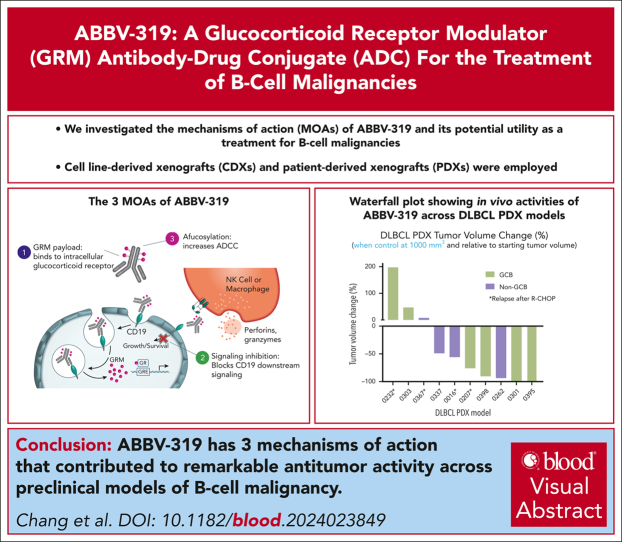
Key Points
-
•
ABBV-319 has 3 mechanisms of action that contributed to significant antitumor activity across preclinical models of B-cell malignancy.
-
•
ABBV-319 delivers a glucocorticoid receptor modulator (GRM) payload that induces pronounced anti-tumor activity in B-cell malignancies.
Visual Abstract
Abstract
Glucocorticoids are key components of the standard-of-care treatment regimens for B-cell malignancy. However, systemic glucocorticoid treatment is associated with several adverse events. ABBV-319 is a CD19-targeting antibody-drug conjugate engineered to reduce glucocorticoid-associated toxicities while possessing 3 distinct mechanisms of action (MOA) to increase therapeutic efficacy: (1) antibody-mediated delivery of a glucocorticoid receptor modulator (GRM) payload to activate apoptosis, (2) inhibition of CD19 signaling, and (3) enhanced fragment crystallizable (Fc)–mediated effector function via afucosylation of the antibody backbone. ABBV-319 elicited potent GRM-driven antitumor activity against multiple malignant B-cell lines in vitro, as well as in cell line-derived xenografts and patient-derived xenografts (PDXs) in vivo. Remarkably, a single dose of ABBV-319 induced sustained tumor regression and enhanced antitumor activity compared with repeated dosing of systemic prednisolone at the maximum tolerated dose in mice. The unconjugated CD19 monoclonal antibody (mAb) also displayed antiproliferative activity in a subset of B-cell lymphoma cell lines through the inhibition of phosphoinositide 3-kinase signaling. Moreover, afucosylation of CD19 mAb enhanced Fc-mediated antibody-dependent cellular cytotoxicity. Notably, ABBV-319 displayed superior efficacy compared with afucosylated CD19 mAb in human CD34+ peripheral blood mononuclear cell–engrafted NSG-Tg(Hu-IL15) transgenic mice, demonstrating enhanced antitumor activity when multiple MOAs are enabled. ABBV-319 also showed durable antitumor activity across multiple B-cell lymphoma PDX models, including nongerminal center B-cell diffuse large B-cell lymphoma and relapsed lymphoma after R-CHOP treatment. Collectively, these data support the ongoing evaluation of ABBV-319 in a phase 1 clinical trial.
Glucocorticoids and monoclonal antibodies are key components for the treatment of B-cell lymphoma. Chang and colleagues report on the preclinical evaluation of ABBV-319, a CD19-targeting antibody-drug conjugate that binds to and activates the glucocorticoid receptor. ABBV-319 also inhibits CD19 signaling and enhances antibody-dependent cytotoxicity in B-cell lymphoma patient-derived xenograft models and may provide an effective therapy that combines multiple mechanisms of antilymphoma action while avoiding the complications of systemic steroid therapy.
Introduction
Glucocorticoids exhibit clinical activity across B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia, follicular lymphoma (FL), and acute lymphoblastic leukemia (ALL).1 The antitumorigenic effects of glucocorticoids on lymphoma and leukemia were first discovered in clinical studies in the 1950s.2,3 Glucocorticoids have demonstrated single-agent and combinatorial antitumor activities with different chemotherapeutic agents.3,4 Glucocorticoids are incorporated into combination regimens for B-cell malignancies, such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), and hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine). Mechanistically, glucocorticoids act as an agonist for glucocorticoid receptors (GR) to facilitate the activation or repression of downstream transcription targets associated with cell cycle progression (eg, MYC and CCND3) and apoptosis genes (eg, BCL2L11 and BCL2), which subsequently result in cell cycle arrest and/or apoptosis of malignant B cells.5, 6, 7, 8 However, the administration of glucocorticoids systemically is associated with a broad range of potentially dose-limiting side effects, such as hyperglycemia, diabetes mellitus, osteoporosis, or psychosis,9 and these side effects limit the full therapeutic potential of glucocorticoids.
CD19 is a well-validated therapeutic target for B-cell malignancies including B-cell non-Hodgkin lymphoma (NHL) and leukemia.10 In normal development, CD19 expression is restricted to the B-cell lineage and its expression increases with B-cell maturation.11 CD19 is a coreceptor for the B-cell receptor (BCR) and the phosphorylation of its cytoplasmic domain mediates the recruitment of phosphoinositide 3-kinase (PI3K), generation of the secondary messenger phosphatidylinositol-3,4,5-triphosphate, and the activation of downstream signaling molecules including Bruton's tyrosine kinase (BTK), phosphatidylinositol-specific phospholipase Cγ2 (PLCγ2), protein kinase B (PKB/AKT), and mammalian target of rapamycin (mTOR).12,13 In B-cell lymphoma and leukemia, CD19 is overexpressed and its expression is often maintained in later lines, even after CD19-targeting therapies.14, 15, 16 Moreover, genetic perturbation studies have revealed that CD19 plays a critical role in the survival of Burkitt lymphoma (BL) and germinal center B-cell (GCB)–like DLBCL cells.17 Therefore, CD19 is an attractive therapeutic target for the development of biologics and immunotherapy.18
Here, we describe the preclinical characterization of ABBV-319, a CD19-targeting glucocorticoid receptor modulator (GRM) agonist, antibody-drug conjugate (ADC), for the treatment of B-cell malignancy. ABBV-319 elicits antitumor activity through 3 distinct mechanisms of action (MOA) that collectively contribute to robust antitumor activities across B-cell lymphoma models, including non-GCB DLBCL and patient-derived xenograft (PDX) samples from relapse/refractory lymphoma with poor clinical outcomes.19,20 ABBV-319 is currently being investigated in a clinical trial (NCT05512390) as a new therapeutic option for B-cell malignancy.
Methods
Antibodies and drug conjugates
CD19 monoclonal antibody (mAb) was discovered at AbbVie and the afucosylated (Af.) mAb was generated by heterologous expression of GDP-6-deoxy-D-lyxo-4-hexulose reductase in the Chinese hamster ovary cell line, which disrupts the formation of GDP-fucose, thus preventing the addition of fucose to the mAb.
The drug substance process conjugating the mAb and linker drug includes a phosphine-based redox reaction and a hydrophobic interaction chromatography purification that results in a mixed drug-to-antibody ratio (DAR) 2/4/6 distribution with a mean DAR of ∼4.0, followed by buffer exchange and formulation.
Compounds and formulation
GRM small molecule was synthesized at AbbVie. Dexamethasone (S1322, Selleck Chemicals) and prednisolone (P6004, Sigma-Aldrich) were purchased commercially. The small molecules were dissolved in dimethyl sulfoxide for in vitro studies and formulated in 0.05% hydroxypropyl methylcellulose and 0.02% Tween-80 in water for in vivo studies. Prednisolone sodium phosphate oral solution (44523-0182-08, BioComp Pharma) was used for in vivo benchmark studies. All antibodies and ADCs were formulated in an appropriate ADC buffer containing lyoprotectant.
Cell lines
All cell lines were obtained from the American Type Culture Collection or the Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Cultures) and cultured in growth media supplemented with either fetal bovine serum (F4135, Sigma-Aldrich) or human serum (H4522, Sigma-Aldrich) at 5% CO2 at 37°C. The growth media for each cell line are described in supplemental Table 1, available on the Blood website.
Detailed protocol for over-expression of CD19 and reporter cell line generation and assays are provided in the supplemental Methods.
In vitro cellular screen
Cells were seeded at 1000 cells per well in 384-well tissue culture plates (Corning) in a total volume of 25 μL of their respective culture media. The plates were dosed the following day with GRM payload, dexamethasone, prednisolone, and Af. isotype mAb, Af. CD19 mAb and ABBV-319 at 0.1 μM, 1 μM, 10 μM, 1 μM, 1 μM, and 1 μM top doses, respectively, using the ECHO Liquid Handler. Each drug was dosed as a 12-point dilution series with a threefold dilution between successive concentrations and as triplicates on the same plate per dosing session. Cell proliferation after 120 hours of drug treatment was determined using the Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) as per the manufacturer’s recommendation. The half maximal effective concentration (EC50) and % maximal effect (% Emax) for all drugs in all cell lines were relative to staurosporine using in-house analysis tools. The median EC50 and %Emax from all repeats were reported.
RNA-seq
RNA was isolated from frozen cell pellets using the Qiagen (Hilden, Germany) RNAeasy kit according to the manufacturer’s protocol. Whole-transcriptome sequencing (RNA sequencing [RNA-seq]) was performed using TruSeq Stranded Total RNA with the Illumina Ribo-Zero Plus rRNA Depletion kit according to the manufacturer’s protocol (Illumina, Inc, San Diego, CA). The analysis workflow is provided in the supplemental Methods.
Cellular indexing of transcriptomes and epitopes (CITE-seq)
Two million peripheral blood mononuclear cells (PBMCs) were treated with vehicle, 66 nM Af. CD19 mAb and 66 nM ABBV-319 for 24 hours in Iscove modified Dulbecco medium (12440053, Thermo Fisher Scientific) supplemented with 10% human serum (H4522, Sigma-Aldrich). Viable cells were washed and resuspended in a cell staining buffer (420201, BioLegend). After 100 μm strainer filtration, cells were blocked with fragment crystallizable (Fc) block (422302, BioLegend) in cell staining buffer and stained with TotalSeq Universal antibody cocktail (399904, BioLegend). The cells were washed and loaded into a 10x Chromium controller at 16 000 cells per sample. Single-cell encapsulation was processed according to the manufacturer’s protocol using 3’ chemistry version 3.1 (10x Genomics). Gene expression libraries were sequenced at a depth of 50 000 reads per cell and surface protein/antibody tag libraries at a depth of 5000 usable reads per cell on a NextSeq and NovaSeq sequencer (Illumina). The analysis workflow is provided in the in supplemental Methods.
Immunoblot
Cells were washed twice with ice-cold phosphate-buffered saline and lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (R0278, Sigma-Aldrich) supplemented with 1× Halt Protease and Phosphatase Inhibitor Cocktail (78446, Thermo Fisher Scientific). Protein concentrations of the lysates were measured using a bicinchoninic acid (BCA) Protein Assay Kit (23227, Thermo Fisher Scientific). Equal amounts of lysates (1-10 μg) were resolved on 4% to 12% gradient gels (NW04120BOX, Thermo Fisher Scientific) and transferred onto a polyvinylidene fluoride (PVDF) membrane (IB24001, Thermo Fisher Scientific). The membranes were incubated with primary and secondary antibodies conjugated with horseradish peroxidase. Enhanced chemiluminescent substrates (Thermo Fisher Scientific) were added and detected using Azure Image Systems C600. Details of immunoblot detection and antibodies used are provided in the supplemental Methods.
GRE reporter activation assay
A total of 50 000 glucocorticoid response element (GRE) luciferase reporter cells were treated with dose-titrated drugs in growth media containing 1% charcoal-stripped fetal bovine serum (12676-029, Thermo Fisher Scientific) until the indicated end point. Dual-Glo Luciferase Assay System (E2920, Promega) substrate and buffer were added for 10 minutes and analyzed for luminescence using MicroBeta (PerkinElmer).
In vitro antibody-dependent cellular cytotoxicity (ADCC) assays
The ADCC Reporter Bioassay (G7010 and G9790, Promega) was performed according to the manufacturer's protocol. The target lymphoma cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) (C34554, Thermo Fisher Scientific) and then opsonized with an antibody or ADC. PBMCs from healthy donors were added to the indicated effector-to-target ratios. After 4-hour incubation, the cells were stained with the Live/Dead Fixable Violet Dead Cell Stain Kit (L34955, Thermo Fisher Scientific) and fixed with 4% paraformaldehyde. The fixed cells were analyzed using a Stratedigm S1000EON flow cytometer. The percentage of specific lysis was calculated by subtracting the percentage of dead target cells (percentage violet dye-positive in CFSE+ cells) in each treated condition from the untreated control containing only the effector and target cells.
In vivo efficacy study
Cell line-derived xenograft (CDX) studies were conducted in-house, whereas DLBCL PDX studies were conducted at the WuXi AppTec (Suzhou, China). Cell lines were inoculated into the flank of female CB17 severe combined immunodeficiency (SCID) or SCID beige mice at 6 to 8 weeks of age (Charles River Laboratories) or CD34+ PBMC–engrafted NSG-Tg(Hu-IL15) mice at 18 to 21 weeks of age (The Jackson Laboratory) with a 1:1 mixture of minimum essential medium Eagle (S-MEM) or Hanks' balanced salt solution (HBSS) (Thermo Fisher Scientific, Waltham, MA) and Matrigel (BD, Franklin Lakes, NJ). DLBCL PDX tumors (30 mm3 tumor slices) were inoculated into the flank of 6- to 8-week-old NOD-SCID mice. DLBCL tumor subtypes (GCB or non-GCB) were determined by immunohistochemical staining for CD10, BCL6, and MUM1.20 Tumors were size-matched at ∼80 to 200 mm3, and the small molecules and biologics (antibody and ADC) were dosed via oral and intraperitoneal route of administration, respectively. Tail vein bleeds were taken for pharmacokinetics (PK) analysis (supplemental Methods). Measurements of length (L) and width (W) of the tumor were obtained via electronic calipers and the volume (V) was calculated according to the following equation: V = (L × W2)/2. Mice were euthanized when the tumor volume reached a maximum of 2000 mm3 or if animal health was compromised, per institutional guidelines.
Percentage tumor growth inhibition (%TGI), percentage tumor volume change, and percentage tumor growth delay (%TGD) were determined with the equations below:
Δ %TGI max was determined when the difference between the treatment and control groups was maximal.
Day X is when vehicle-treated tumors reached 1000 mm3.
TTEt and TTEc are the median time periods of treated and control groups to reach tumor volumes of (1 cm3) following the onset of treatment.
All experiments were conducted in compliance with AbbVie's institutional animal care and use committee and the National Institutes of Health Guide for Care and Use of Laboratory Animals guidelines.
Results
Generation and characterization of ABBV-319
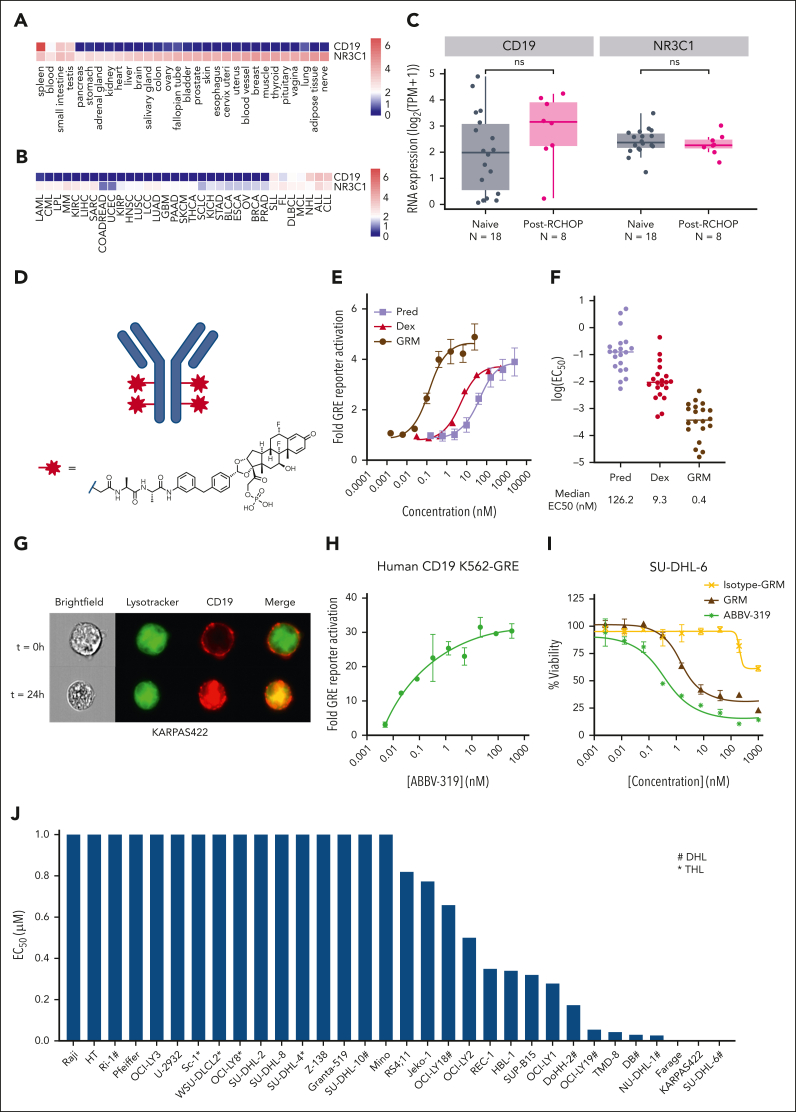
Transcriptomic analysis showed that CD19 expression is restricted to a few normal tissues (eg, spleen and blood), whereas NR3C1, a gene encoding GR, is expressed ubiquitously (Figure 1A). In malignant settings, CD19 is predominantly expressed in B-cell malignancies, such as FL, DLBCL, chronic lymphocytic leukemia, and mantle cell lymphoma (Figure 1B), whereas NR3C1 is also expressed across B-cell malignancies. Importantly, both CD19 and NR3C1 expressions were maintained in patients with DLBCL after R-CHOP treatment (Figure 1C).
Figure 1.
Characterization of ABBV-319. (A) Analysis of CD19 and NR3C1 gene expression in normal tissues using GTEx data sets. (B) Analysis of CD19 and NR3C1 gene expression across different cancer indications using Aster ORIEN data sets. (C) Analysis of CD19 and NR3C1 gene expression in patients that are treatment naïve or post–R-CHOP treatment using Aster ORIEN data sets. Statistical analysis with Wilcoxon test. ns, not significant. (D) Structure of the GRM linker drug. (E) Fold change in GRE activity compared with the untreated control after treatment of K562 GRE reporter cells with prednisolone, dexamethasone, and GRM payload for 24 hours. Mean ± standard error of the mean (SEM) are depicted. (F) Summary of EC50 for prednisolone, dexamethasone, and GRM payload across 20 glucocorticoid-sensitive cell lines. Each dot represents the log(EC50) of a cell line and the median log(EC50) is displayed. (G) Imaging analysis of CD19 localization after treatment of KARPAS422 with an Alexa Fluor 647-labeled ABBV-319 for the indicated time. Brightfield, LysoTracker (green), CD19 (red), and merged images are displayed. (H) The fold change in GRE activity relative to the untreated control after treating K562-GRE reporter cells with ABBV-319 for 24 hours. (I) Percentage viability of SU-DHL-6 cells relative to the untreated control after treatment with GRM payload, isotype-GRM ADC, and ABBV-319 for 5 days. (J) EC50 of ABBV-319 across a panel of malignant B-cell lines with a range of Emax from supplemental Table 2. # denotes double-hit lymphoma (DHL) and ∗ denotes triple-hit lymphoma (THL) based on published annotations.21
ABBV-319 comprises a GRM payload conjugated to an Af. CD19 antibody via an alanine-alanine protease-cleavable dipeptide linker (Figure 1D). The GRM payload was more potent than clinical glucocorticoids (dexamethasone and prednisolone) in a GRE reporter and in vitro cell proliferation assays in a panel of glucocorticoid-sensitive malignant B-cell lines (Figure 1E-F). By comparing the median EC50, the GRM payload was 25 and >300 times more potent compared with dexamethasone and prednisolone, respectively.
Af. CD19 mAb and ABBV-319 were cross-reactive with both human and cynomolgus monkey CD19 (supplemental Figure 1A-E). Twenty-four-hour treatment of ABBV-319 on KARPAS422 cells resulted in CD19 internalization and lysosomal trafficking, as shown by the colocalization of CD19 and Lysotracker (Figure 1G). Furthermore, there was dose-dependent activation of GRE reporter activity after ABBV-319 treatment on K562 cells (Figure 1H; supplemental Figure 1D-E), which confirmed the subsequent release of GRM payload in the lysosome, followed by induction of GR transcription. ABBV-319 treatment elicited dose-dependent cytotoxicity that was dependent on CD19, as isotype-GRM ADC showed a significant reduction in activity in SU-DHL-6 (Figure 1I). In the screening of a panel of B-cell malignant cell lines, ABBV-319 showed potent antiproliferative activity in cell lines across a range of indications, including DLBCL, mantle cell lymphoma, FL, and ALL (Figure 1J; supplemental Table 2). There was not a significant association between ABBV-319 sensitivity and the expression of CD19 or GR (supplemental Figure 2A-D). B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement are known as double-hit lymphoma (DHL) and triple-hit lymphoma, respectively.22 DHL and triple-hit lymphoma are considered high-grade B-cell lymphoma with inferior survival outcomes when treated with R-CHOP.22, 23, 24 Notably, several DHL cell lines (DB, OCI-LY19, DoHH-2, and OCI-LY18) with MYC plus BCL2 or BCL6 rearrangements were still responsive to ABBV-319 (Figure 1J).
ABBV-319 engaged and activated GR in DLBCL cell lines
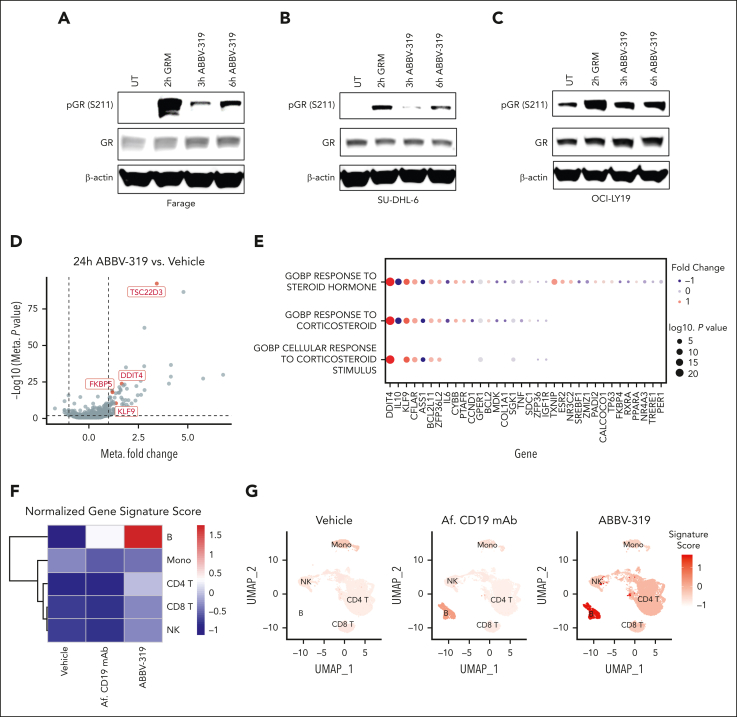
We carried out a pharmacodynamic analysis to evaluate the ability of the GRM payload to engage endogenous GR in malignant B cells. GR is phosphorylated on serine 211 in response to glucocorticoid treatment and this phosphorylation event is required for GR transcriptional activity.25,26 Both GRM payload and ABBV-319 treatment elicited a marked increase in serine 211 phosphorylation on GR in Farage, SU-DHL-6, and OCI-LY19 (Figure 2A-C).
Figure 2.
ABBV-319 engages and activates GR in DLBCL cell lines. (A-C) Immunoblot analysis of GR phosphorylation on serine 211 (S211) and GR expression after treatment with 10 nM GRM and 100 nM ABBV-319 for indicated time in Farage (A), SU-DHL-6 (B), and OCI-LY19 (C). β-actin was used as the loading control. (D) Volcano plot showing the fold change and P value from the meta-analysis of differentially expressed genes (DEGs) between 24-hour ABBV-319 treatment and vehicle control in GRM-sensitive cell lines. Each dot represents a DEG and the selected known GR targets are highlighted in red. (E) Pathways and genes enriched in the meta-analysis of DEGs between the ABBV-319 and vehicle treatment. The color represents the directionality of the fold change, and the size of the circle represents the log(P value). (F) Heat map showing the expression of the 8-gene glucocorticoid gene signature in different immune subsets in PMBC after 24 hours of indicated treatment. (G) Uniform manifold approximation and projection (UMAP) of immune cells within PBMC after the indicated treatment for 24 hours. Color indicates the expression of the 8-gene glucocorticoid gene signature.
We also performed RNA-seq analysis to characterize the transcriptomic changes in GRM-sensitive cell lines (OCI-LY19, DoHH2, Farage, Pfeiffer, SU-DHL6, OCI-LY3, TMD-8, and U-2932) after ABBV-319 treatment. Compared with the vehicle control, ABBV-319 treatment elevated the expression of several reported GR targets (eg, TSC22D3, DDIT4, FKBP5, and KLF9) (Figure 2D). Moreover, a meta-analysis of the differential expressed genes between ABBV-319 treatment and vehicle control revealed enrichment of gene sets related to steroid/corticosteroid response (Figure 2E).
To investigate the specificity of ABBV-319, we carried out CITE-seq experiments by treating PMBCs with vehicle, Af. CD19 mAb and ABBV-319. We used a published 8-gene glucocorticoid gene signature27 to evaluate the pharmacodynamic effects of ABBV-319 on PBMC. ABBV-319 treatment for 24 hours resulted in the most prominent activation of glucocorticoid gene signatures in the B-cell population (Figure 2F-G). Other immune subsets (T, NK, and monocytes) displayed minimal glucocorticoid gene signature activation compared with the B cells. Collectively, our data demonstrated that ABBV-319 can specifically deliver GRM payload to CD19+ B cells and release GRM payloads to activate GR transcriptional activity.
ABBV-319 inhibited prosurvival signaling and induces apoptotic cell death
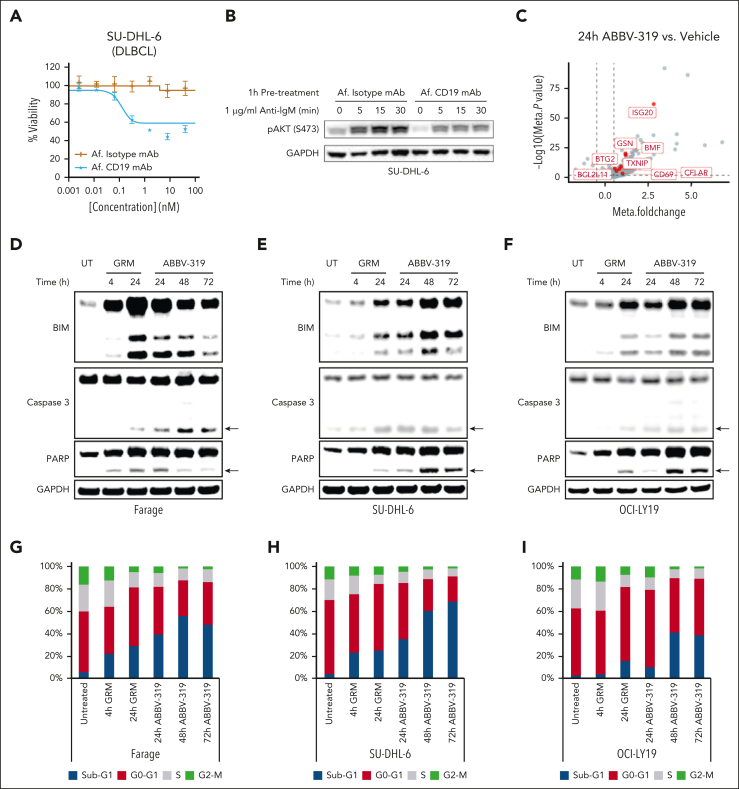
In our in vitro screening, some B-cell NHL cell lines (OCI-LY2, TMD-8, Jeko-1, JVM-2, and SU-DHL-6) were sensitive to the unconjugated CD19 mAb (Af. CD19 mAb) treatment (Figure 3A; supplemental Table 2). We hypothesize that Af. CD19 mAb could block BCR–mediated PI3K activation. Indeed, Af. CD19 mAb pretreatment blunted anti-IgM–stimulated phosphorylation on AKT (S473) (Figure 3B), suggesting that the unconjugated CD19 mAb can block BCR–mediated PI3K prosurvival signaling. Notably, Af. CD19 mAb–mediated signaling effects were not observed in the nonresponsive cell line SU-DHL-4 (supplemental Figure 3A).
Figure 3.
ABBV-319 inhibits prosurvival signaling and induces apoptotic cell death in DLBCL. (A) The percentage viability relative to the untreated control after treatment of SU-DHL-6 cells with Af. isotype mAb and Af. CD19 mAb. Mean ± SEM is displayed. (B) SU-DHL-6 cells were pretreated with 100 nM Af. Isotype mAb or Af. CD19 mAb for an hour and then stimulated with 1 μg/ml anti-immunoglobulin M (anti-IgM) for the indicated time. Cell lysates were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblot analysis for phospho-AKT (Ser473) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are displayed. GAPDH is used as a loading control. (C) Volcano plot showing the fold change and P value from the meta-analysis of DEGs between 24-hour ABBV-319 treatment and vehicle control in ABBV-319-sensitive cell lines. Each dot represents a DEG and the genes involved in apoptosis are highlighted in red. (D-F) Immunoblot analysis of BIM, caspase 3, PARP, and GAPDH after treating Farage (D), SU-DHL-6 (E), and OCI-LY19 (F) with 10 nM GRM payload and 100 nM ABBV-319 for the indicated time. Arrows show the cleaved product of caspase 3 and PARP. (G-I) Cell cycle analysis of Farage (G), SU-DHL-6 (H), and OCI-LY19 (I) after treatment with 10 nM GRM and 100 nM ABBV-319 for the indicated time. The percentage of cells from sub-G1, G0-G1, S, and G2-M phases of the cell cycle are displayed.
In the meta-analysis of RNA-seq data, we found that ABBV-319 elevated the expression of genes involved in apoptosis, such as BCL2L11, BMF, and TXNIP (Figure 3C). BCL2L11 encodes Bcl-2 Interacting Mediator of cell death (BIM) and has previously been shown to be involved in glucocorticoid-induced apoptosis.7 Consistent with published reports, BIM expression was upregulated by both GRM and ABBV-319 treatment in Farage, SU-DHL-6, and OCI-LY19 (Figure 3D-F). Notably, GRM and ABBV-319 treatment led to an increase in all 3 BIM splice isoforms (BIMEL, BIML, and BIMS) that have been shown to be involved in apoptosis.28 The increase in BIM expression also correlated with the increase in cleaved caspase 3, cleaved PARP, and sub-G1 population (dead cells) from the western blot and flow cytometric analysis (Figure 3D-I). Importantly, these GRM- and ABBV-319–driven apoptotic and cytotoxic effects were specific to the responsive, but not resistant, cell lines (supplemental Figure 3B-C).
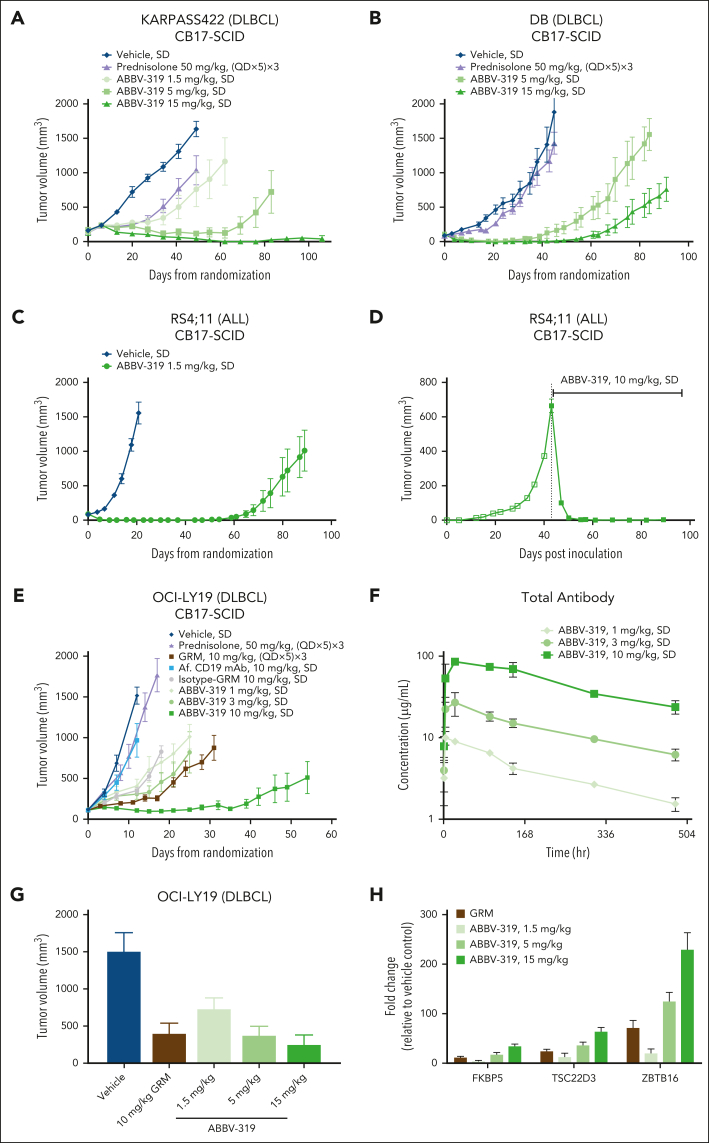
ABBV-319 elicited potent and durable antitumor activity in vivo
In multiple DLBCL and ALL CDX, a single dose of ABBV-319 induced dose-dependent tumor regression and durable tumor control (Figure 4A-C,E; supplemental Figure 4A-C). In the RS4;11 tumor model in which tumors were allowed to grow to large sizes (>600 mm3), a single dose of ABBV-319 at 10 mg/kg resulted in durable tumor regression for >40 days (Figure 4D), suggesting that ABBV-319 is efficacious in settings that are typically difficult to treat. Remarkably, the antitumor activity of a single dose of ABBV-319 was superior to that of multiple doses (n = 15) of prednisolone at 50 mg/kg (maximum tolerated dose) or multiple doses of GRM (n = 15) at 10 mg/kg (Figure 4A-B,E; supplemental Figure 4C).
Figure 4.
ABBV-319 elicits potent and durable antitumor activity in CDX models. (A-E) Growth of xenografted KARPAS422 (A), DB (B), RS4;11 (C-D), and OCI-LY19 (E) tumors after the indicated treatment regimen. Drug treatments were initiated within 24 hours after tumor size matching and randomization (A-C,E), whereas the large RS4;11 tumor (D) was dosed at day 43 after inoculation. Means ± SEM of tumor volumes were plotted for each treatment group vs days from randomization or days after inoculation. (F) Total antibody detected in mouse whole blood from the OCI-LY19 study (E). Means ± SEM are shown. (G) Volume of xenografted OCI-LY19 tumors after indicated treatment for 7 days. GRM was dosed at (QD × 5) × 3, whereas ABBV-319 was dosed as a SD. Means ± SEM of tumor volumes were plotted for each treatment group. (H) Quantitative reverse transcription polymerase chain reaction analysis of FKBP5, TSC22D3, and ZBTB16 expression in tumors after the indicated treatments. Means ± SEM of fold change relative to the vehicle control are displayed. QD, once daily; SD, single dose.
The isotype-GRM ADC, the nontargeting control, displayed marginal antitumor activity at 10 mg/kg, but to a much lesser extent than the antitumor activity observed with ABBV-319 dosed at 10 mg/kg, suggesting a CD19-dependent delivery of GRM to the tumor (Figure 4E; supplemental Figure 4C). Hematological tumor models show higher levels of Fc receptors, which could potentially result in nonspecific uptake of isotype-GRM ADC.
There was a dose-dependent increase in the total antibody concentration and area under the curve, suggesting that ABBV-319 exhibits linear pharmacokinetics in mice (Figure 4F; supplemental Figure 4D). Importantly, the increase in total antibody serum concentration correlated with the antitumor activities of ABBV-319. Moreover, tumor control in OCI-LY19 tumors correlated with the induction of selected GR targets, including FKBP5, TSC22D3, and ZBTB16 (Figure 4G-H), demonstrating the on-target pharmacodynamic effects of GRM in vivo.
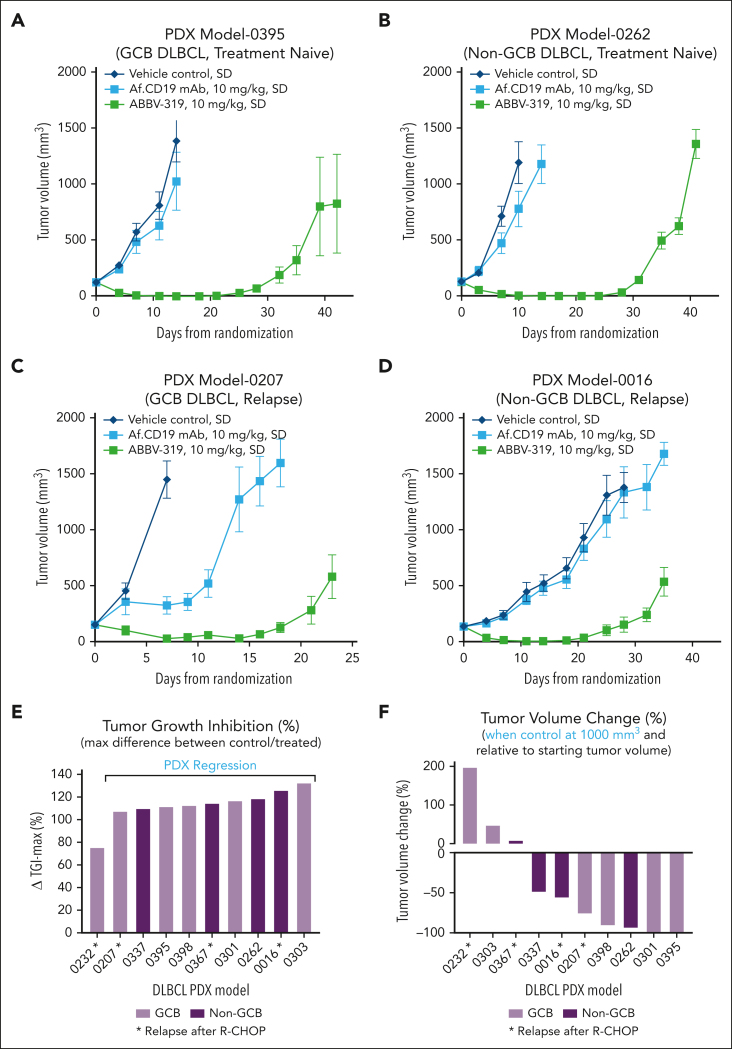
ABBV-319 was tested in DLBCL PDXs to examine its activity in clinically relevant B-cell NHL models. A single dose of ABBV-319 at 10 mg/kg elicited tumor regression in PDXs that were treatment naïve or relapsed after 4 to 7 rounds of R-CHOP treatment (Figure 5A-F). In the PDX study, ABBV-319 elicited tumor growth inhibition compared with the vehicle control in 10 of 10 PDXs and regression in 9 of 10 PDXs (Figure 5E). Robust tumor regression induced by ABBV-319 was observed in 7 of 10 PDXs when vehicle-treated tumors reached 1000 mm3 (Figure 5F). Similar to our in vitro cell line observations, Af. CD19 mAb treatment resulted in tumor growth inhibition in 1 of the 10 DLBCL PDX (Figure 5C).
Figure 5.
ABBV-319 exhibits antitumor activity in non-GCB DLBCL and relapsed DLBCL PDX models. (A-D) Growth of xenografted PDX models 0395 (A), 0262 (B), 0207 (C), and 0016 (D) in NOD-SCID mice after the indicated treatment regimen. Means ± SEM of tumor volumes were plotted for each treatment group vs days from randomization. (A) and 0262 (B) were treatment naïve whereas PDX models 0207 (C) and 0016 (D) were from relapsed disease after the 4 R-CHOP treatments. GCB and non-GCB subtyping were determined via immunohistochemistry methods, as described in “Methods.” (E) Maximal percentage tumor growth inhibition relative to the vehicle control in each PDX model is displayed. Models showing tumor regression after ABBV-319 treatment are shown in the figure. (F) Percentage tumor volume change relative to the starting tumor volume when the vehicle control reaches 1000 mm3. GCB and non-GCB DLBCL are shown as different colors. ∗Denotes PDX samples from patients with relapsed disease after R-CHOP treatment.
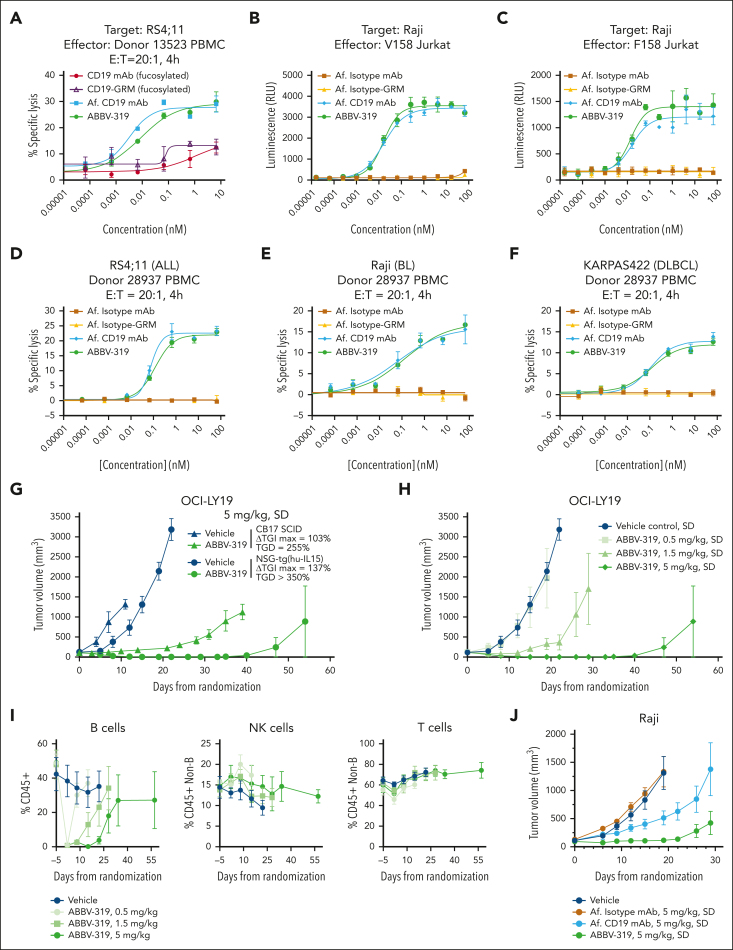
ABBV-319 induced ADCC in vitro and in vivo
Therapeutic antibodies can elicit antitumor activity via Fc-mediated effector functions, such as ADCC, antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC).29 We first assessed the ability of ABBV-319 to engage in ADCP and CDC in vitro. The treatment with unconjugated Af. CD19 mAb and ABBV-319 resulted in an increase in macrophage-mediated ADCP in Raji and NU-DHL-1 cells (supplemental Figure 5A-C). Consistent with the literature report,30 rituximab induced CDC in response to the addition of complements in Raji, Ramos, and SU-DHL-6 (supplemental Figure 5D-F). However, neither Af. CD19 mAb nor ABBV-319 showed CDC activity in these experiments. These data therefore demonstrated that ABBV-319 can engage ADCP but not CDC in vitro.
The antibody backbone of ABBV-319 was Af. to increase the Fc-mediated effector function. Af. CD19 mAb and ABBV-319 induced higher specific lysis of tumor cells than their fucosylated counterparts when cocultured with PBMC (Figure 6A). ABBV-319, and Af. CD19 mAb, also activated NFAT reporter activity with similar potency in Jurkat reporter cells expressing V158 (high affinity) and F158 (low affinity) Fc gamma receptor IIIa (FcγRIIIa) (Figure 6B-C). Moreover, ABBV-319 elicited potent specific lysis of B-cell lymphoma (ALL, BL, and DLBCL) cell lines cocultured with PBMC (Figure 6D-F). Notably, FcγRIIIa binding and the ADCC activity of ABBV-319 were comparable to the Af. CD19 mAb, indicating that the conjugation of GRM payloads at DAR4 did not negatively impact ADCC activity.
Figure 6.
ABBV-319 induces ADCC in vitro and in vivo. (A) Percentage specific lysis of RS4;11 cells in coculture with PBMC at an effector-to-target (E:T) ratio of 20:1 after 4-hour treatment with the indicated agents. Mean ± SEM are displayed. (B-C) Luciferase reporter activation in Jurkat cells expressing V158 (B) and F158 (C) FcγRIIIa after treatment with the indicated agents for 4 and 16 hours, respectively. Mean ± SEM are displayed. (D-F) Percentage specific lysis of RS4;11 (D), Raji (E), and KARPAS422 (F) in coculturing with PBMC at an E:T ratio of 20:1 after treatment with the indicated agent for 4 hours. Mean ± SEM are displayed. (G) Growth of OCI-LY19 tumors in CB17 SCID or CD34+ PBMC–engrafted NSG-Tg(Hu-IL15) mice after treatment with vehicle or a SD of ABBV-319 at 5 mg/kg. Mean ± SEM of tumor volumes were plotted for each treatment group vs days from randomization. ΔTGImax and TGD1000 were calculated as described in the Methods. (H) Growth of OCI-LY19 tumors in CD34+ PBMC–engrafted NSG-Tg(Hu-IL15) mice after treatment with vehicle or a SD of ABBV-319 at 0.5, 1.5, and 5 mg/kg. Means ± SEM of tumor volumes were plotted for each treatment group vs days from randomization. (I) Flow cytometric immunophenotyping analysis of tail vein bleeds from OCI-LY19 tumor-bearing mice (H). B cells are presented as the percentage of CD45+ cells, whereas T and NK cells are presented as percentages of CD45+ cells without B cells. Details of the immunophenotyping methods are in supplemental Methods. (J) Growth of Raji tumors in CD34+ PMBC–engrafted NSG-Tg(Hu-IL15) mice after the indicated treatment regimen. Means ± SEM of tumor volumes were plotted for each treatment group vs days from randomization.
Due to the differences in human and mouse FcɣR network, humanized mouse models have been developed to better model human NK cell biology.31, 32, 33 In particular, NSG-Tg(Hu-IL15) is a humanized NSG mouse that is engineered to express human interleukin 15 at physiological levels, which enables differentiation and development of functional human NK cells after CD34+ hematopoietic stem cell engraftment.33 A single dose of ABBV-319 at 5 mg/kg elicited deeper tumor growth inhibition and more durable antitumor activity in CD34+ PBMC-engrafted NSG-Tg(hu-IL15) compared with CB17 SCID mice (Figure 6G). The antitumor effects of ABBV-319 were dose-dependent and durable tumor regression was observed at the 5 mg/kg dose in OCI-LY19 CDX in NSG-Tg(hu-IL15) (Figure 6H). Notably, flow cytometric immunophenotyping of human cells in the periphery showed that ABBV-319 specifically depleted normal human B but not NK or T cells (Figure 6I). The depletion of normal human B cells is transient, and peripheral B-cell counts rebound over time. ABBV-319 also elicited superior antitumor efficacy compared with Af. CD19 mAb in the Raji CDX model in NSG-Tg(hu-IL15) when both agents were dosed at 5 mg/kg (Figure 6J). Collectively, these data demonstrate the robust ADCC activity of ABBV-319 and support additive, or synergistic, antitumor activities from the combination of different MOAs.
Discussion
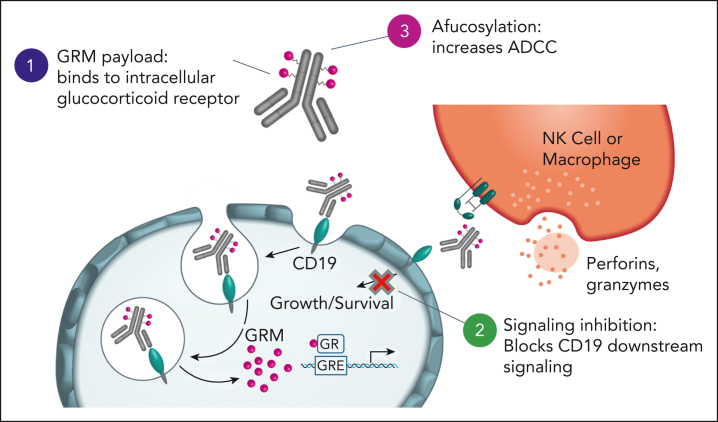
To our knowledge, this is the first study demonstrating the therapeutic potential of GRM ADC in oncology. Our study demonstrated that ABBV-319 consists of 3 distinct MOAs that drive antitumor activity in B-cell malignancy (Figure 7): (1) delivery of GRM payload via CD19 to activate apoptotic cell death, (2) CD19 downstream signaling inhibition, and (3) enhanced ADCC from afucosylation of the antibody backbone.
Figure 7.
ABBV-319 elicits antitumor effects through 3 distinct MOA. ABBV-319 results in CD19 internalization and lysosomal trafficking to release GRM payload. GRM payload drives the transcriptional activation of GR targets (eg, BCL2L11) to activate apoptotic cell death. ABBV-319 also blocks CD19-mediated activation of the PI3K pathway. Lastly, afucosylation of the Fc region enhances ADCC driven by effector cells.
The GRM-mediated antitumor activity of ABBV-319 was demonstrated in vitro and in immune-compromised mice, in which Af. CD19 mAb showed modest efficacy. This is further supported by pharmacodynamic analysis showing GR engagement and target gene induction after ABBV-319 treatment. Remarkably, a single dose of ABBV-319 imparts superior antitumor efficacy compared with repeated daily dosing of prednisolone at its maximum tolerated dose in vivo (Figure 4A-B,E; supplemental Figure 4C). The striking improvement in efficacy is likely attributed to the targeted delivery of a potency-enhanced GRM payload to the CD19+ malignant cells, as well as the PK and safety benefits of improved ADC exposure and lower systemic GRM payload levels relative to the small-molecule prednisolone. There was not a significant correlation between ABBV-319 sensitivity and CD19 or GR expression in vitro. There are conflicting reports on the correlation of CD19 ADC sensitivity to antigen expression, which may be influenced by the affinity of the CD19 antibody, potency of the payload, and uptake/processing of the ADC.34,35 ABBV-319 consists of multiple MOAs that are difficult to model simultaneously in vitro and in mouse model systems. Thus, future studies are needed to further define the predictive biomarkers for ABBV-319 using clinical data sets.
A recent functional genomic study reported that CD19 is essential for the survival of BL and GCB DLBCL cell lines.17 In part, this essentiality is driven by BCR-mediated phosphorylation of the YXXM motif on CD19, which can result in the recruitment and activation of prosurvival PI3K signaling.13 Our data demonstrated that the Af. CD19 mAb is capable of blocking BCR–mediated PI3K activation (Figure 3B). However, the antiproliferative activity evoked by Af. CD19 mAb was not restricted to BL or GCB DLBCL, as shown in functional genomic studies.17 It is conceivable that the inhibitory effects of the mAb could be partially compared with the complete deletion of the target. Future studies are needed to better understand the biomarkers that could predict the response to the CD19 antibody–mediated antitumor mechanism within ABBV-319.
Glucocorticoids combine well with chemotherapy and therapeutic antibodies because of their synergistic efficacy and manageable toxicity.1 Glucocorticoids are part of the standard-of-care chemoimmunotherapy regimens (ie, R-CHOP, hyper-CVAD, or EPOCH-R), and it is anticipated that ABBV-319 could be a viable combination partner in B-cell malignancies. ABBV-319 showed superior antitumor activity compared with that of the unconjugated Af. CD19 mAb in the immune-competent NSG-Tg(Hu-IL15) model (Figure 6J), demonstrating that different MOAs of ABBV-319 (GRM payload activity, CD19 inhibition, and ADCC) combined optimally to enhance its antitumor activity in vivo. This was likely due to the ability of the GRM payload to induce apoptosis, as mitochondrial apoptosis has been shown to be associated with NK-mediated killing of tumor cells.36 Contrary to published reports with systemic glucocorticoids,37, 38, 39 total NK cell numbers were not impacted by ABBV-319 treatment in the NSG-Tg(Hu-IL15) model, demonstrating that ABBV-319 treatment did not negatively impact NK cell health.
ABBV-319 displayed remarkable efficacy in clinically relevant B-cell malignancy models. ABBV-319 treatment led to tumor regression and durable tumor control in both non-GCB and GCB DLBCL PDXs, with all models showing sensitivity to ADC (Figure 5E-F). Non-GCB DLBCL represents a more aggressive disease, as it has worse 5-year overall survival than GCB DLBCL.20 Furthermore, PDXs with relapsed disease after R-CHOP treatment were still responsive to ABBV-319, suggesting a potential role for ABBV-319 in the relapse/refractory setting. Collectively, these positive preclinical data support the ongoing evaluation of ABBV-319 safety, tolerability, and preliminary activity in a phase 1 clinical trial (NCT05512390).
Conflict-of-interest disclosure: C.A.C., E.E., A.L.D., W.Z., C.C., K.P., D.M., E.P., A.O., P.E., L.R., R.D., C.H., L.L., W.A., P.Z., W.L., A.H.J., K.M., Z.Z., G.R., Z.C., Y.L., J.C., G.Z., T.C., M.B., C.C.M., A.H., M. McPherson, T.U., M.A.P., X.Z., J.H., M. McDevitt, K.J.F., S.M.-L., and J.W.P. are employees of AbbVie. O.P. and A.B. were employees of AbbVie at the time of the study. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of the data, review, and approval of the manuscript.
Acknowledgments
The authors thank John Silva, Isabella Sturdevant and Mandy Meng-Shan Wu of AbbVie for their assistance on the in vivo pharmacology figures.
Authorship
Contribution: C.A.C., E.E., A.L.D., W.Z., T.U., and J.W.P. contributed to the conception and design; C.A.C., A.L.D., C.C., D.M., E.P., A.O., P.E., L.R., C.H., L.L., W.A., P.Z., A.H.J., K.M., Z.Z., G.R., Z.C., O.P., and J.C. contributed to the acquisition of the data; C.A.C., E.E., A.L.D., W.Z., C.C., D.M., R.D., A.H.J., K.M., T.U., and J.W.P. contributed to the analysis and interpretation of data (eg, statistical analysis, biostatistics, and computational analysis); C.A.C. wrote the manuscript; and all authors reviewed the manuscript before submission.
Footnotes
The RNA sequencing and CITE-seq data are available in Gene Expression Omnibus database under accession numbers GSE249023 and GSE249543, respectively.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
Chewei Anderson Chang, Email: anderson.chang@abbvie.com.
James W. Purcell, Email: james.purcell@abbvie.com.
Supplementary Material
References
- 1.Livingston RB, Carter SK. 1st ed. Springer; 1970. Single Agents in Cancer Chemotherapy. [Google Scholar]
- 2.Pearson OH, Eliel LP. Use of pituitary adrenocorticotropic hormone (ACTH) and cortisone in lymphomas and leukemias. J Am Med Assoc. 1950;144(16):1349–1353. doi: 10.1001/jama.1950.02920160023005. [DOI] [PubMed] [Google Scholar]
- 3.Kofman S, Perlia CP, Boesen E, Eisenstein R, Taylor SG., 3rd The role of corticosteroids in the treatment of malignant lymphomas. Cancer. 1962;15:338–345. doi: 10.1002/1097-0142(196203/04)15:2<338::aid-cncr2820150217>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328(14):1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 5.Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol. 2017;18(3):159–174. doi: 10.1038/nrm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pufall MA. Glucocorticoids and Cancer. Adv Exp Med Biol. 2015;872:315–333. doi: 10.1007/978-1-4939-2895-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploner C, Rainer J, Niederegger H, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22(2):370–377. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee K, Bresnahan W, Hirai A, Hirai M, Thompson EA. c-Myc and cyclin D3 (CcnD3) genes are independent targets for glucocorticoid inhibition of lymphoid cell proliferation. Cancer Res. 1995;55(18):4188–4195. [PubMed] [Google Scholar]
- 9.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 10.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5-6):385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 11.Tedder TF, Inaoki M, Sato S. The CD19–CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6(2):107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 12.Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148–167. doi: 10.1038/nrc.2017.121. [DOI] [PubMed] [Google Scholar]
- 13.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260(5110):986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Yamaguchi M, Nakamura S, et al. Clinicopathologic significance of loss of CD19 expression in diffuse large B-cell lymphoma. Int J Hematol. 2007;85(1):41–48. doi: 10.1532/IJH97.06148. [DOI] [PubMed] [Google Scholar]
- 15.Johnson NA, Boyle M, Bashashati A, et al. Diffuse large B-cell lymphoma: reduced CD20 expression is associated with an inferior survival. Blood. 2009;113(16):3773–3780. doi: 10.1182/blood-2008-09-177469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Sun Q, Liang X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 2019;10:2664. doi: 10.3389/fimmu.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560(7718):387–391. doi: 10.1038/s41586-018-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer O. CD19 as an attractive target for antibody-based therapy. mAbs. 2012;4(5):571–577. doi: 10.4161/mabs.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi: 10.1056/NEJMra2027612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 21.Drexler HG, Eberth S, Nagel S, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for double-hit B-cell lymphomas. Leuk Lymphoma. 2016;57(5):1015–1020. doi: 10.3109/10428194.2015.1108414. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31(2):37–42. doi: 10.1016/j.blre.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114(11):2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation "code" of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277(29):26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- 26.Miller AL, Webb MS, Copik AJ, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19(6):1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Carman JA, Holloway D, et al. Development of a molecular signature to monitor pharmacodynamic responses mediated by in vivo administration of glucocorticoids. Arthritis Rheumatol. 2018;70(8):1331–1342. doi: 10.1002/art.40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goydel RS, Rader C. Antibody-based cancer therapy. Oncogene. 2021;40(21):3655–3664. doi: 10.1038/s41388-021-01811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res. 2006;12(13):4027–4035. doi: 10.1158/1078-0432.CCR-06-0066. [DOI] [PubMed] [Google Scholar]
- 31.Casey E, Bournazos S, Mo G, et al. A new mouse expressing human Fcgamma receptors to better predict therapeutic efficacy of human anti-cancer antibodies. Leukemia. 2018;32(2):547–549. doi: 10.1038/leu.2017.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 33.Aryee KE, Burzenski LM, Yao LC, et al. Enhanced development of functional human NK cells in NOD-scid-IL2rg(null) mice expressing human IL15. FASEB J. 2022;36(9) doi: 10.1096/fj.202200045R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan MC, Palanca-Wessels MC, Schimpf B, et al. Therapeutic potential of SGN-CD19B, a PBD-based anti-CD19 drug conjugate, for treatment of B-cell malignancies. Blood. 2017;130(18):2018–2026. doi: 10.1182/blood-2017-04-779389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zammarchi F, Corbett S, Adams L, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131(10):1094–1105. doi: 10.1182/blood-2017-10-813493. [DOI] [PubMed] [Google Scholar]
- 36.Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185(9):1521–1538.e18. doi: 10.1016/j.cell.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100(5):1765–1773. [PubMed] [Google Scholar]
- 38.Gatti G, Cavallo R, Sartori ML, et al. Inhibition by cortisol of human natural killer (NK) cell activity. J Steroid Biochem. 1987;26(1):49–58. doi: 10.1016/0022-4731(87)90030-6. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen BK, Beyer JM. Characterization of the in vitro effects of glucocorticosteroids on NK cell activity. Allergy. 1986;41(3):220–224. doi: 10.1111/j.1398-9995.1986.tb00303.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.