LMM fixed effects |
|||
|---|---|---|---|---|---|
| End | End | Treatment (HON) | Time (End) | Treatment × Time Interaction | |
| Object-location binding1 | 2.82 ± 0.11 (61) | 2.76 ± 0.13 (62) | β: 0.01 CI: −0.12, 0.15 P = 0.87 |
β: 0.10 CI: −0.04, 0.23 P = 0.17 |
β: −0.15 CI: −0.34, 0.05 P = 0.14 |
Abbreviations: CI, confidence interval; HON, Honey; LMM, linear mixed-effect model; NC, Negative Control; SEM, standard error of the mean.
Data were analyzed by LMMs and are presented as mean ± SEM (n). The LMM fixed effects columns denote the main treatment effect (HON treatment), time (end), and the treatment by time interaction term (Treatment, Time, Interaction).
Object-location binding: The number of objects correctly placed within a predefined radius around the original location, with a higher score indicating better performance.
Fecal microbiota
Diversity and higher taxonomic classifications
No differences were detected between the HON and NC conditions at end in alpha (Supplemental Figure 7) or beta diversity (Supplemental Figures 8–11). Also, there were no differences from baseline to end in alpha diversity (Supplemental Figure 12) or beta diversity (Supplemental Figures 13 and 14). LEfSe analyses detected differential abundances within the HON and NC conditions at the genus level (Supplemental Figure 15). Supplemental Tables 5 and 6 display means and LMM results at the phylum and genus levels, and family level, respectively, for the HON and NC conditions. Notably, the time effect at end predicted enriched bifidobacteria at the phylum (Actinobacteriota), family (Bifidobacteriaceae), and genus (Bifidobacterium) levels using LMMs.
Multinomial regression and taxa rankings
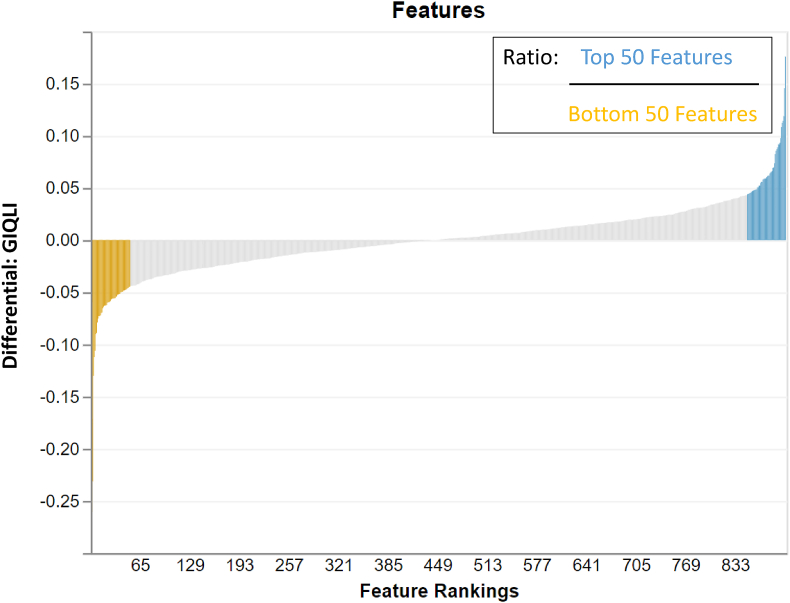
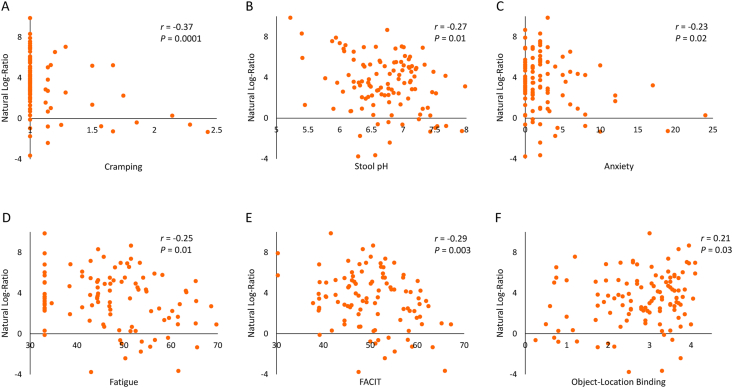
Although the primary outcome, intestinal transit time, did not improve the model fit in Songbird multinomial regression, GIQLI did improve the model fit. The top and bottom 50 ranked features (taxa) of the GIQLI differential (Figure 3) were used to create natural log-ratios by sample to explore in relation to other study outcomes. Having a higher log-ratio of microbial features making up the GIQLI differential was related to easier passage of bowel movements and more acidic stool (r = −0.22; P = 0.01); lower anxiety (r =−0.30; P = 0.001), cramping (r = −0.34; P = 0.0001), fatigue (FACIT: r = −0.25; P = 0.01; Fatigue: r = −0.22; P = 0.01), and stress (r = −0.20; P = 0.02); and better memory task performance (object-location binding: r = 0.18; P = 0.03) (Figure 4).
FIGURE 3.
Top and bottom ranked features of the GIQLI differential. The top and bottom 50 taxa are presented using the tool Qurro. These differentials were used to create natural log-ratios by sample to explore in relation to other study outcomes. GIQLI, Gastrointestinal Quality of Life Index; Qurro, Quantitative Rank/Ratio Observation.
FIGURE 4.
Correlations between study outcomes and GIQLI natural log-ratios. Having a higher log-ratio of microbial features making up the GIQLI differential was related to easier passage of bowel movements and more acidic stool (r = −0.22, P = 0.01); lower anxiety (r = −0.30, P = 0.001), cramping (r = −0.34, P = 0.0001), fatigue (FACIT: r = −0.25, P = 0.01; fatigue: r = −0.22, P = 0.01), and stress (r = −0.20, P = 0.02); and better memory task performance (object-location binding: r = 0.18, P = 0.03). FACIT, PROMIS SF v1.0 - Fatigue 13a; GIQLI, Gastrointestinal Quality of Life Index.
Intestinal transit time regression models with microbial covariates
There was no association between intestinal transit time and B. animalis percent sequences at end (r = 0.04; P = 0.67). Using LMM at the first baseline period, Catenibacillus (β: 89.5; P = 0.02) and Oscillibacter (β: 26.5; P = 0.01) were both found to be predictors of longer intestinal transit time. The other genera with baseline positive correlations with intestinal transit time were not found to be predictors of intestinal transit time at end using LMMs.
Study extension: Positive Control (PC) comparisons with Negative Control (NC) and Honey (HON)
Participant characteristics
Of the 62 participants who completed the randomized controlled trial, which included the NC and HON conditions, 37 began the nonrandomized study extension, of which 36 completed the study extension. Characteristics of participants that began the extension study compared to those that did not revealed that the cohort who elected to participate in all 3 conditions had slightly less positive affect and higher anger scores than individuals who chose to only complete the first 2 primary conditions (Supplemental Table 7).
Probiotic enrichment
Using LEfSe at the species level at the end timepoint, B. animalis was enriched with HON, compared to NC and PC (d = 3.54; P < 0.001) (Supplemental Figure 16). Yogurt consumption itself (the time effect at end) predicted higher abundance of B. animalis using LMM with all participants (β: 0.36; P = 0.001). The treatment by time interaction of HON at end predicted higher B. animalis with all participants (β: 0.58; P < 0.001), and the subsample of 36 participants that completed all 3 conditions (β: 0.60; P = 0.01). Similarly, for PC, the treatment by time interaction predicted higher B. animalis using all participants (β: 0.57; P = 0.002) and the subsample of 36 participants that completed all 3 conditions (β: 0.66; P = 0.002). Means of B. animalis and other yogurt species are displayed in Supplemental Table 8.
Intestinal transit time
No differences in intestinal transit time were detected between the 3 study conditions. Supplemental Table 9 displays end intestinal transit time means from all participants and LMM fixed effects.
Digestive health
There were no differences in Gastrointestinal Tolerability or GIQLI digestive health scores (Supplemental Table 10), gastrointestinal symptoms (Supplemental Table 11), or function (Supplemental Table 12). However, among the subsample of 36 participants who completed all 3 conditions, the interaction of the PC at end was predictive of higher stool pH (β: 0.27; P = 0.04) (Supplemental Table 13).
Mood states and cognitive function
The PANAS-SF analyses revealed that the time effect at end predicted lower negative affect (better mood) using all participants (Supplemental Table 14) but not in the subsample of 36 participants who completed all 3 conditions. There were no treatment, time, or interaction effects for DASS-42 mood states using all participants (Supplemental Table 15); however, the time effect at end predicted lower stress in the subsample of 36 participants who completed all 3 conditions (β: −1.51; P = 0.05). Analyses of the PROMIS questionnaire scores revealed several time and interaction effects (Supplemental Table 16). Cognitive analyses revealed that the effect of time at end predicted lower arousal to negative images in the subsample of 36 participants who completed all 3 conditions (β: −0.32; P = 0.03) (Supplemental Table 17). In contrast to the PROMIS cognitive questionnaire, no LMM effects predicted lower cognitive function as measured by the reconstruction task (Supplemental Table 18).
Fecal microbiota
No differences were detected among the HON, NC, and PC conditions at end in alpha (Supplemental Figure 17) or beta diversity (Supplemental Figures 18–21). There were no differences within the 3 conditions from baseline to end in alpha (Supplemental Figure 22) or beta diversity (Supplemental Figures 23–25). Genus differential abundances using LEfSe are reported in Supplemental Figure 26. Supplemental Tables 19 and 20 display means and LMM results at the phylum and genus levels, and family level, respectively. The time effect at end predicted enriched bifidobacteria at the phylum (Actinobacteriota), family (Bifidobacteriaceae), and genus (Bifidobacterium) levels using LMMs.
Discussion
Our study aimed to determine the effects of yogurt plus honey on the primary outcome of intestinal transit time and secondary outcomes of probiotic enrichment, digestive health, mood states and cognitive function, and gastrointestinal microbiota in healthy adults. We hypothesized that yogurt plus honey would enrich the probiotic in vivo, thereby reducing intestinal transit time; improve digestive health measures, mood, and cognition; and alter gastrointestinal microbiota composition. To our knowledge, this is the first attempt to translate in vitro research demonstrating enhanced probiotic survival with honey into a human study.
Although consuming the yogurt beverage with honey did not reduce intestinal transit time, we observed enriched fecal B. animalis, which suggests the effect previously observed in vitro [17] translated in vivo. However, although yogurt with honey supported probiotic enrichment, it did not result in other functional outcomes in the study. Notably, yogurt consumption itself (regardless of condition) predicted enriched bifidobacteria. Although the NC yogurt was pasteurized prior to consumption to kill the bifidobacteria, there was a marginal increase of fecal bifidobacterial in NC, which indicates that yogurt itself can stimulate bifidobacteria in the gastrointestinal tract. As a potential complication, bifidobacteria DNA was likely present in the pasteurized yogurt and may have contributed to the bifidobacteria DNA identified in the stool samples in the NC group. However, this requires the DNA in the pasteurized yogurt to survive host digestion in the stomach and small intestine and microbial metabolism in the colon. Alternatively, small numbers of bifidobacteria may have survived our pasteurization step and entered a viable but nonculturable state, as Quigley et al. [40] described, and perhaps survived gastrointestinal transit. However, in both possibilities, the contribution to our bifidobacteria numbers in the NC yogurt were likely small; however, we cannot rule out the possibility. In this study, although a yogurt control was necessary for adequate study blinding, future studies could include different preparations to compare probiotic yogurt plus honey, probiotic yogurt without honey, and a control with no live or inactivated probiotics.
Although several previous clinical trials reported that consuming B. animalis reduced intestinal transit time [[3], [4], [5], [6]], this finding was not replicated in the present study. This may have been related to our participants’ shorter intestinal transit times. Having <3 bowel movements per week is a criterion for functional constipation [41]. Although a self-reported frequency of 3 to 6 bowel movements per week was an inclusion criterion to participate in the study, objective measures revealed that the participants’ intestinal transit time averaged 26.5 h (SD: 8.82 h; range: 10.3–49.1 h), which equates to 6.3 bowel movements per week. Confidence intervals are a useful complement to assess nonsignificant tests [42]. To further examine the intestinal transit time outcome, NC and HON had means of 27.9 ± 1.79 h and 26.7 ± 1.44 h and 95% confidence intervals of 24.3 to 31.5 h and 23.4 to 28.2 h, respectively. Due to the comparable means and relatively narrow width of the confidence intervals (∼5–7 h), we can conclude any undetected effect to have a small effect size with reasonable confidence. Moreover, intention-to-treat analyses (n = 66) did not reveal any condition differences in intestinal transit time.
Three previous B. animalis intestinal transit time studies were conducted in elderly participants (aged 50–75 y) [4,6] and only females [5], populations both found to have greater potential for improved intestinal transit time with probiotic consumption [8]. In a B. animalis intestinal transit time study conducted in healthy males and females [3], mean group intestinal transit times were 30 and 33 h before consumption, compared to our baseline mean intestinal transit time of 26.5 h. However, like our results, healthy females that consumed yogurt with a different B. animalis strain for 2 wk (with control and active study periods with colonic transit times of 43.3 and 42.1 h, respectively) did not have reduced intestinal transit time, even with a history of straining during bowel movements or hard stools [43]. In contrast, in adults with functional gastrointestinal symptoms given a B. animalis strain for 2 wk, both low (1.8 billion CFU) and high (17.2 billion CFU) doses of the probiotic reduced intestinal transit time, while the placebo did not have this effect [44]. Similarly, in adults with constipation, 2 wk of yogurt containing a B. animalis strain with polydextrose reduced intestinal transit time [45]. In contrast, in another study of adults with constipation, a B. animalis strain mixed into a dairy product for 4 wk did not improve intestinal transit time; however, a post hoc analysis detected improved frequency in participants who initially had <3 bowel movements a week [46]. Thus, when considering our findings in the context of the overall literature, B. animalis and other strains appear to be most efficacious in decreasing intestinal transit time in individuals with constipation or consistently slower intestinal transit times. However, future work focused on intestinal transit time would benefit from objective measures at the time of enrollment to confirm that participants’ recall of the frequency of bowel movements is objectively verified.
Our work revealed no differences among the conditions in tolerability, gastrointestinal quality of life, digestive symptoms, or stool characteristics. This demonstrates the favorable digestive profile of our yogurt beverages because our healthy population did not experience increased gastrointestinal symptoms or effects from consumption. Although we hypothesized that yogurt plus honey would improve digestive symptoms and stool characteristics, our participants had, on average, no or mild symptoms at baseline and optimal stool consistency; therefore, improvement was not needed. In a systematic review of studies examining health effects of yogurt and fermented milk, 26 studies reported effects on gastrointestinal health and disease [47]. Of these studies, 20 showed improved digestive health measures [47]. However, most of these studies were conducted in participants with IBS, lactose intolerance, diarrhea, constipation, increased age, otherwise healthy participants who reported digestive symptoms, or to alleviate antibiotic side effects. Therefore, it would be beneficial for future studies to test the yogurt plus honey combination in these populations with gastrointestinal dysfunction.
Our work revealed no treatment effects on mood states or cognitive function. In contrast, studies have reported that fermented dairy consumption was related to decreased anxiety in young adults [48] and improved relational memory in healthy adults [10]. Also, in females and adults aged ≥60 y, a cross-sectional study found that yogurt consumption and depressive symptoms had an inverse relationship [49]. However, another study reported that a probiotic milk drink only improved mood for participants who initially had poor mood [50]. Likewise, in a meta-analysis of studies examining the effects of probiotics on depressive symptoms, no improved outcomes were found for healthy individuals; however, improvements were observed for people who already presented with depressive symptoms [51]. Therefore, future studies examining the effects of yogurt plus honey to support health will likely be best suited in populations with mental health conditions.
The beneficial effects of time on mood and cognition outcomes in our study demonstrate the potential benefits of yogurt or fermented dairy consumption. Similar beneficial effects of dairy consumption have been reported in cross-sectional [11] and interventional [10] trials. In contrast, the interaction effects of HON and PC at end predicted lower cognitive function and positive affect, respectively. However, the lower cognitive effect was not replicated with cognitive performance in the spatial reconstruction task. The lower positive affect predicted at the end of the study extension (i.e., the final condition [PC]) may have been due to the participants who elected to complete all conditions being reluctant to finish the 16-wk study. Additionally, it is possible that individuals who completed all 3 arms of the trial were different in temperament than those who elected to not to continue on to the study extension. Our comparison of these cohorts revealed that the cohort who elected to participate in all 3 conditions reported slightly less positive affect and higher anger scores than individuals who chose to only complete the first 2 primary conditions.
Although this is the first trial in humans, to our knowledge, to test the effect of honey on probiotic enrichment, previous studies have examined synbiotics. A synbiotic is a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host [52]. Like honey, different fruits and other plants contain prebiotics, polyphenols, and antioxidants that may feed and protect probiotic microorganisms. Indeed, fruits and plant extracts have been added to yogurt to test improved probiotic survival. In one in vitro digestion experiment, yogurt fortified with polyphenols from cactus pear extract did not enhance lactic acid bacteria survival but did protect bioactive compounds and antioxidant activity [53]. In another study, plant extract made from olive, garlic, onion, and citrus improved yogurt culture viability [54].
Strengths of this study included the thorough approach to assessing gastrointestinal effects and tolerance through symptom ratings, stool characteristics, and gastrointestinal questionnaires [55]. Additionally, this trial adds novel findings to the literature by contributing the first honey plus yogurt study investigating the translational efficacy of this combination in vitro to humans. Limitations include the participants’ objectively measured stool frequency being slightly longer on average (∼6.3 bowel movements per week) compared to the self-reported screening criterion (3–6 bowel movements per week). The limitation of self-reported stool frequency can be addressed in the future by measuring intestinal transit time or tracking bowel movements during a screening period. In addition, as probiotics have been found to improve function, such as mood and gastrointestinal health, in people with health conditions, future studies should include testing the effects of yogurt plus honey in patient populations. Lastly, future studies should further explore the effects of yogurt and probiotics combined with honey and other food ingredients. These whole food ingredients are readily available to the public and have the potential to boost probiotic efficacy to help maintain well-being in healthy populations and improve health in people with gastrointestinal and mental health conditions.
In conclusion, this work reveals that yogurt plus honey supports probiotic enrichment in vivo; however, the enrichment did not result in any gastrointestinal, cognitive, or mood improvements. Ultimately, it is challenging to induce meaningful changes in human adult microbiota that further elicit changes in physiological outcomes.
Acknowledgments
We thank Jedi Brown and the FSHN Pilot Processing Plant for training us to use their homogenizer and tunnel pasteurizer to prepare our study beverages. We thank Ella Beal and Maggie Oleksiak for metabolic kitchen management. We acknowledge Mara Perez Tamayo, Alexis Baldeon, David Revilla, Harini Ramaswamy, Karly Miller, Julie Mathews, Diana Gomez, and Yasmine Haynes for assistance in conducting testing procedures and preparing yogurt conditions.
Author contributions
The authors’ responsibilities were as follows – HDH, NAK, MJM, ARM, DAA: designed the research; MDB: recruited and enrolled participants and coordinated study procedures; ARM, KG: prepared study beverages; ARM, DAA, ME, TA, JZ: conducted the experiments and testing appointments; EC: managed biological sample collection; ARM, EC, ME, KG: managed study data; ARM, CNC: analyzed the data; ARM, HDH: wrote the manuscript; HDH: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This work was supported by the National Honey Board and the USDA National Institute of Food and Agriculture, Hatch Project 1009249.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2024.05.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shaikh S.D., Sun N., Canakis A., Park W.Y., Weber H.C. Irritable bowel syndrome and the gut microbiome: a comprehensive review. J. Clin. Med. 2023;12(7):2558. doi: 10.3390/jcm12072558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salari N., Ghasemianrad M., Ammari-Allahyari M., Rasoulpoor S., Shohaimi S., Mohammadi M. Global prevalence of constipation in older adults: a systematic review and meta-analysis. Wien. Klin. Wochenschr. 2023;135(15–16):389–398. doi: 10.1007/s00508-023-02156-w. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier M., Meance S., Bouley C., Berta J.L., Grimaud J.C. Effects of consumptionof a milk fermented by the probiotic strain Bifidobacterium animalis DN-173 010 on colonic transit times in healthy humans. Biosci. Microflora. 2001;20(2):43–48. doi: 10.12938/bifidus1996.20.43. [DOI] [Google Scholar]
- 4.Meance S., Cayuela C., Turchet P., Raimondi A., Lucas C., Antoine J.M. A fermented milk with a Bifidobacterium probiotic strain DN-173 010 shortened oro-fecal gut transit time in elderly. Microb. Ecol. Health Dis. 2001;13(4):217–222. doi: 10.1080/089106001753341291. [DOI] [Google Scholar]
- 5.Marteau P., Cuillerier E., Meance S., Gerhardt M.F., Myara A., Bouvier M., et al. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study, Aliment. Pharmacol. Ther. 2002;16(3):587–593. doi: 10.1046/j.1365-2036.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- 6.Meance S., Cayuela C., Raimondi A., Turchet P., Lucas C., Antoine J.M. Recent advances in the use of functional foods: effects of the commercial fermented milk with Bifidobacterium animalis strain DN-173 010 and yogurt strains on gut transit time in the elderly. Microb Ecol Health Dis. 2003;15(1):15–22. doi: 10.1080/08910600310015565. [DOI] [Google Scholar]
- 7.Guyonnet D., Chassany O., Ducrotte P., Picard C., Mouret M., Mercier C.H., et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial, Aliment. Pharmacol. Ther. 2007;26(3):475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller L.E., Ouwehand A.C. Probiotic supplementation decreases intestinal transit time: meta-analysis of randomized controlled trials. World J. Gastroenterol. 2013;19(29):4718–4725. doi: 10.3748/wjg.v19.i29.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannavale C.N., Mysonhimer A.R., Bailey M.A., Cohen N.J., Holscher H.D., Khan N.A. Consumption of a fermented dairy beverage improves hippocampal-dependent relational memory in a randomized, controlled cross-over trial. Nutr. Neurosci. 2023;26(3):265–274. doi: 10.1080/1028415X.2022.2046963. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A.M., Thompson S.V., Edwards C.G., Musaad S.M.A., Khan N.A., Holscher H.D. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2020;23(12):983–992. doi: 10.1080/1028415X.2019.1582578. [DOI] [PubMed] [Google Scholar]
- 12.Liu R.T., Walsh R.F.L., Sheehan A.E. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chick H., Shin H.S., Ustunol Z. Growth and acid production by lactic acid bacteria and bifidobacteria grown in skim milk containing honey. J. Food Sci. 2001;66(3):478–481. doi: 10.1111/j.1365-2621.2001.tb16134.x. [DOI] [Google Scholar]
- 14.Ustunol Z., Gandhi H. Growth and viability of commercial Bifidobacterium spp. in honey-sweetened skim milk. J. Food Prot. 2001;64(11):1775–1779. doi: 10.4315/0362-028x-64.11.1775. [DOI] [PubMed] [Google Scholar]
- 15.Kajiwara S., Gandhi H., Ustunol Z. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: an in vitro comparison with commercial oligosaccharides and inulin. J. Food Prot. 2002;65(1):214–218. doi: 10.4315/0362-028x-65.1.214. [DOI] [PubMed] [Google Scholar]
- 16.Favarin L., Laureano-Melo R., Luchese R.H. Survival of free and microencapsulated Bifidobacterium: effect of honey addition. J. Microencapsul. 2015;32(4):329–335. doi: 10.3109/02652048.2015.1017620. [DOI] [PubMed] [Google Scholar]
- 17.Alvarado D.A., Ibarra-Sánchez L.A., Mysonhimer A.R., Khan T.A., Cao R., Miller M.J., et al. Honey varietals differentially impact Bifidobacterium animalis ssp. lactis survivability in yogurt through simulated in vitro digestion. J. Nutr. 2024;154(3):866–874. doi: 10.1016/j.tjnut.2024.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Dairy Foods Association . IDFA; Washington, DC: 2019. Live and Active Culture Yogurt Seal Program: procedures and guidelines.https://www.idfa.org/wordpress/wp-content/uploads/2020/03/8.19.19_LAC_Seal_Guidelines_and_Appendices.pdf Available from: [Google Scholar]
- 19.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry S.E., Valdes A.M., Drew D.A., Asnicar F., Mazidi M., Wolf J., et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020;26(6):964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baer D.J., Gebauer S.K., Novotny J.A. Measured energy value of pistachios in the human diet. Br. J. Nutr. 2012;107(1):120–125. doi: 10.1017/S0007114511002649. [DOI] [PubMed] [Google Scholar]
- 24.Holscher H.D., Doligale J.L., Bauer L.L., Gourineni V., Pelkman C.L., Fahey G.C., et al. Gastrointestinal tolerance and utilization of agave inulin by healthy adults. Food Funct. 2014;5(6):1142–1149. doi: 10.1039/c3fo60666j. [DOI] [PubMed] [Google Scholar]
- 25.Maki K.C., Rains T.M., Kelley K.M., Cook C.M., Schild A.L., Gietl E. Fibermalt is well tolerated in healthy men and women at intakes up to 60 g/d: a randomized, double-blind, crossover trial. Int. J. Food Sci. Nutr. 2013;64(3):274–281. doi: 10.3109/09637486.2012.738652. [DOI] [PubMed] [Google Scholar]
- 26.Eypasch E., Williams J.I., Wood-Dauphinee S., Ure B.M., Schmülling C., Neugebauer E., et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br. J. Surg. 1995;82(2):216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 27.Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 28.Brown T.A., Chorpita B.F., Korotitsch W., Barlow D.H. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav. Res. Ther. 1997;35(1):79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 29.Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 31.Bradley M.M., Lang P.J. Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 32.Lang P.J., Bradley M.M., Cuthbert B.N. 1997. International Affective Picture System (IAPS): technical manual and affective ratings, NIMH Center for the Study of Emotional Attention; pp. 39–58. [Google Scholar]
- 33.R Core Team, R . R Foundation for Statistical Computing; Vienna, Austria: 2019. A language and environment for statistical computing. [Google Scholar]
- 34.Del Re A.C., Maisel N.C., Blodgett J.C., Finney J.W. Intention-to-treat analyses and missing data approaches in pharmacotherapy trials for alcohol use disorders. BMJ Open. 2013;3(11) doi: 10.1136/bmjopen-2013-003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White I.R., Horton N.J., Carpenter J., Pocock S.J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMurdie P.J., Holmes S. Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac. Symp. Biocomput. 2012:235–246. [PMC free article] [PubMed] [Google Scholar]
- 38.Morton J.T., Marotz C., Washburne A., Silverman J., Zaramela LS, Edlund A, et al. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 2019;10(1):2719. doi: 10.1038/s41467-019-10656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedarko M.W., Martino C., Morton J.T., González A., Rahman G., Marotz C.A., et al. Visualizing 'omic feature rankings and log-ratios using Qurro. NAR Genom. Bioinform. 2020;2(2) doi: 10.1093/nargab/lqaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quigley L., McCarthy R., O’Sullivan O., Beresford T.P., Fitzgerald G.F., Ross R.P., et al. The microbial content of raw and pasteurized cow milk as determined by molecular approaches. J. Dairy Sci. 2013;96(8):4928–4937. doi: 10.3168/jds.2013-6688. [DOI] [PubMed] [Google Scholar]
- 41.Aziz I., Whitehead W.E., Palsson O.S., Törnblom H., Simrén M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev. Gastroenterol. Hepatol. 2020;14(1):39–46. doi: 10.1080/17474124.2020.1708718. [DOI] [PubMed] [Google Scholar]
- 42.Colegrave N., Ruxton G.D. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav. Ecol. 2003;14(3):446–447. doi: 10.1093/beheco/14.3.446. [DOI] [Google Scholar]
- 43.Merenstein D.J., D’Amico F., Palese C., Hahn A., Sparenborg J., Tan T., et al. Short-term, daily intake of yogurt containing Bifidobacterium animalis ssp. lactis Bf-6 (LMG 24384) does not affect colonic transit time in women. Br. J. Nutr. 2014;111(2):279–286. doi: 10.1017/S0007114513002237. [DOI] [PubMed] [Google Scholar]
- 44.Waller P.A., Gopal P.K., Leyer G.J., Ouwehand A.C., Reifer C., Stewart M.E., et al. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011;46(9):1057–1064. doi: 10.3109/00365521.2011.584895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magro D.O., de Oliveira L.M., Bernasconi I., Ruela Mde S., Credidio L., Barcelos I.K., et al. Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: a randomized, double-blind, controlled study in chronic constipation. Nutr. J. 2014;13(1):75. doi: 10.1186/1475-2891-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibarra A., Latreille-Barbier M., Donazzolo Y., Pelletier X., Ouwehand A.C. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236–251. doi: 10.1080/19490976.2017.1412908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savaiano D.A., Hutkins R.W. Yogurt, cultured fermented milk, and health: a systematic review. Nutr. Rev. 2021;79(5):599–614. doi: 10.1093/nutrit/nuaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa R.J.M., Baptista J.A.B., Silva C.C.G. Consumption of fermented dairy products is associated with lower anxiety levels in Azorean university students. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.930949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J., Wang W., Zhang D. Associations of different types of dairy intakes with depressive symptoms in adults. J. Affect. Disord. 2020;274:326–333. doi: 10.1016/j.jad.2020.05.095. [DOI] [PubMed] [Google Scholar]
- 50.Benton D., Williams C., Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007;61(3):355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 51.Ng Q.X., Peters C., Ho C.Y.X., Lim D.Y., Yeo W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018;228:13–19. doi: 10.1016/j.jad.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 52.Swanson K.S., Gibson G.R., Hutkins R., Reimer R.A., Reid G., Verbeke K., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020;17(11):687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cenobio-Galindo A.J., Díaz-Monroy G., Medina-Pérez G., Franco-Fernández M.J., Ludeña-Urquizo F.E., Vieyra-Alberto R., et al. Multiple emulsions with extracts of cactus pear added in a yogurt: antioxidant activity, in vitro simulated digestion and shelf life. Foods. 2019;8(10):429. doi: 10.3390/foods8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michael M., Phebus R.K., Schmidt K.A. Plant extract enhances the viability of Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus acidophilus in probiotic nonfat yogurt. Food Sci. Nutr. 2015;3(1):48–55. doi: 10.1002/fsn3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holscher H.D., Chumpitazi B.P., Dahl W.J., Fahey G.C., Liska D.J., Slavin J.L., et al. Perspective: assessing tolerance to nondigestible carbohydrate consumption. Adv. Nutr. 2022;13(6):2084–2097. doi: 10.1093/advances/nmac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.