Key Points
Question
Does use of a wheelchair plus an exoskeleton for 4 months in the home and community environments compared with use of a wheelchair only improve patient-reported mental and physical health outcomes for veterans with spinal cord injury?
Findings
In this randomized clinical trial of 161 veterans with paralysis, no significant differences were found in mental or physical health for those using a wheelchair plus an exoskeleton vs those using a wheelchair only. The exoskeleton group reported low use of the device.
Meaning
Personal use of an exoskeleton in the home and community did not result in improved patient-reported outcomes in veterans with paralysis; safe device modifications to reduce companion requirements are needed to increase personal use.
This randomized clinical trial examines whether the use of an exoskeletal-assisted walking device improves patient-reported mental and physical health outcomes among veterans with paralysis.
Abstract
Importance
Robotic exoskeletons leverage technology that assists people with spinal cord injury (SCI) to walk. The efficacy of home and community exoskeletal use has not been studied in a randomized clinical trial (RCT).
Objective
To examine whether use of a wheelchair plus an exoskeleton compared with use of only a wheelchair led to clinically meaningful net improvements in patient-reported outcomes for mental and physical health.
Design, Setting, and Participants
This RCT of veterans with SCI was conducted at 15 Veterans Affairs medical centers in the US from September 6, 2016, to September 27, 2021. Data analysis was performed from March 10, 2022, to June 20, 2024.
Interventions
Participants were randomized (1:1) to standard of care (SOC) wheelchair use or SOC plus at-will use of a US Food and Drug Administration (FDA)–cleared exoskeletal-assisted walking (EAW) device for 4 months in the home and community.
Main Outcomes and Measures
Two primary outcomes were studied: 4.0-point or greater improvement in the mental component summary score on the Veterans RAND 36-Item Health Survey (MCS/VR-36) and 10% improvement in the total T score of the Spinal Cord Injury–Quality of Life (SCI-QOL) physical and medical health domain and reported as the proportion who achieved clinically meaningful changes. The primary outcomes were measured at baseline, post randomization after advanced EAW training sessions, and at 2 months and 4 months (primary end point) in the intervention period. Device usage, reasons for not using, and adverse events were collected.
Results
A total of 161 veterans with SCI were randomized to the EAW (n = 78) or SOC (n = 83) group; 151 (94%) were male, the median age was 47 (IQR, 35-56) years, and median time since SCI was 7.3 (IQR, 0.5 to 46.5) years. The difference in proportion of successes between the EAW and SOC groups on the MCS/VR-36 (12 of 78 [15.4%] vs 14 of 83 [16.9%]; relative risk, 0.91; 95% CI, 0.45-1.85) and SCI-QOL physical and medical health domain (10 of 78 [12.8%] vs 11 of 83 [13.3%]; relative risk, 0.97; 95% CI, 0.44-2.15) was not statistically different. Device use was lower than expected (mean [SD] distance, 1.53 [0.02] miles per month), primarily due to the FDA-mandated companion being unavailable 43.9% of the time (177 of 403 instances). Two EAW-related foot fractures and 9 unrelated fractures (mostly during wheelchair transfers) were reported.
Conclusions and Relevance
In this RCT of veterans with SCI, the lack of improved outcomes with EAW device use may have been related to the relatively low device usage. Solutions for companion requirements and user-friendly technological adaptations should be considered for improved personal use of these devices.
Trial Registration
ClinicalTrials.gov Identifier: NCT02658656
Introduction
Paralysis from spinal cord injury (SCI) is a catastrophic condition with diminishing gains in functional recovery over time, often with severe walking limitations and some degree of permanent disability.1,2 The Veterans Health Administration (VHA) cares for the single largest population of people with SCI in the US. Approximately 17 000 veterans with SCI are seen annually in 1 of 25 spinal cord injuries and disorders (SCI/D) centers (eFigure 1 in Supplement 1).3
Historically, strategies for walking after SCI have included use of leg bracing4 or functional electrical stimulation with crutches or a walker,5 requiring high energy consumption and minimal long-term user adoption.5,6,7,8,9,10 Robotic or manual body weight–supported treadmill training offers a form of assisted-walking but is not performed over ground.11,12,13,14 The first-generation exoskeletal devices potentially provide an energy-efficient solution to support over-ground walking by using computer algorithm–controlled external leg bracing with motors at the hip and knees along with use of assistive devices for balance.
Improvements in outcomes after 2 to 4 months of in-hospital, supervised exoskeletal-assisted walking (EAW) were reported for body composition,15,16 energy expenditure,16,17 and bowel function.18,19 The safety and efficacy of EAW in the home and community have not been prospectively studied. We performed a randomized clinical trial (RCT) to examine the efficacy of supervised training sessions followed by 4 months of EAW in the home and community environments in veterans with SCI on clinically meaningful net improvements in patient-reported outcomes for mental and physical health and an array of exploratory outcome measures.
Methods
Trial Design
A parallel, 2-group RCT was conducted at 15 VHA SCI/D centers (eFigure 1 in Supplement 1). Eligible participants were randomly assigned within site (1:1 allocation) using an interactive touch-tone randomization system generated by the coordinating center.20 Participant and trainer blinding were not possible due to the visibility of exoskeleton use. However, outcome assessors at each site were blinded. The study chairpersons were blinded to enrollment without access to participants or results until study closure to data collection. The study was reviewed and approved by the VHA Central Institutional Review Board. This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for RCTs.21 Study design details may be found in the full trial protocol (Supplement 2) and a prior publication.20
Participants and Companions
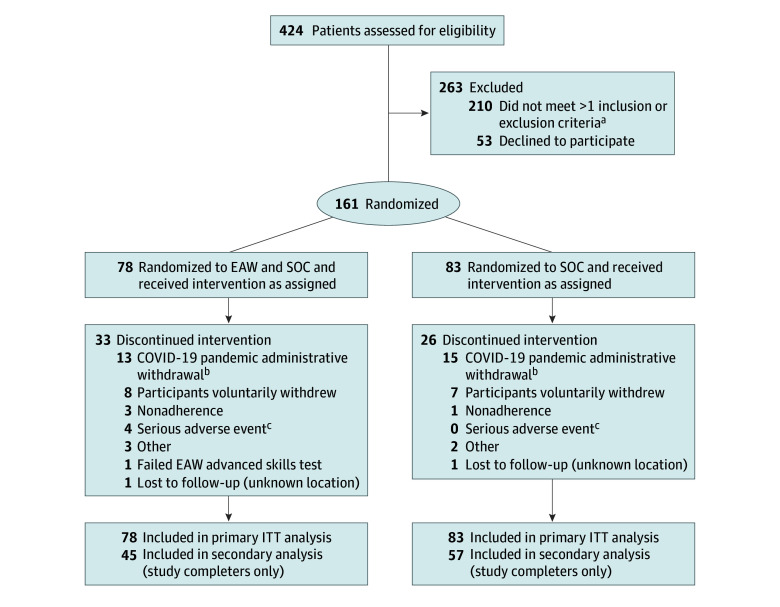
This study was conducted between September 6, 2016, and September 27, 2021. During this time, 424 veterans with SCI were screened, 210 were excluded, 53 declined, and 161 consented to participate and were randomized (Figure; eTable 3 in Supplement 1). Twenty-eight were treated as early terminators due to the COVID-19 pandemic (Figure).
Figure. Participant Flow Diagram.
EAW indicates exoskeletal-assisted walking; ITT, intention to treat; and SOC, standard of care control group. Note that the EAW group is labeled “EAW and SOC” but is referred to in analyses as the EAW group.
aThe major reasons for 1 or more screening failures were bone mineral density loss (n = 64), range of motion or spasticity (n = 39), medical contraindications (n = 31), failed EAW basic skills test (n = 32), anthropometric or weight limits (n = 27), fracture history (n = 21), level of spinal cord injury or neurologic status (n = 21), no companion available (n = 15), and other (n = 9).
bThe COVID-19 early administrative withdrawals were participants who were actively enrolled in the study when the pandemic necessitated a study hold and were eventually terminated by the data monitoring committee due to an inability to restart the study.
cThe 4 serious adverse events in the EAW group that resulted in early withdrawals were 2 fractures, including 1 femur fracture that occurred during transfer from wheelchair to toilet (not related) and 1 foot stress fracture (related) diagnosed as a probable preexisting stress fracture reoccurring during EAW; the other 2 serious adverse events were due to substance abuse and a medical issue, both of which were not study related. There were no adverse event–related early terminations in either group.
Veterans or active-duty soldiers 18 years or older with SCI of more than 6 months and who used a wheelchair for mobility were recruited. Key exclusion criteria included weight greater than 100 kg, history of lower-extremity fracture(s), extremely low hip (less than −3.5 total hip T score) or knee bone mineral density (BMD) (proximal tibia or distal femur BMD<0.60 g/cm2),22 severe spasms or contractures, and medical or health conditions for which walking was contraindicated. Participants were asked to self-report their race and ethnicity identity for diversity inclusion in accordance with Veterans Affairs research policy. A complete list of eligibility criteria is provided (eTables 1 and 2 in Supplement 1).20
The US Food and Drug Administration (FDA) requires a trained companion to accompany the personal exoskeletal device user. The site team determined whether each companion was physically able to assist the participant with the required tasks. Each participant had up to 3 companions who trained with them during the EAW training sessions. Veteran participants and companions provided separate written informed consents for screening and randomization.
Procedures and Intervention
The study consisted of screening, randomization, orientation for the standard of care (SOC) group and advanced exoskeletal in-hospital training for the EAW group (phase 1), and 4 months of intervention (phase 2). The SOC group was offered poststudy training sessions in the exoskeleton; no outcome data were collected. Adverse events were recorded for all study components.
To ensure members of both groups were eligible and able to use an exoskeleton, all participants were fitted for the device, received 5 preliminary EAW training sessions, and were required to pass the EAW basic skills test before randomization.20 The SOC group received orientation by attending weekly meetings and/or were contacted to review their usual activities as an attention balance (while the EAW group was being trained on the exoskeleton). The EAW group received 30 or fewer sessions of advanced training and were required to pass the advanced skills test20 with their companion(s) before taking the exoskeleton home. During the home and community intervention, the EAW group continued with usual activities using their wheelchair but were also provided with a ReWalk 6.0 powered exoskeleton (ReWalk Robotics Inc) for at-will use. The SOC group continued with usual activities. Both groups received weekly contact from the research coordinators to review their weekly log forms.
Primary and Major Secondary Outcomes
On the basis of preliminary data, 2 primary outcome measurements were chosen: (1) mental component summary (questions 4, 5, 8, and 9) of the Veterans RAND 36-Item Health Survey (MCS/VR-36), for which a 4.0-point or greater improvement was considered to indicate greater vitality, social functioning, and improved role-emotional and mental health23,24; and (2) the summary T score of the Spinal Cord Injury–Quality of Life (SCI-QOL) physical and medical health domain for bladder management difficulties, bladder complications, bowel management difficulties, and pain interference item banks, for which a 10% improvement was considered a clinically relevant change.25 The major secondary outcome was a 1.0-kg or greater total body fat mass loss (TBFmass).26,27 These outcome measures were analyzed as the proportion of group participants who did or did not achieve the clinically meaningful change from baseline to month 4.
Secondary Outcomes
All secondary outcomes were intended to be exploratory and included 6 patient-reported outcome surveys: (1) Global Impression of Change Scale for Severity and Improvement, completed by participants and companions; (2) Patient-Reported Outcomes Measurement Information System sleep disturbance scale28; (3) SCI Functional Index29; (4) SCI-QOL emotional health domain30,31; (5) SCI-QOL social participation domain32; and (6) self-reported measures of bowel function. Objective secondary outcomes included change in visceral adipose tissue,27 high-density lipoprotein cholesterol,33,34 low-density lipoprotein cholesterol,35,36 triglycerides, total cholesterol, fasting plasma glucose,36,37,38 fasting plasma insulin,36,37,38 and calculation of Homeostasis Model Assessment for Insulin Resistance.39
All outcomes were assessed at baseline and 3 time points after randomization: orientation and training, 2 months, and 4 months (primary outcome time point). For the EAW group, number of steps from the exoskeleton step log, amount of time the device was used, locations of use, and reasons why the device was not used were recorded on a weekly log form. Usual activities were recorded weekly for both groups using a fixed-answer format.
Sample Size
Sample size estimation and power analysis were based on hypothesis testing of the 2 primary and major secondary outcomes for change from baseline to 4 months. Using hypothesized expected outcomes, we hypothesized that 33% of the intervention group and 10% of the control group would achieve a 4.0-point or greater change on the MCS/VR-36; 42% and 10%, respectively, were hypothesized to have a 10% improvement on the summary T score in the combined SCI-QOL physical and medical health domain; and 35% and 10%, respectively, were hypothesized to maintain a 1.0-kg or greater TBFmass loss. Two-tailed superiority tests for the primary and major secondary end points with a sample size of 160 individuals (80 per group) had 80% power at a P < .025 significance level, assuming an attrition rate of 15%. In March 2019, an interim analysis was performed from 86 evaluable participants and found none of the primary or major secondary end points met the study stopping criteria (eMethods in Supplement 1).
Statistical Analysis
The primary end point was change from baseline to month 4, and an intention-to-treat (ITT) analysis was performed, including all randomized participants. By design, there were no missing cases: participants either met the outcome criteria (success), did not meet these criteria (failure), or terminated early (failure). For the 2 primary and the major secondary outcomes, the proportion of successes was compared between the EAW and SOC groups using Pearson χ2 tests, constituting the primary and secondary efficacy analyses. Secondary analysis included only participants who completed the study (excluding early terminators) and was performed the same as the primary analysis. Additional secondary analyses used all data available and compared mean and mean difference scores from baseline to each time point, using 2-tailed, unpaired t tests. Wilcoxon tests were also performed for variables for which normality assumption was violated. Repeated-measures analyses were completed using generalized linear mixed models. Exploratory post hoc analyses were conducted to determine relationships between the number of steps taken in the exoskeleton with all outcomes. All statistical tests were 2-sided. The 2 primary and major secondary outcomes were tested at a P < .0125 level of significance. Due to the number of secondary outcomes, all were tested at a significance level of P < .01 to reduce type 1 error. SAS software, version 9.4 (SAS Institute Inc) was used to conduct all analyses. Data analysis was performed from March 10, 2022 to June 20, 2024.
Results
Study Participants
A total of 161 veterans with SCI were randomized to EAW (n = 78) or SOC (n = 83), of whom 151 (94%) were male and 10 (6%) were female. The median age was 47 (IQR, 35-56) years, and median time since injury was 7.3 (IQR, 0.5-46.5) years. Two participants (1.2%) were American Indian or Alaska Native, 1 (0.6%) Asian, 27 (16.8%) Black, 109 (67.7%) White, 19 (11.8%) of other race (including multiple or unspecified), and 3 (1.9%) of unknown or unreported race (Table 1).
Table 1. Demographic and Clinical Characteristics of the Participants by Group at Baselinea.
| Characteristic | EAW group (n = 78) | SOC group (n = 83) |
|---|---|---|
| Age, median (IQR), y | 47 (34-56) | 46 (36-56) |
| Height, mean (SD), cm | 176.5 (6.1) | 177.0 (7.1) |
| Weight, median (IQR), kg | 82.1 (70.3-90.7) | 80.3 (72.6-90.3) |
| BMI, mean (SD) | 25.8 (3.7) | 25.7 (3.7) |
| Total body fat mass, mean (SD), kg | 27.3 (9.1) | 27.0 (8.6) |
| Visceral fat mass, median (IQR), kg | 1.60 (0.69-2.39) | 1.50 (0.67-2.23) |
| Duration of SCI, median (IQR), y | 7.4 (0.5-44.1) | 6.3 (0.5-46.5) |
| Sex | ||
| Male | 72 (92.3) | 79 (95.2) |
| Female | 6 (7.7) | 4 (4.8) |
| Ethnicity | ||
| Hispanic or Latino | 8 (10.3) | 19 (22.9) |
| Not Hispanic or Latino | 66 (84.6) | 63 (75.9) |
| Unknown or not given | 4 (5.1) | 1 (1.2) |
| Race | ||
| Asian | 0 | 1 (1.2) |
| Black or African American | 14 (17.9) | 13 (15.7) |
| American Indian or Alaska Native | 2 (2.6) | 0 |
| White | 55 (70.5) | 54 (65.1) |
| Otherb | 6 (7.7) | 13 (15.7) |
| Unknown or not given | 1 (1.3) | 2 (2.4) |
| Cause of SCI | ||
| Traumatic | 66 (84.6) | 74 (89.2) |
| Nontraumatic | 12 (15.4) | 9 (10.8) |
| Paraplegia | 68 (87.2) | 71 (85.5) |
| Complete motor | 45 (66.2) | 57 (80.3) |
| Incomplete motor | 23 (33.8) | 14 (19.7) |
| Tetraplegia | 10 (12.8) | 12 (14.5) |
| Complete motor | 4 (40.0) | 6 (50.0) |
| Incomplete motor | 6 (60.0) | 6 (50.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EAW, exoskeletal-assisted walking; SCI, spinal cord injury; SOC, standard of care.
Data are presented as number (percentage) of participants unless otherwise indicated.
This category included participants who identified with multiple races (n = 8) and those who selected “other” but did not specify race (n = 11).
Primary and Major Secondary Outcome Results
All 161 randomized participants were included in the ITT analysis. The proportions of successes were not statistically different between the groups for the MCS/VR-36 (EAW: 12 of 78 [15.4%]; SOC: 14 of 83 [16.9%]; relative risk [RR], 0.91; 95% CI, 0.45-1.85; P = .80); SCI-QOL physical and medical health domain (EAW: 10 of 78 [12.8%]; SOC: 11 of 83 [13.3%]; RR, 0.97; 95% CI, 0.44-2.15; P = .93), and TBFmass loss (EAW: 14 of 78 [17.9%]; SOC: 16 of 83 [19.3%]; RR 0.93, 95% CI, 0.49 to 1.78; P = .83) (Table 2). Secondary analysis of study completers (45 in the EAW group and 57 in the SOC group) demonstrated a similar percentage of successes in both groups as the primary analyses but failed to reach significance (MCS/VR-36: 12 of 45 [26.7%] vs 14 of 57 [24.6%]; P = .81; SCI-QOL physical and medical health: 10 of 45 [22.2%] vs 11 of 57 [19.3%]; P = .72); and TBFmass loss: 14 of 45 [31.1%] vs 16 of 57 [28.1%]; P = .74).
Table 2. Results of the Primary and Major Secondary Outcome Measures by Time Point and Group (Primary Analysis, All Participants)a.
| Outcome | EAW group (n = 78) | SOC group (n = 83) | RR (95% CI) | P value |
|---|---|---|---|---|
| Mental component summary of the Veterans RAND 36-Item Health Surveyb | ||||
| Baseline score, median (IQR) | 65.4 (58.4 to 67.3) (n = 78) | 63.4 (53.9 to 67.2) (n = 82) | .34 | |
| Training and orientation | 66.5 (59.8 to 68.6) (n = 54) | 62.4 (55.8 to 66.1) (n = 68) | .01 | |
| 2 Months (intervention) | 65.3 (58.0 to 68.3) (n = 48) | 63.7 (49.6 to 67.6) (n = 60) | .17 | |
| 4 Months (intervention) | 65.4 (60.2 to 69.1) (n = 45) | 62.1 (48.1 to 67.8) (n = 57) | .15 | |
| Change from baseline by time point | ||||
| Training and orientation | 1.3 (−2.2 to 6.1) (n = 54) | −1.2 (−4.7 to 3.4) (n = 67) | .08 | |
| 2 Months (intervention) | 1.1 (−2.4 to 4.3) (n = 48) | 0.3 (−4.3 to 3.8) (n = 59) | .36 | |
| 4 Months (intervention) | 0.0 (−4.8 to 4.3) (n = 45) | 0.8 (−6.7 to 3.9) (n = 56) | .88 | |
| Primary outcome (≥4-point improvement), No. (%) | ||||
| Success | 12 (15.4) | 14 (16.9) | 0.91 (0.45 to 1.85) | .80 |
| Failure | 66 (84.6) | 69 (83.1) | ||
| SCI-QOL physical medical health domainc | ||||
| Baseline score, median (IQR) | 152 (137 to 167) (n = 78) | 149 (135 to 170) (n = 83) | .86 | |
| Training and orientation | 146 (137 to 162) (n = 54) | 153 (134 to 173) (n = 67) | .61 | |
| 2 Months (intervention) | 147 (130 to 161) (n = 47) | 145 (134 to 169) (n = 58) | .41 | |
| 4 Months (intervention) | 146 (133 to 163) (n = 43) | 147 (129 to 170) (N = 57) | .58 | |
| Change from baseline by time point | ||||
| Training and orientation | −3.2 (−13.4 to 5.9) (n = 54) | 0.0 (−11.6 to 1.9) (n = 67) | .19 | |
| 2 Months (intervention) | −1.3 (−12.4 to 3.0) [47] | −1.4 (−12.9 to 9.4) (n = 58) | .26 | |
| 4 Months (intervention) | −4.1 (−17.2 to 3.3) (n = 43) | −1.0 (−1.5 to 12.0) (n = 57) | .16 | |
| Primary outcome (10% improvement in summary T score), No. (%) | ||||
| Success | 10 (12.8) | 11 (13.3) | 0.97 (0.44 to 2.15) | .94 |
| Failure | 68 (87.2) | 72 (86.7) | ||
| Total body fat massd | ||||
| Baseline, mean (SD), kg | 27.3 (9.1) (n = 78) | 27.0 (8.6) (n = 83) | .86 | |
| Training and orientation | 28.4 (9.5) (n = 54) | 27.4 (8.7) (n = 65) | .57 | |
| 2 Months (intervention) | 28.5 (9.6) (n = 47) | 27.0 (8.5) (n = 57) | .41 | |
| 4 Months (intervention) | 28.5 (9.7) (n = 45) | 26.5 (7.7) (n = 55) | .26 | |
| Change from baseline by time point, median (IQR) | ||||
| Training and orientation | 0.4 (−0.9 to 1.2) (n = 54) | 0.2 (−0.8 to 1.3) (n = 65) | .84 | |
| 2 Months (intervention) | 0.6 (−1.1 to 2.2) (n = 47) | 0.0 (−1.3 to 1.2) (n = 57) | .45 | |
| 4 Months (intervention) | 0.4 (−1.7 to 1.9) (n = 45) | −0.2 (−1.2 to 1.9) (n = 55) | .79 | |
| Major secondary outcome (≥1.0 kg loss), No. (%) | ||||
| Success | 14 (17.9) | 16 (19.3) | 0.93 (0.49 to 1.78) | .83 |
| Failure | 64 (82.1) | 67 (80.7) |
Abbreviations: EAW, exoskeletal-assisted walking; RR, relative risk; SCI-QOL, Spinal Cord Injury–Quality of Life; SOC, standard of care.
The 2 primary and the major secondary outcome measurements were based on the percentage of individuals in each group who achieved the described specific criteria (success) for each variable at 4 months after randomization. Continuous data with normal distribution are presented as mean (SD) using unpaired, 2-tailed t tests, and nonnormally distributed data are presented as median (IQR) using Wilcoxon tests.
Mental component summary of the Veterans RAND 36-Item Health Survey for vitality, social functioning, role-emotional, and mental health.22,23
Summary T score for the SCI-QOL physical, medical, and health domain for 4 of the item banks: bladder management difficulties, bladder complications, bowel management difficulties, and pain interference.24
Secondary Outcomes
The participants’ Global Impression of Severity of SCI at baseline was not statistically different between the 2 groups (Table 3). The EAW participants and companions reported significant global impression of improvement at each time point compared with the SOC group (Table 3). The EAW group compared with the SOC group reported significant reduction in sleep disturbance at training and orientation (median change from baseline T score, −1.9 [IQR, −5.7 to 3.0] vs 1.3 [IQR, −3.2 to 7.0]); P = .01) and 2 months (median change from baseline T score, −2.7 [IQR, −7.0 to 2.2] vs 1.8 (IQR, −3.2 to 5.7); P = .01) but not at 4 months. No other significant differences between the groups were noted for change in SCI Functional Index, SCI-QOL emotional health domain, and SCI-QOL social participation domain (Table 3). Self-reported measures of bowel function (eTable 4 in Supplement 1) and change in visceral adipose tissue, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, fasting plasma glucose, fasting plasma insulin, and Homeostasis Model Assessment for Insulin Resistance (eTable 5 in Supplement 1) were not statistically different between the groups at any time point.
Table 3. Results of the Secondary Outcome Measures by Time Point and Groupa.
| Outcome | EAW group | SOC group | P value |
|---|---|---|---|
| Global impression of severity of SCI | |||
| Participant’s impression | |||
| Baseline score, median (IQR) | 2 (2 to 3) (n = 78) | 2 (2 to 3) (n = 82) | .74 |
| Training and orientation | 2 (1 to 3) (n = 54) | 3 (2 to 3) (n = 67) | .01 |
| 2 Months (intervention) | 2 (2 to 3) (n = 47) | 3 (2 to 3) (n = 59) | .15 |
| 4 Months (intervention) | 2 (1.5 to 3) (n = 44) | 3 (2 to 4) (n = 57) | .07 |
| Change from baseline score | |||
| Training and orientation | 0 (−1 to 0) (n = 54) | 0 (0 to 1) (n = 66) | .01 |
| 2 Months (intervention) | 0 (−1 to 1) (n = 47) | 0 (0 to 1) (n = 58) | .25 |
| 4 Months (intervention) | 0 (−0.5 to 0) (n = 44) | 0 (0 to 1) (n = 56) | .06 |
| Companion’s impression | |||
| Baseline score, median (IQR) | 2 (2 to 3) (n = 77) | 2 (2 to 3) (n = 81) | .93 |
| Training and orientation | 2 (2 to 3) (n = 51) | 2 (1 to 3) (n = 64) | .34 |
| 2 Months (intervention) | 2 (2 to 3) (n = 45) | 2 (2 to 3) (n = 56) | .63 |
| 4 Months (intervention) | 2 (1 to 3) [41] | 2 (2 to 4) [51] | .92 |
| Change from baseline score | |||
| Training and orientation | 0 (−1 to 1) (n = 51) | 0 (−1 to 1) (n = 63) | .99 |
| 2 Months (intervention) | 0 (−1 to 1) (n = 45) | 0 (−1 to 1 (n = 56) | .92 |
| 4 Months (intervention) | 0 (0 to 1) (n = 41) | 0 (0 to 1) (n = 51) | .89 |
| Global impression of improvement of SCI | |||
| Participant’s change from baseline score, median (IQR) | |||
| Training and orientation | 5 (3 to 6) (n = 54) | 2 (1 to 3) (n = 69) | <.001b |
| 2 Months (intervention phase) | 4 (3 to 6) (n = 47) | 2 (1 to 5) (n = 58) | <.001b |
| 4 Months (postrandomization) | 5 (3 to 6) (n = 43) | 3 (2 to 4) (n = 55) | <.001b |
| Companion’s change from baseline score, median (IQR) | |||
| Training and orientation | 5 (3 to 6) (n = 51) | 2.5 (1 to 4.5) (n = 64) | <.001b |
| 2 Months (intervention) | 5 (3 to 6) (n = 45) | 3 (2 to 4) (n = 55) | <.001b |
| 4 Months (intervention) | 5 (4 to 6) (n = 40) | 3 (1 to 5) (51) | .01b |
| PROMIS sleep disturbance | |||
| Baseline T score, median (IQR) | 46 (38 to 53) (n = 78) | 46 (38 to 54) (83) | .83 |
| Training and orientation | 44 (38 to 54) (n = 54) | 50 (41 to 55) (68) | .20 |
| 2 Months (intervention) | 46 (38 to 55) (n = 48) | 49 (40 to 54) (59) | .92 |
| 4 Months (intervention) | 47 (40 to 58) (n = 45) | 48 (41 to 56) (57) | .94 |
| Change from baseline T score, median (IQR) | |||
| Training and orientation | −1.9 (−5.7 to 3.0) (n = 54) | 1.3 (−3.2 to 7.0) (68) | .01 |
| 2 Months (intervention) | −2.7 (−7.0 to 2.2) (n = 48) | 1.8 (−3.2 to 5.7) (59) | .01 |
| 4 Months (intervention) | 0.0 (−4.8 to 4.0) (n = 45) | 1.5 (−4.1 to 5.3) (57) | .50 |
| SCI-FI | |||
| Baseline summary T score, mean (SD) | 344 (24.50) (n = 29) | 343 (20.6) (n = 31) | .92 |
| Training and orientation | 345 (23.72) (n = 20) | 338 (17.6) (n = 24) | .24 |
| 2 Months (intervention) | 348 (24.92) (n = 18) | 335 (20.6) (n = 24) | .09 |
| 4 Months (intervention) | 348 (16.53) (n = 22) | 339 (24.0) (n = 22) | .17 |
| Change from baseline T score, mean (SD) | |||
| Training and orientation | 5.4 (20.97) (n = 18) | −5.8 (11.63) (n = 19) | .06 |
| 2 Months (intervention) | 7.3 (16.00) (n = 16) | −4.5 (12.99) (n = 19) | .02 |
| 4 Months (intervention) | 3.8 (16.36) (n = 17) | −8.6 (20.60) (n = 17) | .06 |
| Emotional health | |||
| Negative constraints | |||
| Baseline summary T score, median (IQR) | 219 (200 to 255) (n = 78) | 226 (201 to 257) (83) | .60 |
| Training and orientation | 220 (199 to 245) (n = 54) | 226 (195 to 272) (68) | .28 |
| 2 Months (intervention) | 208 (194 to 233) (n = 48) | 219 (188 to 273) (60) | .35 |
| 4 Months (intervention) | 207 (191 to 226) (n = 44) | 223 (193 to 263) (57) | .10 |
| Change from baseline summary T score, median (IQR) | |||
| Training and orientation | −5.3 (−19.0 to 7.1) (n = 54) | 1.1 (−10.3 to 14.2) (n = 68) | .06 |
| 2 mo (intervention) | −6.4 (−21.3 to 6.5) (n = 48) | −0.7 (−10.6 to 14.0) (n = 60) | .08 |
| 4 mo (intervention) | −8.7 (−21.3 to 4.0) (n = 44) | −3.0 (−13.0 to 10.6) (n = 57) | .06 |
| Positive aspects | |||
| Baseline score, median (IQR) | 173 (159 to 189) (n = 78) | 167 (150 to 192) (n = 82) | .70 |
| Training and orientation | 173 (155 to 187) (n = 54) | 160 (144 to 199) (n = 68) | .70 |
| 2 Months (intervention) | 178 (154 to 192) (n = 48) | 165 (141 to 191) (n = 60) | .30 |
| 4 Months (intervention) | 176 (160 to 192) (n = 45) | 162 (141 to 189) (n = 57) | .19 |
| Change from baseline score, median (IQR) | |||
| Training and orientation | 0.0 (−7.9 to 7.6) (n = 54) | 0.0 (−11.3 to 5.9) (n = 67) | .50 |
| 2 Months (intervention) | 0.2 (−2.9 to 11.6) (n = 48) | 0.0 (−10.6 to 6.7) (n = 59) | .21 |
| 4 Months (intervention) | 2.4 (−2.7 to 12.7) (n = 45) | 0.0 (−10.0 to 7.1) (n = 56) | .12 |
| Social participation | |||
| Baseline summary T score, median (IQR) | 155 (141 to 169) (n = 77) | 155 (143 to 178) (n = 83) | .33 |
| Training and orientation | 154 (145 to 169) (n = 53) | 153 (141 to 177) (n = 68) | .87 |
| 2 Months (intervention) | 149 (142 to 173) (n = 47) | 155 (137 to 179) (n = 59) | .98 |
| 4 Months (intervention) | 158 (145 to 178) (n = 44) | 152 (139 to 176) (n = 57) | .37 |
| Change from baseline summary T score, median (IQR) | |||
| Training and orientation | 2.4 (−7.8 to 10.5) (n = 52) | 0.0 (−8.3 to 4.8) (n = 68) | .12 |
| 2 Months (intervention) | 0.0 (−7.5 to 9.5) (n = 46) | −0.2 (−7.7 to 4.4) (n = 59) | .23 |
| 4 Months (intervention) | 4.8 (−4.3 to 14.5) (n = 43) | 0.0 (−13.4 to 6.3) (n = 57) | .04 |
Abbreviations: EAW, exoskeletal-assisted walking; PROMIS, Patient-Reported Outcomes Measurement Information System (short form); SCI, spinal cord injury; SCI-FI, Spinal Cord Injury–Functional Index; SOC, stand of care.
Continuous data with normal distribution are presented as mean (SD) using unpaired, 2-tailed t tests, and nonnormally distributed data are presented as median (IQR) using Wilcoxon tests.
Significant results (P ≤ .001) are in favor of the EAW group. Any other nonsignificant results (P ≤ .01) are in favor of the EAW group at those time points.
Exoskeletal Device Use and Usual Activities
The EAW group reported using the exoskeletal device a mean (SD) of 86 (46) minutes per week (range, 0-248 minutes per week) for 7.7 (5.3) weeks (range, 0-16 weeks). The mean (SD) cumulative total step count ranged from 4321 (4654) to 6192 (10 707) steps per month (range across all EAW participants, 250-57 766 steps per month), which is a mean (SD) distance of 1.53 (0.02) miles per month (range, 0.07-16.40 miles per month). The EAW group reported walking in the exoskeleton on tile, wood, carpet, and smooth indoor surfaces and concrete, asphalt, cement, dirt, gravel, and cobblestone outdoor surfaces; in their homes; on sidewalks; and in shopping centers, restaurants, places of worship, and parks (eTable 6 in Supplement 1). Among 403 reported instances, the predominant reason for not using the exoskeleton was companion unavailability (177 [43.9%]). Other nonuse reasons were illness (70 [17.4%]), being busy (58 [14.4%]), travel (37 [9.2%]), and inclement weather (24 [6.0%]) (eTable 6 in Supplement 1). No significant relationships were found between the number of EAW steps and any of the outcome measures.
The mean (SD) times of participation in usual activities were similar between the EAW (830 [666] minutes per week) and SOC (937 [754] minutes per week) groups (eTable 7 in Supplement 1). Usual activities included household chores, pushing a wheelchair for exercise, stationary arm cycling, weightlifting, stretching, wheelchair sports, and some non–wheelchair-based activities (eTable 7 in Supplement 1).
Safety and Adverse Events
During screening, 34 serious adverse events (SAEs) occurred in 27 participants; 145 adverse events (AEs) occurred in 87 participants (eTable 8 in Supplement 1). During the postrandomization period, 18 SAEs and 157 AEs occurred in the EAW group and 28 SAEs and 165 AEs in the SOC group. Twelve of 78 participants (15.4%) in the EAW group and 14 of 83 (16.9%) in SOC group experienced 1 or more SAEs (eTable 8 in Supplement 1). Overall, 11 fractures occurred, including 9 non–study-related and 2 exoskeleton-related fractures (eTable 8 in Supplement 1). The 2 study-related fractures occurred during initial standing and stepping (no fall or trauma) in the first year of enrollment. Because both cases were possibly undiagnosed preexisting calcaneus fractures from the radiology report, bilateral foot radiographs were added to screening with no other foot or other device-related fractures occurring (eResults in Supplement 1). To determine the number of exoskeletal-related SAEs and AEs, we reported on the whole study group during screening and separately by randomized group (eTable 8 in Supplement 1).
Discussion
In this trial, the use of an exoskeleton in the home and community of veterans with chronic SCI did not result in significantly greater proportions of clinically meaningful changes in mental as well as physical and medical health outcomes compared with the control group who used only their wheelchairs. Likewise, there was no significant difference between the groups for TBFmass loss. Secondary analysis of study completers also failed to reach statistical significance between groups. Because both groups had a small proportion of improvement in the primary outcomes, the positive changes may have been attributed to participation in a clinical trial. The lack of difference in the primary and major secondary outcomes are speculated to be explained by the relatively low device usage: a mean of 86 minutes per week for a total mean walking distance of 1.53 miles per month. This low amount of walking may have been insufficient to elicit a change and is considerably less than the approximately 5000 steps per day reported in ambulatory sedentary populations.40 Van Dijsseldonk et al41 surveyed 14 motor-complete SCI users of the ReWalk exoskeleton in their homes and communities and reported similar findings of low use but good satisfaction with use for social events and exercise. A previous report of home- and community-based use of other ambulatory devices in patients with SCI also showed poor long-term use.42 The low amount of device use in this trial is in contrast to the institutional-based RCT in which EAW was performed under supervision for 3 to 5 hours per week with the mean (SD) steps being much higher at 1420 (491) per session (approximately 17 000 steps per month).43 In that study and others with supervised training, favorable changes in body composition, bowel function, energy expenditure, and quality of life were noted.15,17,18,19,44 Among our participants, EAW demonstrated extreme nonuse and use for any given month (0.07-16.40 miles).
After training in the exoskeleton and at 2 and 4 months of personal use, veterans with SCI reported a significant impression of global improvement in their SCI, suggesting that even with minimal use of an exoskeleton, or possibly from being in the study intervention arm, there was an associated positive change in their global impression of SCI. These findings are similar to those of van Dijsseldonk et al,41 who also reported low use but good satisfaction. However, use of the exoskeleton in the home and community was not associated with statistically significant improvements in functional independence, emotional health, and social participation.
Potential exoskeletal device–related safety concerns included falls resulting in a fracture or other injury and fragility fractures from weightbearing while standing and stepping in those with extremely low BMD. Reduction of long-bone fracture risk was addressed with strict hip and knee BMD exclusion criteria.22 No exoskeleton-related long bone fractures occurred during this study. Two device-related calcaneus fractures occurred with initial standing in the device, which were likely preexisting. Nine fractures occurred that were related to wheelchair falls, transfers, or other nonexoskeletal device activities.
Future Considerations and Lessons Learned
Research for a cure for SCI has made little progress in helping patients recover the ability to walk, and few other interventions have made over-ground walking safe and feasible in this population. Powered exoskeletons are assistive devices and not a cure for SCI. To increase usability, exoskeletal devices require further improvements, such as easier donning and doffing, increased walking speed, hands-free or self-balancing technology, lateral and backward stepping for better maneuverability, stair climbing, and, most importantly, enhancement of safety features that reduce or eliminate the need for a companion. Although the EAW group only walked a little more than a 1.5 miles per month on average, that is an amount of walking they could not have accomplished without the exoskeleton. Although a few small studies have shown that EAW sessions in a supervised hospital setting have demonstrated health benefits in participants with SCI,15,18 device innovations are needed to improve usability in the home and community.
Lessons from this study include the need to estimate (a priori) high screen failure and withdrawal rates due to the daily challenges faced by people living with paralysis. Clinicians treating patients with SCI and individuals with SCI should be made aware of the importance of maintaining bone health and adequate range of motion in the lower extremities for eligibility to participate in future walking strategies.
Limitations
The high number of screen failures and withdrawals and the low amount of device use were limitations to this study. Of the veterans with chronic SCI who wished to participate, more than half did not meet the BMD and fracture history and/or range of motion criteria at screening. Although it is widely known that people with SCI lose significant BMD,22,45,46,47,48 the high percentage of screen failures due to significant bone loss and/or fracture history was unexpected. A major contributor to the withdrawal rate was the interruption to the study by the COVID-19 pandemic. The most influential factor associated with low exoskeleton use was the unavailability of companion(s). Additionally, the study was underpowered because when the sample size was calculated it did not account for any predefined interim analyses. Despite these limitations, no other technology to date has offered over-ground walking on this scale to people with SCI. With appropriate screening and training, using an exoskeleton in the home and community can be safely accomplished. The importance of this technology is evidenced by the decisions of the VHA and Centers for Medicare & Medicaid Services to provide FDA-cleared exoskeletal devices to eligible veterans and nonveterans with SCI.49,50
Conclusions
Given current health care trends of identifying positive quality-of-life outcomes in many populations,51,52,53,54 home and community use of this first-generation personal exoskeleton in the SCI population failed to support improved quality of life. The VHA Cooperative Studies Program and SCI/D centers provided an appropriate infrastructure to conduct this RCT. Safety-focused eligibility criteria were implemented successfully to minimize AEs. Lessons learned from this trial may be implemented for people with SCI in future protocols of more advanced exoskeletal devices and other walking strategies, such as spinal stimulation for people with SCI.
eMethods. Supplemental Methods
eResults. Supplemental Results
eFigure. Geographic Locations of the Veterans Health Administration Spinal Cord Injury/Disorders (SCI/D) System of Care and the Participating Sites
eTable 1. Participant and Companion Eligibility Criteria
eTable 2. Fracture Definition for Eligibility Criteria
eTable 3. Reasons for Screen Failures and Study Withdrawals
eTable 4. Results of Self-reported Bowel Function for Each Time Point Assessment and Group
eTable 5. Results of Visceral Adipose Tissue Mass, Lipid Profile, and HOMA-IR for Each Time Point Assessment and Group
eTable 6. Location, Surface, and Step Count for Exoskeletal Device Usage and Reasons for Not Using the Device
eTable 7. Self-Reported Record of Usual Weekly Activities During the Intervention Phase by Group
eTable 8. Serious Adverse and Adverse Events During Screening and Post Randomization
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25-57. doi: 10.1016/j.pneurobio.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18 [DOI] [PubMed] [Google Scholar]
- 3.Sippel JL, Daly JE, Poggensee L, et al. Modernization of a large spinal cord injuries and disorders registry: the Veterans Administration experience. Arch Rehabil Res Clin Transl. 2022;4(4):100237. doi: 10.1016/j.arrct.2022.100237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann AW, Magiera-Planey R, Schiro-Geist C, Gimines G. Mobility for persons with spinal cord injury: an evaluation of two systems. Arch Phys Med Rehabil. 1987;68(2):90-93. [PubMed] [Google Scholar]
- 5.Kapadia N, Masani K, Catharine Craven B, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med. 2014;37(5):511-524. doi: 10.1179/2045772314Y.0000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massucci M, Brunetti G, Piperno R, Betti L, Franceschini M. Walking with the advanced reciprocating gait orthosis (ARGO) in thoracic paraplegic patients: energy expenditure and cardiorespiratory performance. Spinal Cord. 1998;36(4):223-227. doi: 10.1038/sj.sc.3100564 [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa S, Solomonow M, Baratta R, D’Ambrosia R. Energy expenditure and fatiguability in paraplegic ambulation using reciprocating gait orthosis and electric stimulation. Disabil Rehabil. 1996;18(3):115-122. doi: 10.3109/09638289609166028 [DOI] [PubMed] [Google Scholar]
- 8.Petrofsky JS, Smith JB. Physiologic costs of computer-controlled walking in persons with paraplegia using a reciprocating-gait orthosis. Arch Phys Med Rehabil. 1991;72(11):890-896. doi: 10.1016/0003-9993(91)90007-6 [DOI] [PubMed] [Google Scholar]
- 9.Hitzig SL, Craven BC, Panjwani A, et al. Randomized trial of functional electrical stimulation therapy for walking in incomplete spinal cord injury: effects on quality of life and community participation. Top Spinal Cord Inj Rehabil. 2013;19(4):245-258. doi: 10.1310/sci1904-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam T, Eng JJ, Wolfe DL, Hsieh JT, Whittaker M; the SCIRE Research Team . A systematic review of the efficacy of gait rehabilitation strategies for spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;13(1):32-57. doi: 10.1310/sci1301-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1508-1517. doi: 10.1016/j.apmr.2011.01.024 [DOI] [PubMed] [Google Scholar]
- 12.Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil. 2012;93(9):1487-1497. doi: 10.1016/j.apmr.2012.04.034 [DOI] [PubMed] [Google Scholar]
- 13.Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14(1):24. doi: 10.1186/s12984-017-0232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggioni S, Lünenburger L, Riener R, Curt A, Bolliger M, Melendez-Calderon A. Assessing walking ability using a robotic gait trainer: opportunities and limitations of assist-as-needed control in spinal cord injury. J Neuroeng Rehabil. 2023;20(1):121. doi: 10.1186/s12984-023-01226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asselin P, Cirnigliaro CM, Kornfeld S, et al. Effect of exoskeletal-assisted walking on soft tissue body composition in persons with spinal cord injury. Arch Phys Med Rehabil. 2021;102(2):196-202. doi: 10.1016/j.apmr.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 16.Asselin P, Knezevic S, Kornfeld S, et al. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52(2):147-158. doi: 10.1682/JRRD.2014.02.0060 [DOI] [PubMed] [Google Scholar]
- 17.Rigoli A, Francis L, Nicholson M, Weber G, Redhead J, Iyer P. A systematic review of the effects of robotic exoskeleton training on energy expenditure and body composition in adults with spinal cord injury. Int J Rehabil Res. 2024;47(2):64-74. doi: 10.1097/MRR.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 18.Chun A, Asselin PK, Knezevic S, et al. Changes in bowel function following exoskeletal-assisted walking in persons with spinal cord injury: an observational pilot study. Spinal Cord. 2020;58(4):459-466. doi: 10.1038/s41393-019-0392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorman PH, Forrest GF, Asselin PK, et al. The effect of exoskeletal-assisted walking on spinal cord injury bowel function: results from a randomized trial and comparison to other physical interventions. J Clin Med. 2021;10(5):964. doi: 10.3390/jcm10050964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spungen AM, Bauman WA, Biswas K, et al. The design of a randomized control trial of exoskeletal-assisted walking in the home and community on quality of life in persons with chronic spinal cord injury. Contemp Clin Trials. 2020;96:106102. doi: 10.1016/j.cct.2020.106102 [DOI] [PubMed] [Google Scholar]
- 21.The CONSORT Group. The CONSORT statement. Updated 2014. Accessed September 23, 2016. https://www.consort-statement.org/consort-2010
- 22.Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int. 2017;28(3):747-765. doi: 10.1007/s00198-016-3798-x [DOI] [PubMed] [Google Scholar]
- 23.Kazis LE, Miller DR, Skinner KM, et al. Applications of methodologies of the Veterans Health Study in the VA healthcare system: conclusions and summary. J Ambul Care Manage. 2006;29(2):182-188. doi: 10.1097/00004479-200604000-00011 [DOI] [PubMed] [Google Scholar]
- 24.Kazis LE, Miller DR, Skinner KM, et al. Patient-reported measures of health: The Veterans Health Study. J Ambul Care Manage. 2004;27(1):70-83. doi: 10.1097/00004479-200401000-00012 [DOI] [PubMed] [Google Scholar]
- 25.Tulsky DS, Kisala PA, Tate DG, Spungen AM, Kirshblum SC. Development and psychometric characteristics of the SCI-QOL Bladder Management Difficulties and Bowel Management Difficulties item banks and short forms and the SCI-QOL Bladder Complications scale. J Spinal Cord Med. 2015;38(3):288-302. doi: 10.1179/2045772315Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse LR, Biering-Soerensen F, Carbone LD, et al. Bone mineral density testing in spinal cord injury: 2019 ISCD official position. J Clin Densitom. 2019;22(4):554-566. doi: 10.1016/j.jocd.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 27.Cirnigliaro CM, LaFountaine MF, Dengel DR, et al. Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring). 2015;23(9):1811-1817. doi: 10.1002/oby.21194 [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10(1):6-24. doi: 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinemann AW, Dijkers MP, Ni P, Tulsky DS, Jette A. Measurement properties of the Spinal Cord Injury-Functional Index (SCI-FI) short forms. Arch Phys Med Rehabil. 2014;95(7):1289-1297.e5. doi: 10.1016/j.apmr.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tulsky DS, Kisala PA, Victorson D, et al. Overview of the Spinal Cord Injury–Quality of Life (SCI-QOL) measurement system. J Spinal Cord Med. 2015;38(3):257-269. doi: 10.1179/2045772315Y.0000000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victorson D, Tulsky DS, Kisala PA, Kalpakjian CZ, Weiland B, Choi SW. Measuring resilience after spinal cord injury: development, validation and psychometric characteristics of the SCI-QOL Resilience item bank and short form. J Spinal Cord Med. 2015;38(3):366-376. doi: 10.1179/2045772315Y.0000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann AW, Kisala PA, Hahn EA, Tulsky DS. Development and psychometric characteristics of the SCI-QOL ability to participate and satisfaction with social roles and activities item banks and short forms. J Spinal Cord Med. 2015;38(3):397-408. doi: 10.1179/2045772315Y.0000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30(10):697-703. [DOI] [PubMed] [Google Scholar]
- 34.Bauman WA, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? observations from a diverse cohort. Spinal Cord. 1999;37(7):485-493. doi: 10.1038/sj.sc.3100862 [DOI] [PubMed] [Google Scholar]
- 35.Bauman WA, Adkins RH, Spungen AM, Maloney P, Gambino R, Waters RL. Ethnicity effect on the serum lipid profile in persons with spinal cord injury. Arch Phys Med Rehabil. 1998;79(2):176-180. doi: 10.1016/S0003-9993(98)90296-9 [DOI] [PubMed] [Google Scholar]
- 36.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266-277. doi: 10.1080/10790268.2001.11753584 [DOI] [PubMed] [Google Scholar]
- 37.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37(11):765-771. doi: 10.1038/sj.sc.3100893 [DOI] [PubMed] [Google Scholar]
- 38.Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11(1):109-140. doi: 10.1016/S1047-9651(18)30150-5 [DOI] [PubMed] [Google Scholar]
- 39.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract. 2011;94(1):146-155. doi: 10.1016/j.diabres.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 40.Sisson SB, Camhi SM, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Characteristics of step-defined physical activity categories in U.S. adults. Am J Health Promot. 2012;26(3):152-159. doi: 10.4278/ajhp.100326-QUAN-95 [DOI] [PubMed] [Google Scholar]
- 41.van Dijsseldonk RB, van Nes IJW, Geurts ACH, Keijsers NLW. Exoskeleton home and community use in people with complete spinal cord injury. Sci Rep. 2020;10(1):15600. doi: 10.1038/s41598-020-72397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Zhang J, Xie Y, et al. Wearable health devices in health care: narrative systematic review. JMIR Mhealth Uhealth. 2020;8(11):e18907. doi: 10.2196/18907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong E, Gorman PH, Forrest GF, et al. mobility skills with exoskeletal-assisted walking in persons with SCI: results from a three center randomized clinical trial. Front Robot AI. 2020;7:93. doi: 10.3389/frobt.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spungen AMAP, Fineberg D, Harel NY, Kornfeld S, Bauman WA. Beneficial changes in body composition after exoskeletal-assisted walking: implications for improved metabolic function. Top Spinal Cord Inj Rehabil. 2013;19(5):8-9. [Google Scholar]
- 45.Bauman WA, Spungen AM, Wang J, Pierson RN Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10(2):123-127. doi: 10.1007/s001980050206 [DOI] [PubMed] [Google Scholar]
- 46.Bauman WA, Wecht JM, Kirshblum S, et al. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev. 2005;42(3):305-313. doi: 10.1682/JRRD.2004.05.0062 [DOI] [PubMed] [Google Scholar]
- 47.Cirnigliaro CM, Myslinski MJ, Asselin P, et al. Progressive sublesional bone loss extends into the second decade after spinal cord injury. J Clin Densitom. 2019;22(2):185-194. doi: 10.1016/j.jocd.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 48.Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27(3):202-206. doi: 10.1080/10790268.2004.11753748 [DOI] [PubMed] [Google Scholar]
- 49.Revised Clinical Protocol for Issuance of Powered Exoskeleton Devices to Veterans with Spinal Cord Injury 1-10. 2018. Accessed July 24, 2024. https://www.sci.va.gov/docs/VA_Exoskeleton_Clincal_Protocol_6-7-18.pdf
- 50.Healthcare Common Procedure Coding System (HCPCS) Level II Final Coding, Benefit Category and Payment Determinations 1-204. 2020). Accessed July 24, 2024. https://www.cms.gov/medicare/coding-billing/healthcare-common-procedure-system
- 51.Daundasekara SS, Arlinghaus KR, Johnston CA. Quality of life: the primary goal of lifestyle intervention. Am J Lifestyle Med. 2020;14(3):267-270. doi: 10.1177/1559827620907309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carson TL, Hidalgo B, Ard JD, Affuso O. Dietary interventions and quality of life: a systematic review of the literature. J Nutr Educ Behav. 2014;46(2):90-101. doi: 10.1016/j.jneb.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillison FB, Skevington SM, Sato A, Standage M, Evangelidou S. The effects of exercise interventions on quality of life in clinical and healthy populations: a meta-analysis. Soc Sci Med. 2009;68(9):1700-1710. doi: 10.1016/j.socscimed.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 54.Nguyen LB, Vu LG, Nguyen XT, et al. Global mapping of interventions to improve quality of life of patients with cancer: a protocol for literature mining and meta-analysis. Int J Environ Res Public Health. 2022;19(23):16155. doi: 10.3390/ijerph192316155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eResults. Supplemental Results
eFigure. Geographic Locations of the Veterans Health Administration Spinal Cord Injury/Disorders (SCI/D) System of Care and the Participating Sites
eTable 1. Participant and Companion Eligibility Criteria
eTable 2. Fracture Definition for Eligibility Criteria
eTable 3. Reasons for Screen Failures and Study Withdrawals
eTable 4. Results of Self-reported Bowel Function for Each Time Point Assessment and Group
eTable 5. Results of Visceral Adipose Tissue Mass, Lipid Profile, and HOMA-IR for Each Time Point Assessment and Group
eTable 6. Location, Surface, and Step Count for Exoskeletal Device Usage and Reasons for Not Using the Device
eTable 7. Self-Reported Record of Usual Weekly Activities During the Intervention Phase by Group
eTable 8. Serious Adverse and Adverse Events During Screening and Post Randomization
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement