Abstract
Background
The in vitro fertilization (IVF) technique is commonly used and is the only treatment option for a proportion of infertile couples. To obtain better outcomes of IVF, it is important to enhance embryo quality by optimizing IVF techniques. In IVF procedures, oocytes and sperm are routinely co‐incubated overnight, which may expose oocytes and zygotes to suboptimal culture conditions with increased reactive oxygen species (ROS) produced by sperm in this long term culture. As an attempt to avoid possible detrimental effects on the oocytes from long exposure to sperm, the brief co‐incubation insemination protocol was developed. However, despite a number of studies in this area, it is unclear whether brief co‐incubation improves the IVF outcomes compared with the standard overnight insemination protocol.
Objectives
This Cochrane review aimed to determine whether brief co‐incubation of sperm and oocytes improves outcomes compared with the standard overnight insemination protocol for women undergoing IVF.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Register (14 June 2012), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, 1st quarter), MEDLINE (1948 to 14 June 2012), EMBASE (1989 to 14 June 2012), PsycINFO (1806 to 14 June 2012) and CINAHL (1980 to 26 July 2012). In addition, we searched trials registers, reference lists of articles, conference proceedings (American Society for Reproductive Medicine (ASRM), European Society of Human Reproduction and Embryology (ESHRE)) and contacted experts in the field.
Selection criteria
We included randomized controlled trials (RCTs) comparing brief co‐incubation of gametes with the standard overnight insemination protocol.
Data collection and analysis
Two review authors independently assessed studies for inclusion and trial quality, and extracted data. Disagreements were resolved by discussion with a third author. Statistical analysis was performed using RevMan software.
Main results
Eight RCTs with 733 women in total that compared brief co‐incubation and the standard insemination protocol were included. Live birth was not reported in the included studies. For ongoing pregnancy rate, there were 127 ongoing pregnancies in two trials including 426 women. The low quality evidence showed that brief co‐incubation was associated with an increased ongoing pregnancy rate compared to the standard insemination protocol (pooled odds ratio (OR) 2.42, 95% confidence interval (CI) 1.55 to 3.77; P < 0.0001, I2 = 0%). Measuring clinical pregnancy rate, there were 93 clinical pregnancies in three trials including 372 women. The low quality evidence showed that brief co‐incubation was associated with a significantly higher clinical pregnancy rate than the overnight insemination protocol (pooled OR 2.36, 95% CI 1.45 to 3.85; P = 0.0006, I2 = 0%). For the miscarriage rate, there were six miscarriages in one trial including 167 women. This low quality evidence suggested no significant difference in the odds of miscarriage between brief co‐incubation and standard insemination (OR 1.98, 95% CI 0.35 to 11.09; P = 0.44).
Authors' conclusions
This review has provided evidence that brief co‐incubation of sperm and oocytes may improve the ongoing pregnancy and clinical pregnancy rates for infertile women undergoing IVF cycles. More RCTs are required to assess whether brief co‐incubation would contribute to a higher live birth rate and a lower miscarriage rate compared to the standard overnight insemination protocol.
Keywords: Female; Humans; Male; Pregnancy; Abortion, Spontaneous; Abortion, Spontaneous/epidemiology; Coculture Techniques; Coculture Techniques/methods; Culture Media; Fertilization in Vitro; Fertilization in Vitro/methods; Pregnancy Rate; Randomized Controlled Trials as Topic; Reactive Oxygen Species; Reactive Oxygen Species/metabolism; Sperm‐Ovum Interactions; Sperm‐Ovum Interactions/physiology; Time Factors
Plain language summary
Brief co‐incubation of sperm and oocytes for in vitro fertilization (IVF) techniques
In standard insemination protocols for IVF, oocytes are exposed to sperm for 15 to 20 hours. Such long term co‐incubation with sperm may expose oocytes and zygotes to suboptimal culture medium due to increased levels of reactive oxygen species (ROS) produced by sperm and other products of metabolism. Shortening the co‐incubation time of oocytes and sperm may possibly improve IVF outcomes by reducing the detrimental effect of ROS on the zygotes and the quality of the embryos. The brief co‐incubation method used in IVF reduces the co‐incubation time of oocytes and sperm to one to four hours. This review identified eight randomized controlled trials involving 733 women. Low quality evidence showed increases in ongoing pregnancy and clinical pregnancy rates with the use of the brief co‐incubation protocol. More studies are needed to assess whether brief co‐incubation would contribute to a higher live‐birth rate and a lower miscarriage rate compared to the standard overnight insemination protocol.
Summary of findings
Summary of findings for the main comparison. Brief co‐incubation compared to standard insemination for in vitro fertilization techniques.
| Brief co‐incubation compared to standard insemination for in vitro fertilization techniques | ||||||
| Patient or population: patients with in vitro fertilization techniques Settings: Intervention: Brief co‐incubation Comparison: standard insemination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard insemination | Brief co‐incubation | |||||
| Ongoing pregnancy per randomized woman | 213 per 1000 | 396 per 1000 (296 to 505) | OR 2.42 (1.55 to 3.77) | 426 (2 studies) | ⊕⊕⊝⊝ low1 | |
| Clinical pregnancy rate per randomized woman | 177 per 1000 | 337 per 1000 (238 to 453) | OR 2.36 (1.45 to 3.85) | 372 (3 studies) | ⊕⊕⊝⊝ low2 | |
| Miscarriage rate per randomized woman | 24 per 1000 | 47 per 1000 (9 to 217) | OR 1.98 (0.35 to 11.09) | 167 (1 study) | ⊕⊕⊝⊝ low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One trial lacked adequate explanation for methods of randomization. Allocation concealment not mentioned in any trial. 2 Two trials lacked adequate explanation for randomization methods. Allocation concealment not mentioned in any trial. 3 One trial only and no method of randomization or allocation concealment stated.
Background
Description of the condition
Infertility affects 10% of couples hoping to conceive and has been recognized as a public health issue worldwide by the World Health Organization (WHO). A proportion of couples with infertility due to tubal disease, endometriosis, ovulation disorders, male factor or unexplained reasons, or who have failed with less invasive forms of treatment such as intra‐uterine insemination or other less costly options, will ultimately decide to have in vitro fertilization (IVF). IVF treatment involves controlled ovarian hyper‐stimulation (COH) followed by oocyte retrieval, fertilization, embryo culture and, finally, embryo transfer (ET). Multiple factors such as female age, duration of infertility, basal follicle stimulating hormone (FSH) levels, quantity of oocytes and embryo morphology, along with other factors, are thought to be predictors of a pregnancy outcome after IVF (De Placido 2002; van Loendersloot 2010).
Since IVF was first developed, techniques are continuously progressing. Recently the development of IVF techniques for the treatment of infertile couples has focused primarily on establishing adequate conditions for facilitating sperm‐oocyte interaction, especially during insemination and fertilization.
Description of the intervention
Insemination is an important procedure in IVF techniques. In conventional IVF techniques, oocytes are routinely inseminated with sperm in a culture medium drop overlaid with oil. The actual final concentration of motile sperm in the drop has been reported to be approximately 0.5 to 5 X 106/ml, based on sperm to oocyte ratios previously described (Fiorentino1994). In standard insemination protocols, oocytes are exposed to sperm for 15 to 20 hours and then their cumulus and corona cells (that is, the cells surrounding the oocyte) are removed. This long duration of co‐incubation was originally established for practical reasons, because it corresponded to the timing for the inspection of pronuclei (the male pronucleus and the female pronucleus).
Since the 1990s, in attempting to avoid possible detrimental effects on the oocytes from long exposure to sperm, the short duration insemination protocols were developed, which involve brief co‐incubation of oocytes and sperm. After a one to four hour exposure to spermatozoa, oocytes are withdrawn from the insemination medium. They are rinsed in culture medium to remove sperm not attached to the cumulus‐corona complex and to detach any already digested cumulus, and then the oocytes are incubated in fresh medium. About 16 hours later, oocytes are decoronated and checked for the presence of two pronuclei to confirm fertilization.
Currently, both types of insemination protocols are used in conventional IVF. However, it remains uncertain whether reducing the co‐incubation time significantly improves IVF success rates or whether it is associated with negative consequences.
How the intervention might work
The co‐incubation time seems to affect the reactive oxygen species (ROS) produced by human spermatozoa. At physiological levels, ROS may play an important role in sperm physiological and signalling processes to ensure fertilization. However, ROS at high concentrations may cause DNA fragmentation (Twigg 1998) and damage to both nuclear and mitochondrial DNA (Venkatesh 2011), adversely affect sperm motility and sperm functional competence, and cause other damage to the sperm cell (Bansal 2010; Evenson 2000; Henkel 2011; Mahfouz 2010; Zorn 2003). Studies indicate that high concentration of ROS in vitro may be harmful to oocytes and zygotes (Agarwal 2005; Aitken 1987; Aitken 1996; Bedaiwy 2004; Krausz 1994; Nasr‐Esfahani 1990), cause hardening of the zona pellucida (Gianoroli 1996a; Gianaroli 1996b; Dirnfeld 2003) and negatively influence fertilization rates, embryo quality (Evenson 2000; Larson 2000; Lopes 1998; Saleh 2003; Simon 2011; Virroet 2004) and the pregnancy rate (Henkel 2003).
Gametes are susceptible to ROS attack. When manipulated in vitro during assisted reproductive techniques, these cells run the risk of generating and being exposed to supra‐physiological levels of ROS (du Plessis 2008). In standard insemination protocols, oocytes and sperm are co‐incubated for as long as 15 to 20 hours. The presence of an extra‐physiological number of sperm for prolonged culture may further increase ROS levels in the culture medium, which leads to suboptimal culture conditions. Studies have indicated that a long oocyte exposure to spermatozoa may be harmful (Aitken 1987; Dumoulin1992; Parinaud 1993). Such damage may be greater still when using semen samples from male factor patients since pathological sperm are likely to generate higher levels of ROS than normal sperm.
In addition, the number of sperm affects the outcomes of IVF. Successful fertilization of a human oocyte requires only a single sperm to penetrate through the cumulus cells, zona pellucida and oolemma. Polyspermy, known as the fertilization of an oocyte by more than one sperm, is a significant problem in human IVF. In humans, polyspermy usually results in abnormal embryos containing three or more copies of each chromosome and is recognized as one of the underlying mechanisms of implantation failure and miscarriage. Discarding embryos arising from polyspermy zygotes leads to a reduced number of embryos available, which may ultimately influence the outcome of IVF. Contrary to the situation in vivo, in IVF oocytes are exposed to excessive numbers of sperm. This has led to the thought that the incidence of polyspermy might be related to high sperm concentrations. In previous studies, polyspermy has been related to the maturation status of the oocytes (Angell 1986; van der Ven 1985), the use of a high concentration of capacitated sperm at the site of fertilization and suboptimal in vitro conditions (Wang 2003). Therefore, the overnight incubation of oocytes with a supra‐physiological number of spermatozoa, as is widely practised in IVF laboratories, may be detrimental.
However, in short insemination protocols the cumulus‐oocyte complex (COC) is rinsed after a brief co‐incubation, causing part of the cumulus to be detached early. As was observed, one disadvantage of removal of the COC could be a disturbance of the important communication between the oocyte and the COC (Canipari 2000). Whether brief co‐incubation improves IVF outcomes through minimizing the detrimental effect of ROS without decreasing the fertilization rate is still open to debate.
Why it is important to do this review
IVF is commonly used and is the only treatment option for a proportion of infertile couples. To obtain better outcomes from IVF, it is important to enhance embryo quality by optimizing IVF techniques. In standard IVF protocols, oocytes are co‐incubated with sperm overnight for insemination, which might expose oocytes to high levels of ROS and other products of metabolism in the prolonged culture conditions. In this regard, shortening the co‐incubation time of oocytes and sperm should have improved IVF outcomes by reducing the possible detrimental effects of ROS on the zygotes and quality of the embryo.
However, despite a number of studies in this area, it is unclear whether brief co‐incubation of oocytes and sperm leads to significantly better outcomes compared with the standard overnight insemination protocol. We therefore performed a systematic review to attempt to answer this question.
Objectives
To determine whether brief co‐incubation of sperm and oocytes improves outcomes compared with the standard overnight insemination protocol in women undergoing IVF.
Methods
Criteria for considering studies for this review
Types of studies
Included studies
Only randomized controlled trials were eligible for inclusion in this review.
Excluded studies
Quasi‐randomized trials were excluded.
Types of participants
Infertile women with known indications for conventional IVF treatment and under the age of 41 years were included. Women with poor ovarian response to gonadotropin stimulation and women whose partner had severe oligozoospermia were excluded.
Types of interventions
Studies that compared the two insemination protocols were included. In the standard insemination protocol, oocytes and sperm were co‐incubated for 15 to 20 hours before removing oocytes from the culture medium. In the brief co‐incubation protocol, oocytes were removed after one to four hours of co‐incubation.
Types of outcome measures
Primary outcomes
Live‐birth rate (LBR)
Live birth, defined as the birth of a live offspring
LBR: live births per randomized woman
Secondary outcomes
1. Ongoing pregnancy rate (OPR)
Ongoing pregnancy, defined as evidence of a gestational sac with fetal heart motion at 12 weeks, confirmed with ultrasound
OPR: the number of ongoing pregnancies per randomized woman
2. Clinical pregnancy rate (CPR)
Clinical pregnancy, defined as the presence of a gestational sac determined by ultrasound examination
CPR: the number of clinical pregnancies per randomized woman
3. Miscarriage rate per randomized woman
The number of pregnancy losses up to 20 weeks gestation per woman
4. Fertilization rate
The percentage of zygotes with two visible pronuclei among inseminated oocytes
5. Polyspermy rate
The percentage of zygotes with more than two visible pronuclei among inseminated oocytes
6. Implantation rate
The percentage of embryos implanted of the embryos transferred
Data for outcome 4, 5, and 6 were not pooled but were collected and reported in a tabular format.
Search methods for identification of studies
We searched for all published and unpublished RCTs of brief co‐incubation of oocytes and sperm in IVF cycles, without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
Electronic searches
Computerized searches were conducted using the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of controlled trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and CINAHL.
The Menstrual Disorders and Subfertility Group Specialised Register of controlled trials was searched by the Group's Trials Search Co‐ordinator using the keywords: contains "IVF" or "in vitro fertilization" or "in vitro fertilization" or "oocyte" or "Sperm" or title contains "IVF" or "in vitro fertilization" or "in vitro fertilization" or "oocyte" or "Sperm", and keywords contains "incubation" or "incubator" or "gamete co‐incubation" or "co incubation" or "co‐culture" or title contains "incubation" or "incubator" or "gamete co‐incubation" or "co incubation "or" co‐culture" (from inception to 14 June 2012).
The following databases were searched in Ovid using the search strategies described in the appendices:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 3rd quarter 2011, 1st quarter 2012) (Appendix 1);
MEDLINE (1948 to 14 June 2012) (Appendix 2);
EMBASE (1989 to 14 June 2012) (Appendix 3);
PsycINFO (1806 to 14 June 2012) (Appendix 4);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1980 to 26 July 2012) (Appendix 5).
There were no language restrictions in these searches.
Searching other resources
In addition, we searched the following sources.
The Cochrane Library (www.cochrane.org/index.htm).
Handsearching of appropriate journals (the lists of journals are found in the MDSG Module).
Trials registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com/); ClinicalTrials.gov, a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home); World Health Organization International Clinical Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx).
Citation indexes (http://scientific.thomson.com/products/sci/).
Open Sigle for grey literature from Europe (http://opensigle.inist.fr/).
National and international research registers including the Register of Controlled Trials (www.controlled‐trials.com) and conference proceedings.
The reference lists of all searched primary studies, review articles, citation lists of relevant publications, abstracts of major scientific meetings (for example European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM) and related studies were checked to identify additional relevant citations.
Experts and specialists in the field were contacted for relevant trials.
Data collection and analysis
Selection of studies
Two review authors (HZY, YJ) independently scanned the titles and abstracts of each record retrieved for potential eligibility according to the inclusion and exclusion criteria. They discarded studies that were clearly not applicable. They then searched for the full texts of all potentially relevant titles and abstracts and screened them for eligibility. Studies were appraised in an unblinded fashion. Where further information was required, review authors contacted the trial authors. Any discrepancies between the two review authors were resolved in consultation with a third author (WL).
Data extraction and management
Two review authors (HZY, YJ) extracted data independently, using a standard data extraction form designed according to Cochrane guidelines. The data extraction forms included methodological quality and allocation information, data on study characteristics and results, including methods, participants, interventions and outcomes. The two sets of extracted data were compared and disagreements were resolved by discussion with a third author (WL).
Assessment of risk of bias in included studies
Risk of bias was assessed independently by two review authors (HZY, LJ) using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). A risk of bias table and summary were developed. The authors assessed risk of bias among the included studies in six domains: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias. They contacted trial authors for missing information about the study design, when required. Discrepancies were resolved by discussion with a third author (WL). All judgments were fully described. The conclusions were presented in the 'Risk of bias' table.
Measures of treatment effect
Dichotomous data were expressed as odds ratios (OR) with 95% confidence intervals (95% CI). Statistical analysis was performed in accordance with the guidelines developed by the Menstrual Disorders and Subfertility Group.
Unit of analysis issues
We included in the meta‐analysis only RCTs in which the unit of analysis was per woman. Where data were reported using per cycle, per embryo or per oocyte we briefly summarized the findings in additional tables with a narrative description. In conditions where per cycle data and the number of cycles were equal to the number of women then we analysed the data as per woman.
Dealing with missing data
We attempted to contact by email the authors of the original studies with any missing data. If there was no reply, and if possible, we reported the data in terms of intention to treat. If this was not possible the trials were placed in 'studies awaiting classification'. If data were missing we reported the data available and did not impute any data.
Assessment of heterogeneity
We used the I2 statistic and Chi2 test to examine statistical heterogeneity across trials. The I2 statistic represents the percentage of total variation across trials that is due to heterogeneity. A value greater than 50% was taken to represent substantial heterogeneity (Higgins 2011). If substantial heterogeneity was detected, possible explanations were explored in sensitivity analyses.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimize their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We used alternative, robust search strategies including electronic searching, handsearching (Hopewell 2007a), a comprehensive search of the grey literature (Hopewell 2007b), alternative sources of data or synthesized evidence, and contacting experts and the research community (Hopewell 2007c). We tried to ask the authors for extra data with incomplete reporting of outcomes, and to avoid duplication bias by contacting the author when there was any suspicion about double publication.
A visual inspection of the funnel plots was to be used to investigate the presence and magnitude of publication and related bias (PRB) (Song 2002) if there were 10 or more studies in the analysis.
Data synthesis
Where studies were sufficiently similar, we combined them for meta‐analysis with Review Manager 5.1 (RevMan 5.1) software using the Peto‐modified Mantel‐Haenszel fixed‐effect model method.
A 'Summary of findings' table was constructed for the pooled data. We used GradePro 2009 to produce evidence profile tables across outcomes and gave a summary of the quality of evidence for each comparison.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed based on sperm quality, if possible.
Sensitivity analysis
Where substantial heterogeneity was detected, we planned to conduct sensitivity analyses based on the quality of the studies.
Results
Description of studies
Results of the search
Two review authors (HZY, YJ) scanned the titles and abstracts of the results of the search strings. After removal of inappropriate and duplicate studies 25 trials remained (Figure 1). Copies of the remaining studies, identified as providing data comparing brief co‐incubation protocol and overnight insemination protocol outcomes, were retrieved and evaluated. Eight trials met the inclusion criteria and were finally included in the review (see the table 'Characteristics of included studies'). Fifteen trials were excluded (see the table 'Characteristics of excluded studies'). Two trials are awaiting classification (see the table 'Characteristics of studies awaiting classification').
1.
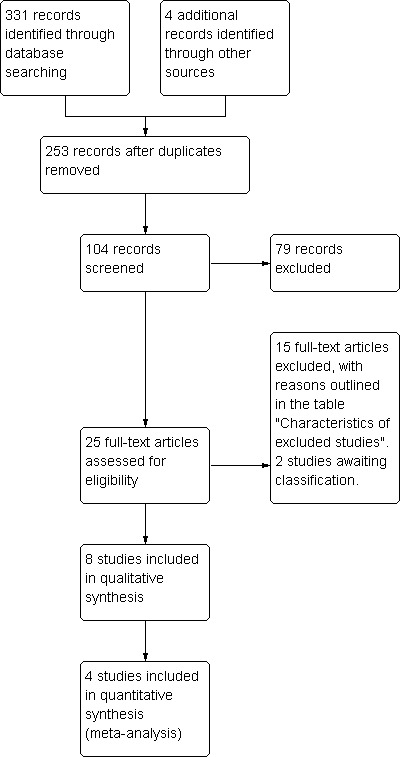
Study flow chart.
Included studies
Study design and setting
There were nine studies identified as randomized controlled trials (RCTs) comparing brief co‐incubation of gametes with a standard insemination protocol and finally eight of them were included in the review.,There was some overlap of participation between two trials (Gianaroli 1996a; Gianaroli 1996b) and only the later one with the higher sample size was included. Full details of these trials can be seen in the table of included studies (see the table 'Characteristics of included studies').
The trials came from seven different countries. Data per randomized woman were able to be extracted from four of these included trials (Dirnfeld 1999; Gianaroli 1996b; Kattera 2003; Waldenström 1998) and were included in the final analysis. The four remaining trials did not report any per woman data (Boone 2001; Coskun 1998; Dirnfeld 2003; Lin 2000). Attempts were made to contact the authors of some of the included trials for further details and clarification.
Participants
The studies included infertile women with known indications for conventional IVF treatment who were under the age of 41 years. Most trials included participants with normo‐ovulatory cycles before the treatment cycle. Women with poor ovarian response to gonadotropin stimulation and women whose partner had severe oligozoospermia were excluded.
Interventions
This review aimed to determine if brief (1 to 4 h) co‐incubation of sperm and oocytes improved outcomes compared with the standard overnight insemination protocol in women undergoing IVF cycles. Brief co‐incubation and overnight insemination were used in the included trials. Four studies (Coskun 1998; Dirnfeld 1999; Gianaroli 1996b; Lin 2000) compared 1 h versus overnight co‐incubation (16 to 20 h). Two studies (Dirnfeld 2003; Kattera 2003) compared 2 h versus overnight co‐incubation. One trial (Waldenström 1998) compared 1.5 to 2 h and another trial (Boone 2001) compared 3 h versus overnight co‐incubation, respectively.
Outcomes
The primary outcome for this review was live birth.
No study reported on the number of live births.
Secondary outcomes for this review were as follows.
1. Ongoing pregnancy rate: two studies reported ongoing pregnancies (Gianaroli 1996b; Kattera 2003).
2. Clinical pregnancy rate: three studies reported clinical pregnancy rate (Dirnfeld 1999; Gianaroli 1996b; Waldenström 1998).
3. Miscarriage rate: one study reported miscarriage rate (Gianaroli 1996b).
4. Fertilization rate: all the included studies reported fertilization rate (Boone 2001; Coskun 1998; Dirnfeld 1999; Dirnfeld 2003; Gianaroli 1996b; Kattera 2003; Lin 2000; Waldenström 1998).
5. Polyspermy rate: five studies reported polyspermy rate (Boone 2001; Coskun 1998; Gianaroli 1996b; Lin 2000; Waldenström 1998).
6. Implantation rate: two studies reported implantation rate (Dirnfeld 1999; Gianaroli 1996b).
Excluded studies
In total, 15 trials were excluded for reasons outlined in the table 'Characteristics of excluded studies'. Twelve studies were excluded because they were not truly randomized controlled trials (Barraud 2003; Barraud 2008; Bungum 2005; Bungum 2006; Hammit 1999; Lundqvist 2001; Navarro 2004; Quinn 1998; Swenson 1998; Swenson 2000; Xiong 2011; Zhang 2009). One trial (Granham 1999) was excluded as it did not have appropriate inclusion criteria for participants. One trial (Swenson 1999) was excluded because it was superseded by a full paper of the trial. A trial of Gianoroli (Gianoroli 1996a) was excluded because the participants in this study were included in another study (Gianaroli 1996b). There are two trials (Jamieson 1999; Pattanayak 2001) awaiting classification; it has not been possible to establish whether or not they were RCTs.
Risk of bias in included studies
Allocation
Generation of random sequence
All the included studies were RCTs. Three studies (Dirnfeld 1999; Dirnfeld 2003; Kattera 2003) were at low risk of selection bias related to random sequence generation as they used standard random number tables. Five studies (Boone 2001; Coskun 1998; Gianaroli 1996b; Lin 2000; Waldenström 1998) were at unclear risk of bias as they did not describe how randomization was carried out. Attempts were made to contact the authors but as yet there has been no reply.
Allocation concealment
The methods of allocation concealment were not described in the included studies. Therefore, there was unclear risk of selection bias in all the trials under review.
Blinding
Blinding of the participants, or the performers of IVF, were not stated in the included studies. It is probable that the women receiving treatment and the clinicians could be blinded but the performing technicians were not blinded. The different duration of oocytes and sperm co‐incubation for each of the experimental groups made it impossible to blind the performing technician to which group a participant was in. We did not consider that blinding was likely to influence the risk of performance bias for the primary review outcome (live birth) and the secondary outcomes. We judged that the outcome measurement was not likely to be influenced by lack of blinding.
Incomplete outcome data
The participants included in the analysis were exactly those who were randomized into the trials. No withdrawals or losses to follow up were mentioned in the studies. There were four studies that did not report per woman data but reported some of the secondary outcomes. So the risk of attrition bias of these studies was rated as unclear.
Selective reporting
We used alternative, robust search strategies including electronic searching, handsearching, a comprehensive search of the grey literature, alternative sources of data or synthesized evidence, and contacting experts and the research community. All included studies did not report live birth, which is the major outcome of the studies, and were rated as at unclear risk of this bias. There were insufficient studies to make a funnel plot feasible.
Other potential sources of bias
There were four studies that did not report per woman data. We did not find potential sources of other bias in the included trials and could not definitively assess other bias.
Effects of interventions
See: Table 1
Comparison of brief co‐incubation versus standard insemination protocol
Primary outcome
Live‐birth rate
Live births were not reported in the included trials.
Secondary outcomes
1. Ongoing pregnancy rate
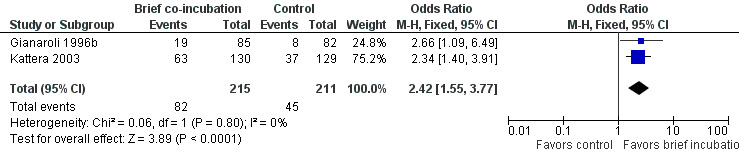
Two trials (Gianaroli 1996b; Kattera 2003) reported ongoing pregnancy rate. The brief co‐incubation protocol was associated with an increased ongoing pregnancy rate compared to the standard overnight insemination protocol (pooled OR 2.42, 95% CI 1.55 to 3.77; P < 0.0001, I2 = 0%) (see Analysis 1.1; Figure 2). There were 426 women in total, 215 in the brief co‐incubation group and 211 in the standard insemination (control) group. In total there were 82 pregnancies (38.1%) in the brief co‐incubation group and 45 (21.3%) in the control group. Heterogeneity was low in this analysis and therefore sensitivity analysis was not performed.
1.1. Analysis.
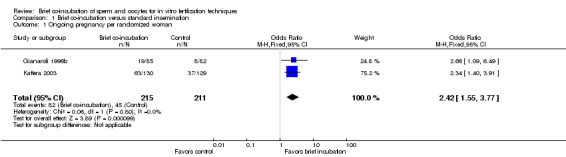
Comparison 1 Brief co‐incubation versus standard insemination, Outcome 1 Ongoing pregnancy per randomized woman.
2.
Forest plot of comparison: 1 Brief co‐incubation versus standard insemination, outcome: 1.1 Ongoing pregnancy per randomized woman.
2. Clinical pregnancy rate
Three studies reported clinical pregnancy rate (Dirnfeld 1999; Gianaroli 1996b; Waldenström 1998). The rate of clinical pregnancy per randomized woman was significantly higher in the brief co‐incubation group than in the overnight insemination group (pooled OR 2.36, 95% CI 1.45 to 3.85; P = 0.0006, I2 = 0%) (see Analysis 1.2; Figure 3). In total there were 59 clinical pregnancies from 372 women randomized, 75 in the brief co‐incubation group (n = 180) and 34 in the control group (n = 192).
1.2. Analysis.
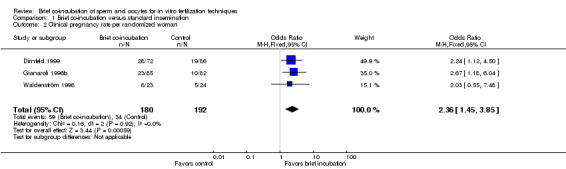
Comparison 1 Brief co‐incubation versus standard insemination, Outcome 2 Clinical pregnancy rate per randomized woman.
3.
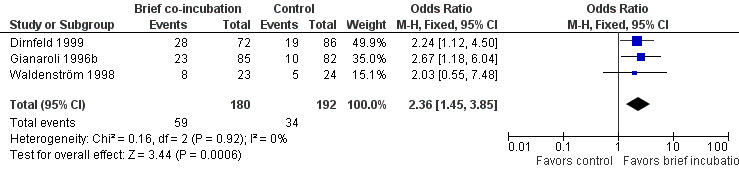
Forest plot of comparison: 1 Brief co‐incubation versus standard insemination, outcome: 1.2 Clinical pregnancy rate per randomized woman.
3. Miscarriage rate
One trial (Gianaroli 1996b) reported miscarriage rate. There were 6 miscarriages in 167 women, 4 in the brief co‐incubation group (n = 85) and 2 in the control group (n = 82). There was no significant difference in the miscarriage rate per randomized woman between the two treatment groups (OR 1.98, 95% CI 0.35 to 11.09; P = 0.44) (see Analysis 1.3; Figure 4).
1.3. Analysis.
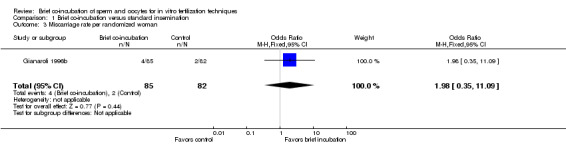
Comparison 1 Brief co‐incubation versus standard insemination, Outcome 3 Miscarriage rate per randomized woman.
4.
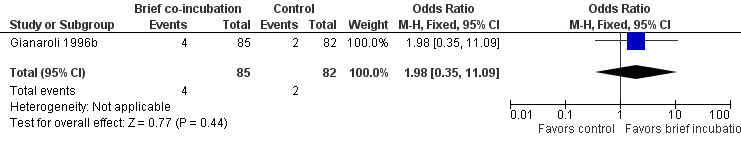
Forest plot of comparison: 1 Brief co‐incubation versus standard insemination, outcome: 1.3 Miscarriage rate per randomized woman.
4. Fertilization rate
All the included studies reported fertilization rate. Fertilization rate varied from 56.1% to 79.5% in the brief co‐incubation group, and from 61% to 85.7% in the control group. Five studies (Boone 2001; Coskun 1998; Dirnfeld 1999; Kattera 2003; Lin 2000) presented a trend for a lower fertilization rate in the brief co‐incubation group. One of them (Boone 2001) reported a significantly lower fertilization rate in the brief co‐incubation group compared with the standard insemination group. Three studies (Dirnfeld 2003; Gianaroli 1996b; Waldenström 1998) showed a trend for a higher fertilization rate in the brief co‐incubation group. Only in one trial (Gianaroli 1996b), brief co‐incubation was associated with a significantly higher fertilization rate compared with the standard insemination protocol (see Additional tables: Table 2).
1. Fertilization rate.
| Study ID | Brief co‐incubation | Standard insemination | P value |
| Boone 2001 | Co‐incubation time:3h Fertilization rate: 70.9% (117/165) |
Co‐incubation time: 19h Fertilization rate: 80.4% (135/168) |
P = 0.001 |
| Coskun 1998 | Co‐incubation time:1h Fertilization rate: 59.0% (135/229) |
Co‐incubation time: 18h Fertilization rate: 63.8% (150/235) |
NS |
| Dirnfeld 1999 | Co‐incubation time:1h Fertilization rate: 56.1% (411/732)) |
Co‐incubation time: 16‐20h Fertilization rate: 61% (501/822) |
NS |
| Dirnfeld 2003 | Co‐incubation time:2h Fertilization rate: 66.8% (20/30) |
Co‐incubation time: 16‐20h Fertilization rate: 65.4% (52/79) |
NS |
| Gianaroli 1996b | Co‐incubation time:1h Fertilization rate: 74.0% (440/595) |
Co‐incubation time: 16h Fertilization rate: 67.7% (376/555) |
P < 0.025 |
| Kattera 2003 | Co‐incubation time:2h Fertilization rate: 75.8% (838/1105) |
Co‐incubation time:20h Fertilization rate: 77.0% (924/1200) |
NS |
| Lin 2000 | Co‐incubation time:1,3h Fertilization rate: 79.5% (93/117) |
Co‐incubation time:16‐18h Fertilization rate: 85.7% (191/223) |
NS |
| Waldenström 1998 | Co‐incubation time:2h Fertilization rate: 69.4% (177/255) |
Co‐incubation time: 16‐18h Fertilization rate: 64.9% (196/302) |
Not stated |
P < 0.05 was defined as statistically significant
NS = not significant
5. Polyspermy rate
Four studies reported polyspermy rate (Boone 2001; Gianaroli 1996b; Lin 2000; Waldenström 1998). The polyspermy rate varied from 0.9% to 6.8% in the brief co‐incubation group, and 2.3% to 6.5% in the control group. Three of the four studies showed a trend for a lower polyspermy rate in the brief co‐incubation group (Boone 2001; Gianaroli 1996b; Waldenström 1998). One trial (Lin 2000) reported a polyspermy rate of 6.8% in the brief co‐incubation group and 4% in the control group, without a significant difference between the groups (see Additional tables: Table 3).
2. Polyspermy rate.
| Study ID | Brief co‐incubation | Standard insemination | P value |
| Boone 2001 | Co‐incubation time: 3h Polyspermy rate: 2.4% (4/165) |
Co‐incubation time: 19h Polyspermy rate: 6.5% (11/168) |
P = 0.110 |
| Gianaroli 1996b | Co‐incubation time: 1h Polyspermy rate: 0.9% (5/555) |
Co‐incubation time: 16h Polyspermy rate: 2.3% (13/555) |
Not stated |
| Lin 2000 | Co‐incubation time: 1, 3h Polyspermy rate: 6.8% (8/117) |
Co‐incubation time: 16‐18h Polyspermy rate: 4.0% (9/223) |
Not stated |
| Waldenström 1998 | Co‐incubation time:2h Polyspermy rate: 1.6% (4/255) |
Co‐incubation time: 16‐18h Polyspermy rate: 3.3% (10/302) |
Not stated |
6. Implantation rate
Two studies (Dirnfeld 1999; Gianaroli 1996b) reported implantation rate and showed a significantly higher implantation rate in the brief co‐incubation group (see Additional tables: Table 4).
3. Implantation rate.
| Study ID | Brief co‐incubation | Standard insemination | P value |
| Dirnfeld 1999 | Co‐incubation time: 1h Implantation rate: 16.2% (31/191) |
Co‐incubation time: 16‐20h Implantation rate: 9.8% (23/234) |
P < 0.05 |
| Gianaroli 1996b | Co‐incubation time: 1h Implantation rate: 11.9% (31/261) |
Co‐incubation time: 16h Implantation rate: 5.6% (14/249) |
P < 0.05 |
Sensitivity analysis
In this review, since no substantial heterogeneity was detected, sensitivity analysis was not performed. There was only one trial which divided the two study groups into two subgroups based on sperm quality, therefore subgroup analysis was not performed.
Discussion
Summary of main results
This systematic review and meta‐analysis aimed to investigate whether brief co‐incubation of oocytes and sperm in IVF techniques made a difference to the outcomes of live birth, pregnancy, and the adverse event of miscarriage. Data per randomized woman were able to be extracted from four of these included trials and were included in the final analysis. Brief co‐incubation appeared to be associated with an increase in ongoing pregnancy and clinical pregnancy rates. No trial reported live births. Only one trial reported miscarriage rate in the comparison of brief co‐incubation versus standard insemination, and no significant difference was found between the brief co‐incubation and the control group.
The data for the outcomes of fertilization rate, polyspermy rate and implantation rate were unable to be pooled and therefore were presented in narrative form. Eight trials reported fertilization rate, and one trial (Boone 2001) reported a significantly lower fertilization rate in the brief co‐incubation group compared with the standard insemination group. One trial (Gianaroli 1996b) showed a significantly higher fertilization rate in the brief co‐incubation group. No significant difference was reported in the polyspermy rate between the brief co‐incubation group and the control in the four trials reporting polyspermy rate. Two trials reporting implantation rate showed a significantly higher implantation rate in the brief co‐incubation group (Dirnfeld 1999; Gianaroli 1996b).
Overall completeness and applicability of evidence
Data per randomized woman were able to be extracted from four of the eight included trials and were included in the final analysis. Live birth was not reported in the included trials. Ongoing pregnancy and clinical pregnancy were reported in two and three trials respectively. Heterogeneity between the trials was low. The adverse event outcome miscarriage was reported in one trial. Comparisons of the brief co‐incubation and standard insemination protocols showed a significantly higher ongoing pregnancy rate and clinical pregnancy rate in the brief co‐incubation group. The comparison did not show any significant difference in the miscarriage rate.
Quality of the evidence
The eight included trials provided low quality evidence. All the included trials were described as randomized, but only 37.5% (3/8) gave information on how the randomization was achieved. All the included studies had unclear methods of allocation concealment. Blinding was not described in the trials, but blinding was not likely to cause performance bias or detection bias in this review. More than half of the included trials were at low risk of attrition bias. Four of the included trials failed to report any relevant per woman clinical outcomes. All of the included trials were at unclear risk of reporting bias for the major outcome, as live birth was not reported. Figure 5 and Figure 6 show the review authors' judgements about the methodological quality of the trials included in this review.
5.
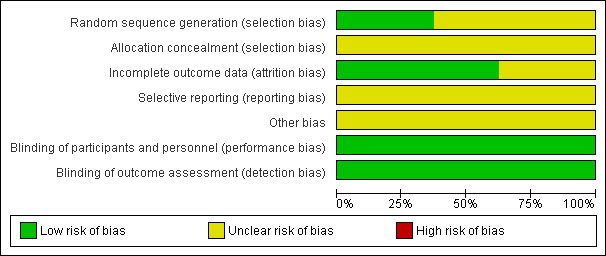
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
6.
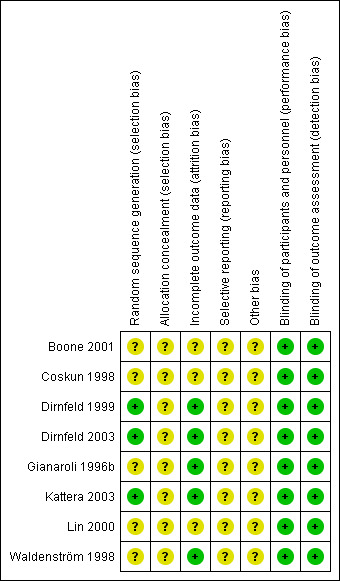
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Potential biases in the review process
We made strenuous efforts to identify all relevant studies. Some bias may have arisen due to the inclusion of trials which included women with mild to moderate male factor infertility and with imbalances between the two groups.
Agreements and disagreements with other studies or reviews
There are no other similar reviews evaluating the effect of brief co‐incubation on IVF outcomes compared to standard insemination protocols.
Authors' conclusions
Implications for practice.
This review provided evidence that brief co‐incubation of sperm and oocytes may improve the ongoing pregnancy rate and clinical pregnancy rate for infertile women undergoing IVF cycles but as there were few studies reporting live birth or miscarriage and the quality of the studies was considered low, more research is required to further substantiate these conclusions.
Implications for research.
More randomized controlled trials are required to assess whether brief co‐incubation contributes to a higher live‐birth rate and a lower miscarriage rate compared with the standard overnight insemination protocol. Full descriptions of the methods including allocation concealment and the method of randomization are required to properly describe the quality of the studies.
Notes
None
Acknowledgements
The authors gratefully acknowledge the Cochrane Menstrual Disorders and Subfertility Group for assistance throughout the protocol and the review process, especially the help of the Managing Editors Jane Clarke and Helen E Nagels, the Trials Search Co‐ordinator Marian Showell, and the Editor Jane Marjoribanks.
Appendices
Appendix 1. CENTRAL search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <3rd Quarter 2011>
1 exp Fertilization in Vitro/ (1422)
2 Vitro Fertilization$.tw. (1174)
3 Vitro Fertilisation$.tw. (113)
4 ivf.tw. (1815)
5 exp Sperm‐Ovum Interactions/ or exp Ovum/ (499)
6 Ovum.tw. (71)
7 exp oocytes/ or exp spermatozoa/ (638)
8 (oocyte$ or sperm$).tw. (2856)
9 gamete$.tw. (82)
10 or/1‐9 (4331)
11 co incubat$.tw. (15)
12 coincubat$.tw. (16)
13 incubat$.tw. (1102)
14 exp Incubators/ (62)
15 or/11‐14 (1134)
16 10 and 15 (102)
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <1st Quarter 2012>
1 exp Fertilization in Vitro/ (1422)
2 Vitro Fertilization$.tw. (1174)
3 Vitro Fertilisation$.tw. (113)
4 ivf.tw. (1815)
5 exp Sperm‐Ovum Interactions/ or exp Ovum/ (499)
6 Ovum.tw. (71)
7 exp oocytes/ or exp spermatozoa/ (638)
8 (oocyte$ or sperm$).tw. (2856)
9 gamete$.tw. (82)
10 or/1‐9 (4331)
11 co incubat$.tw. (15)
12 coincubat$.tw. (16)
13 incubat$.tw. (1102)
14 exp Incubators/ (62)
15 or/11‐14 (1134)
16 10 and 15 (102)
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1948 to 22.09.11>
1 exp Fertilization in Vitro/ (25010)
2 Vitro Fertilization$.tw. (13856)
3 Vitro Fertilisation$.tw. (1162)
4 ivf.tw. (13792)
5 exp Sperm‐Ovum Interactions/ or exp Ovum/ (67802)
6 Ovum.tw. (2775)
7 exp oocytes/ or exp spermatozoa/ (84285)
8 (oocyte$ or sperm$).tw. (136219)
9 gamete$.tw. (7773)
10 or/1‐9 (192250)
11 co incubat$.tw. (3263)
12 coincubat$.tw. (3439)
13 incubat$.tw. (241449)
14 exp Incubators/ (1371)
15 or/11‐14 (244305)
16 10 and 15 (8626)
17 randomized controlled trial.pt. (317580)
18 controlled clinical trial.pt. (83546)
19 randomized.ab. (233020)
20 placebo.tw. (136458)
21 clinical trials as topic.sh. (158204)
22 randomly.ab. (170925)
23 trial.ti. (99809)
24 (crossover or cross‐over or cross over).tw. (52130)
25 or/17‐24 (777956)
26 exp animals/ not humans.sh. (3671821)
27 25 not 26 (718567)
28 16 and 27 (121)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to 14.06.12 >
1 exp Fertilization in Vitro/ (25767)
2 Vitro Fertilization$.tw. (14174)
3 Vitro Fertilisation$.tw. (1237)
4 ivf.tw. (14385)
5 exp Sperm‐Ovum Interactions/ or exp Ovum/ (69219)
6 Ovum.tw. (2809)
7 exp oocytes/ or exp spermatozoa/ (86031)
8 (oocyte$ or sperm$).tw. (139804)
9 gamete$.tw. (8121)
10 or/1‐9 (197302)
11 co incubat$.tw. (3397)
12 coincubat$.tw. (3489)
13 incubat$.tw. (246383)
14 exp Incubators/ (1383)
15 or/11‐14 (249281)
16 10 and 15 (8804)
17 randomized controlled trial.pt. (330191)
18 controlled clinical trial.pt. (84357)
19 randomized.ab. (245374)
20 placebo.tw. (140874)
21 clinical trials as topic.sh. (160749)
22 randomly.ab. (179900)
23 trial.ti. (105611)
24 (crossover or cross‐over or cross over).tw. (53748)
25 or/17‐24 (808768)
26 exp animals/ not humans.sh. (3734130)
27 25 not 26 (746338)
28 16 and 27 (125)
29 (20119$ or 201110$ or 201111$ or 201112$).ed. (219454)
30 2012$.ed. (393447)
31 29 or 30 (612901)
32 28 and 31 (5)
Appendix 3. EMBASE search strategy
Database: Embase <1980 to 2011 Week 37>
1 exp Fertilization in Vitro/ (32563)
2 Vitro Fertilization$.tw. (15641)
3 Vitro Fertilisation$.tw. (1468)
4 ivf.tw. (18321)
5 Ovum.tw. (2695)
6 exp oocytes/ (50525)
7 exp spermatozoon/ (29643)
8 (oocyte$ or sperm$).tw. (140629)
9 gamete$.tw. (8303)
10 or/1‐9 (187131)
11 exp INCUBATOR/ (1735)
12 co incubat$.tw. (3703)
13 coincubat$.tw. (3649)
14 incubat$.tw. (243929)
15 or/11‐14 (246800)
16 10 and 15 (8108)
17 Clinical Trial/ (814550)
18 Randomized Controlled Trial/ (286688)
19 exp randomization/ (53948)
20 Single Blind Procedure/ (14015)
21 Double Blind Procedure/ (100257)
22 Crossover Procedure/ (30553)
23 Placebo/ (184198)
24 Randomi?ed controlled trial$.tw. (63407)
25 Rct.tw. (7522)
26 random allocation.tw. (1044)
27 randomly allocated.tw. (15386)
28 allocated randomly.tw. (1694)
29 (allocated adj2 random).tw. (686)
30 Single blind$.tw. (10976)
31 Double blind$.tw. (117390)
32 (treble or triple) adj blind$).tw. (242)
33 placebo$.tw. (158558)
34 prospective study/ (170289)
35 or/17‐34 (1136238)
36 case study/ (13275)
37 case report.tw. (206022)
38 abstract report/ or letter/ (791249)
39 or/36‐38 (1006560)
40 35 not 39 (1102983)
41 16 and 40 (177)
42 (2010$ or 2011$).em. (2041700)
43 41 and 42 (43)
Database: Embase <1980 to 2012 Week 23>
1 exp Fertilization in Vitro/ (34764)
2 Vitro Fertilization$.tw. (16722)
3 Vitro Fertilisation$.tw. (1676)
4 ivf.tw. (20136)
5 Ovum.tw. (2815)
6 exp oocytes/ (54770)
7 exp spermatozoon/ (31795)
8 (oocyte$ or sperm$).tw. (150540)
9 gamete$.tw. (8988)
10 or/1‐9 (200565)
11 exp INCUBATOR/ (1996)
12 co incubat$.tw. (4166)
13 coincubat$.tw. (3894)
14 incubat$.tw. (259760)
15 or/11‐14 (262839)
16 10 and 15 (8597)
17 Clinical Trial/ (866364)
18 Randomized Controlled Trial/ (323003)
19 exp randomization/ (58330)
20 Single Blind Procedure/ (15953)
21 Double Blind Procedure/ (109131)
22 Crossover Procedure/ (34020)
23 Placebo/ (199298)
24 Randomi?ed controlled trial$.tw. (75150)
25 Rct.tw. (9305)
26 random allocation.tw. (1147)
27 randomly allocated.tw. (17143)
28 allocated randomly.tw. (1807)
29 (allocated adj2 random).tw. (706)
30 Single blind$.tw. (12185)
31 Double blind$.tw. (127866)
32 ((treble or triple) adj blind$).tw. (269)
33 placebo$.tw. (174830)
34 prospective study/ (205050)
35 or/17‐34 (1249368)
36 case study/ (15738)
37 case report.tw. (225200)
38 abstract report/ or letter/ (833427)
39 or/36‐38 (1069815)
40 35 not 39 (1214471)
41 16 and 40 (187)
42 (2011$ or 2012$).em. (1601825)
43 41 and 42 (16)
Appendix 4. PsycINFO search strategy
Database: PsycINFO <1806 to September Week 3 2011>
1 exp Reproductive Technology/ (1090)
2 Vitro Fertilization$.tw. (408)
3 Vitro Fertilisation$.tw. (51)
4 ivf.tw. (300)
5 Ovum.tw. (110)
6 (oocyte$ or sperm$).tw. (2506)
7 gamete$.tw. (179)
8 or/1‐7 (3690)
9 co incubat$.tw. (37)
10 coincubat$.tw. (32)
11 incubat$.tw. (2894)
12 or/9‐11 (2916)
13 8 and 12 (26)
Database: PsycINFO <1806 to June Week 1 2012>
1 exp Reproductive Technology/ (1143)
2 Vitro Fertilization$.tw. (428)
3 Vitro Fertilisation$.tw. (63)
4 ivf.tw. (321)
5 Ovum.tw. (113)
6 (oocyte$ or sperm$).tw. (2665)
7 gamete$.tw. (190)
8 or/1‐7 (3907)
9 co incubat$.tw. (44)
10 coincubat$.tw. (34)
11 incubat$.tw. (3062)
12 or/9‐11 (3085)
13 8 and 12 (31)
14 limit 13 to yr="2011 ‐Current" (7)
Appendix 5. CINAHL search strategy
CINAHL search 26.07.12
S1 (MM "Fertilization in Vitro") OR "ivf" 1434
S2 (MM "Spermatozoa") 377S12 S5 and S11 11
S3 TX spermatozoa 762
S4 (MM "Ovum") OR "oocyte" 666
S5 S1 or S2 or S3 or S4 2618
S6 "co incubation" 38
S7 TX co incubat* 81
S8 "incubation" 1290
S9 "incubator" 252
S10 TX incubat* 2830
S11 S6 or S7 or S8 or S9 or S10 2830
S12 S5 and S11 11
Data and analyses
Comparison 1. Brief co‐incubation versus standard insemination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ongoing pregnancy per randomized woman | 2 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.55, 3.77] |
| 2 Clinical pregnancy rate per randomized woman | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.45, 3.85] |
| 3 Miscarriage rate per randomized woman | 1 | 167 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.35, 11.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boone 2001.
| Methods | Randomized controlled trial | |
| Participants | Country: USA
Female infertility
Mean age: 32.8 years ( age range 23‐40 years)
n=20 recruited
Inclusion criteria: Infertile women with known indications for conventional IVF, under the age of 41 years. Patients provided at least 9 oocytes retrieved on the day of oocyte recovery during in vitro fertilization treatment were included. |
|
| Interventions | Short exposure (3h) versus control standard IVF procedure (19h) |
|
| Outcomes | Fertilization rate, polyploid rate, embryo cell stages and quality scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Oocytes randomized into two treatment groups; randomization method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per woman data not reported |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Coskun 1998.
| Methods | Randomized controlled trial | |
| Participants | Country: Saudi Arabia
Infertile women
mean age 32.1 (32.1 ± 4.9 years)
n=36 recruited Inclusion criteria: Infertile women with known indications for conventional IVF. Patients with six or more oocytes retrieved on the day of oocyte recovery during in vitro fertilization treatment were included. |
|
| Interventions | Reduced insemination (1h ) versus regular insemination (18h) | |
| Outcomes | Fertilization rate, embryo cell stages and embryo quality grading | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Oocytes from each patient were randomly allocated to two treatment groups; randomization method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per woman data not reported |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Dirnfeld 1999.
| Methods | Randomized controlled trial | |
| Participants | Country: Israel Female infertility Mean age: 32.8 ± 3.8 years for short exposure group, 33.2 ± 4.2 years for standard exposure group (range 23‐34 years) n= 158 recruited Inclusion criteria: infertile women with known indications for conventional IVF, with normo‐ovulatory, 23‐41 years, with normal uterine morphology and endometrial line (assessed by hysteron‐salpingography and ultrasound) Exclusion criteria: very poor responders, patients with polycystic ovary syndrome and men with severe oligozoospermia |
|
| Interventions | Short exposure (1h) versus control standard IVF procedure (16‐20h) |
|
| Outcomes | Clinical pregnancy rate, fertilization rate, cleavage rate, embryo quality grading and implantation rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized into two study groups using standard random number tables |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Dirnfeld 2003.
| Methods | Randomized controlled trial | |
| Participants | Country: Israel Female infertility Mean age: 30±4.5 years for short exposure group, 30±6.1 years for standard exposure group n=23 recruited Inclusion criteria: infertile women with known indications for conventional IVF, with normal cycles and a normal endometrial lining (demonstrated by a previous hysterosalpingography) Exclusion criteria: very poor responders, patients with unexplained infertility and patients who were regularly taking any drugs other than those for infertility |
|
| Interventions | Short exposure (2h) versus control standard exposure (16‐20h) |
|
| Outcomes | Fertilization rate, cleavage rate and embryo quality grading | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized into two treatment groups using standard random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Gianaroli 1996b.
| Methods | Randomized controlled trial | |
| Participants | Country: Italy Female infertility n=167 recruited Inclusion criteria: infertile women with known indications for conventional IVF, with normo‐ovulatory, aged ≤38years, normal uterine morphology and endometrial biopsies. Duration of study: 18 months | |
| Interventions | Short exposure (1h) versus control standard exposure (16h) |
|
| Outcomes | Ongoing pregnancy rate, clinical pregnancy rate, miscarriage rate, fertilization rate, polypronuclear rate, implantation rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of how randomization was carried out |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not mentioned, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Kattera 2003.
| Methods | Randomized controlled trial | |
| Participants | Country: Singapore Female infertility (range: 25–44 years) Mean age: 35.4 ± 4.1 years for short exposure group, 35.1 ± 3.9 years for standard exposure group n=259 recruited Inclusion criteria: infertile women with known indications for conventional IVF Exclusion criteria: very poor responders (those who produced fewer than three follicles) and men with severe oligoasthenoteratozoospermia Duration of study: 18 months |
|
| Interventions | Short co‐incubation (2h) versus long co‐incubation (20h) |
|
| Outcomes | Ongoing pregnancy rate, fertilization rate, abnormal fertilization rate, embryo grading and implantation rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomized into two groups using standard random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not mentioned, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Lin 2000.
| Methods | Randomized controlled trial | |
| Participants | Country: Taiwan, China Female infertility n=23 recruited Inclusion criteria: subfertile women with known indications for conventional IVF Exclusion criteria: women with male infertility factors |
|
| Interventions | Short time co‐incubation (1h or 3h) versus standard overnight gamete co‐incubation (16‐18h) |
|
| Outcomes | Fertilization rate, abnormal fertilzation rate, cleavage rate and embryo quality grading | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The patients were randomly allocated to two groups. No details of how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Per woman data not reported |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not mentioned, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Waldenström 1998.
| Methods | Randomized controlled trial | |
| Participants | Country: Sweden Female infertility Mean age: 32 years (range 25–40 years) n=47 recruited Inclusion criteria: infertile women with known indications for standard IVF Exclusion criteria: infertility with male factors, poor responders |
|
| Interventions | Short time sperm exposure (1.5‐2h) versus long time sperm exposure (16‐18h) |
|
| Outcomes | Clinical pregnancy rate, fertilization rate, polyspermy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomized to two treatment groups. How randomization performed was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Did not report live birth, the primary outcome of the review |
| Other bias | Unclear risk | Can not definitively assess other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not stated, but the review authors considered that blinding was not likely to influence the risk of performance bias for the primary review outcome and secondary outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barraud 2003 | Quasi‐randomized controlled trial. |
| Barraud 2008 | Quasi‐randomized controlled trial. |
| Bungum 2005 | Not a randomized controlled trial. Interventions did not meet the requirements. |
| Bungum 2006 | Not a randomized controlled trial. Interventions did not meet the requirements. |
| Gianoroli 1996a | There were some overlap of participation between the two studies (Gianoroli 1996a and 1996b), the former one with smaller sample size was excluded after contacting authors for detailed information. |
| Granham 1999 | Lacked appropriate inclusion criteria for population. |
| Hammit 1999 | Not a randomized controlled trial. |
| Lundqvist 2001 | Not a randomized controlled trial. |
| Navarro 2004 | Quasi‐randomized controlled trial. |
| Quinn 1998 | Quasi‐randomized controlled trial. |
| Swenson 2000 | Quasi‐randomized controlled trial. |
| Swenson 1998 | Not a randomized controlled trial. |
| Swenson 1999 | Superseded by full paper of the trial ‐ Swenson 2000. |
| Xiong 2011 | Not a randomized controlled trial. |
| Zhang 2009 | Not a randomized controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Jamieson 1999.
| Methods | To be added when confirmed. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pattanayak 2001.
| Methods | To be added when confirmed. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
Secondary outcomes of fertilization rate and implantation rate were added as they are thought to be two important outcomes relating to insemination protocols. Considering multiple pregnancy rate was to a large extent influenced by number of embryos transferred and was less likely associated with insemination methods, it was removed from the secondary outcomes in the protocol.
Contributions of authors
HZY: proposed the original title and developed the draft of the protocol, performed the searches, selected trials for inclusion, assessed quality, performed data extraction, entered data and wrote the final review.
LJ: advised and supervised both the protocol and review, assisted in developing the protocol, selecting trials for inclusion, assessing quality, and was consultant on methodological issues.
WL: advised and supervised both the protocol and review, assisted in selecting trials for inclusion, assessing quality, and was consultant on methodological issues.
YJ: assisted in selecting trials for inclusion and extracting the data.
SYJ: assisted in revising and editing the protocol and the review.
LSW: assisted in revising the protocol.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None
New
References
References to studies included in this review
Boone 2001 {published data only}
- Boone WR, Johnson JE. Extending the coincubation time of gametes improves invitro fertilization. Journal of Assisted Reproduction and Genetics 20001;18(1):18‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coskun 1998 {published data only}
- Coskun S, Roca GL, Elnour AM, al Mayman H, Hollanders JM, Jaroudi KA. Effects of reducing insemination time in human in vitro fertilization and embryo development by using sibling oocytes. Journal of Assisted Reproduction and Genetics 1998;15(10):605‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dirnfeld 1999 {published data only}
- Dirnfeld M, Bider D, Koifman M, Calderon I, Abramovici H. Shortened exposure of oocytes to spermatozoa improves in vitro fertilization outcome: a prospective randomized, controlled study. Human Reproduction 1999;14(10):2562‐4. [DOI] [PubMed] [Google Scholar]
Dirnfeld 2003 {published data only}
- Dirnfeld M, Shiloh H, Bider D, Harari E, Koifman M, Lahav‐Baratz S, Abramovici H. A prospective randomized controlled study of the effect of short coincubation of gametes during insemination on zona pellucida thickness. Gynecological Endocrinology 2003;17(5):397‐403. [DOI] [PubMed] [Google Scholar]
Gianaroli 1996b {published data only}
- Gianaroli L, Fiorentino A, Magli MC, Ferraretti AP, Montanaro N. Prolonged sperm‐oocyte exposure and high sperm concentration affect human embryo viability and pregnancy rate. Human Reproduction 1996;11(11):2507‐11. [DOI] [PubMed] [Google Scholar]
Kattera 2003 {published data only}
- Kattera S, Chen C. Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertility and Sterility 2003;80(4):1017‐21. [DOI] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin SP, Lee RK, Su JT, Lin MH, Hwu YM. The effects of brief gamete co‐incubation in human in vitro fertilization. Journal of Assisted Reproduction and Genetics 2000;17(6):344‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waldenström 1998 {published data only}
- Waldenström U, Hamberger L, Nilsson L, Ryding M. Short‐time sperm‐egg exposure to prevent zona hardening. Journal of Assisted Reproduction and Genetics 1998;70:190. [Google Scholar]
References to studies excluded from this review
Barraud 2003 {published data only}
- Barraud V, Sifer C, Lemkecher B, Martin‐Pont T, Sellami A, Porcher R, et al. Short time sperm‐oocyte exposure during IVF improves embryo quality. Proceedings Title: The 19th Annual Meeting of the ESHRE, Madrid, Spain. 2003:i156‐7.
Barraud 2008 {published data only}
- Barraud‐Lange V, Sifer C, Pocaté K, Ziyyat A, Martin‐Pont B, Porcher R, et al. Short gamete co‐incubation during in vitro fertilization decreases the fertilization rate and does not improve embryo quality: a prospective auto controlled study. Journal of Assisted Reproduction and Genetics 2008;25(7):305‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bungum 2005 {published data only}
- Bungum L, Humaidan P, Bungum M. Effect of ultrashort coincubation (30 seconds) of gametes in IVF. Proceedings Title: The 21st Annual Meeting of the ESHRE, Copenhagen, Denmark. 2005:i157.
Bungum 2006 {published data only}
- Bungum M, Bungum L, Humaidan P. A prospective study, using sibling oocytes, examining the effect of 30 seconds versus 90 minutes gamete co‐incubation in IVF. Human Reproduction 2006;21(2):518‐23. [DOI] [PubMed] [Google Scholar]
Gianoroli 1996a {published data only}
- Gianaroli L, Cristina Magli M, Ferraretti AP, Fiorentino A, Tosti E, Panzella S, Dale B. Reducing the time of sperm‐oocyte interaction in human in‐vitro fertilization improves the implantation rate. Human Reproduction 1996;11(1):166‐71. [DOI] [PubMed] [Google Scholar]
Granham 1999 {published data only}
- Graham MC, Phipps WR, Partridge AB, Paulhamus LA. Lack of effect of shortened sperm‐oocyte exposure time on in vitro fertilization (IVF) and embryo quality. Fertility and Sterility 1999;72(3 Suppl 1):S15. [Google Scholar]
Hammit 1999 {published data only}
- Hammitt DG, Barud KM, Galanits TM, Wentworth MA, Walker DL, Damario MA, et al. Comparison of short and long sperm‐oocyte coincubation in organ culture dishes (OCD) and tubes (TU). Fertility and Sterility 1999;72(3 Suppl 1):S15. [Google Scholar]
Lundqvist 2001 {published data only}
- Lundqvist M, Johansson U, Lundkvist O, Milton K, Westin C, Simberg N. Reducing the time of co‐incubation of gametes in human in‐vitro fertilization has no beneficial effects. Reproductive Biomedicine Online 2001;3(1):21‐4. [DOI] [PubMed] [Google Scholar]
Navarro 2004 {published data only}
- Navarro C, Lopez E, Stone BA, Marrs RP. Short co‐incubation of sperm with oocytes is associated with an increased incidence of polyspermy in human in vitro fertilization (IVF). Fertility and Sterility 2004;82:S325. [Google Scholar]
Quinn 1998 {published data only}
- Quinn P, Lydic ML, Ho M, Bastuba M, Hendee F, Brody SA. Confirmation of the beneficial effects of brief coincubation of gametes in human in vitro fertilization. Fertility and Sterility 1998;69(3):399‐402. [DOI] [PubMed] [Google Scholar]
Swenson 1998 {published data only}
- Swenson K, Summers‐Chase D, Check JH, Choe J. Preliminary evidence that shortening the incubation time of sperm and oocyte may improve implantation rates with in vitro fertilization (IVF). Fertility and Sterility 1998;69(3):594. [Google Scholar]
Swenson 1999 {published data only}
- Swenson K, Check JH, Summers‐Chase D, Choe JK, Nazari A. A randomized study comparing the effect of standard versus short incubation of sperm and oocyte on subsequent pregnancy and implantation rates following in vitro fertilization‐embryo transfer (IVF‐ET). Fertility and Sterility 1999;72 Suppl 1:16S‐7S. [DOI] [PubMed] [Google Scholar]
Swenson 2000 {published data only}
- Swenson K, Check JH, Summers‐Chase D, Choe JK, Check ML. A randomized study comparing the effect of standard versus short incubation of sperm and oocyte on subsequent pregnancy and implantation rates following in vitro fertilization embryo transfer. Archives of Andrology 2000;45(1):73‐6. [DOI] [PubMed] [Google Scholar]
Xiong 2011 {published data only}
- Xiong S, Han W, Liu JX, Zhang XD, Liu WW, Liu H, Huang GN. Effects of cumulus cells removal after 6 h co‐incubation of gametes on the outcomes of human IVF. Journal of Assisted Reproduction and Genetics 2011;28:1205‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang NY, Sun HX, Hu YL, Wang B, Xu ZP. Combination of short‐period sperm‐oocyte coincubation and early rescue intracytoplasmic sperm injection after total failure of in vitro fertilization. Zhonghua Nan Ke Xue 2009;15(6):538‐41. [PubMed] [Google Scholar]
References to studies awaiting assessment
Jamieson 1999 {published data only}
- Jamieson ME, George MA, Butcher L, Grossart E, Mitchell PAL, Mowat L, et al. The effect of reduced sperm :oocyte exposure time on embryo development. Proceedings Title: 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics 1999:115.
Pattanayak 2001 {published data only}
- Pattanayak M, Ray R, Santosh L. A comparative study of short and standard exposures of oocytes to sperm in in vitro fertilisation. Proceedings Title: 17th World Congress on Fertility and Sterility 2001:24.
Additional references
Agarwal 2005
- Agarwal A, Allamaneni S, Nallella K, George A, Mascha E. Correlation of reactive oxygen species levels with the fertilisation rate after in vitro fertilisation: a qualified meta‐analysis. Fertility and Sterility 2005;84:228‐31. [DOI] [PubMed] [Google Scholar]
Aitken 1996
- Aitken RJ, Buckingham DW, West K, Brindle J. On the use of paramagnetic beads and ferro fluids to assess and eliminate the leukocytic contribution to oxygen radical generation by human sperm suspensions. American Journal of Reproductive Immunology 1996;35:541‐51. [DOI] [PubMed] [Google Scholar]
Aitken 1987
- Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Journal of Reproduction and Fertility 1987;81:459‐69. [DOI] [PubMed] [Google Scholar]
Angell 1986
- Angell RR, Templeton AA, Messinis IE. Consequences of polyspermy in man. Cytogenetics and Cell Genetics 1986;42:1‐7. [DOI] [PubMed] [Google Scholar]
Bansal 2010
- Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Veterinary Medicine International 2010;pii:686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bedaiwy 2004
- Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertility and Sterility 2004;82:593‐600. [DOI] [PubMed] [Google Scholar]
Canipari 2000
- Canipari R. Oocyte‐‐granulosa cell interactions. Human Reproduction Update 2000;6:279‐89. [DOI] [PubMed] [Google Scholar]
De Placido 2002
- Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, et al. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Human Reproduction 2002;17:2402‐9. [DOI] [PubMed] [Google Scholar]
du Plessis 2008
- du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expert Review of Obstetrics and Gynecology 2008;3:539‐54. [Google Scholar]
Dumoulin1992
- Dumoulin JCM, Bras M, Land JA, Pieters MH, Enginsu ME, Gerardts JP, Evers JL. Effect of the number of inseminated spermatozoa on subsequent human and mouse embryonic development in vitro. Human Reproduction 1992;7:1010‐3. [DOI] [PubMed] [Google Scholar]
Evenson 2000
- Evenson DP, Jost LK. Sperm chromatin structure assay is useful for fertility assessment. Methods in Cell Science 2000;22:169‐89. [DOI] [PubMed] [Google Scholar]
Fiorentino1994
- Fiorentino A, Magli MC, Fortini D, Feliciani E, Ferraretti AP, Dale B, Gianaroli L. Sperm:oocyte ratios in an in vitro fertilization (IVF) program. Journal of Assisted Reproduction and Genetics 1994;11:97‐103. [DOI] [PubMed] [Google Scholar]
Gianaroli 1996a
- Gianaroli L, Cristina Magli M, Ferraretti AP, Fiorentino A, Tosti E, Panzella S, Dale B. Reducing the time of sperm‐oocyte interaction in human in‐vitro fertilization improves the implantation rate. Human Reproduction 1996;11(1):166‐71. [DOI] [PubMed] [Google Scholar]
Henkel 2003
- Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reproductive Biomedicine Online 2003;7:477‐84. [DOI] [PubMed] [Google Scholar]
Henkel 2011
- Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian Journal of Andrology 2011;13:43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration, 2011. [Google Scholar]
Hopewell 2007a
- Hopewell S, Clarke MJ, Lefebvre C, Scherer RW. Handsearching versus electronic searching to identify reports of randomised trials. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopewell 2007b
- Hopewell S, McDonald S, Clarke MJ, Egger M. Grey literature in meta‐analyses of randomized trials of health care interventions. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopewell 2007c
- Hopewell S, Clarke MJ, Stewart L, Tierney J. Time to publication for results of clinical trials. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krausz 1994
- Krausz C, Mills C, Rogers S, Tan SL, Aiken RJ. Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: Relationships with motility and fertilization in vitro. Fertility and Sterility 1994;62:599‐605. [DOI] [PubMed] [Google Scholar]
Larson 2000
- Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Human Reproduction 2000;15:1717‐22. [DOI] [PubMed] [Google Scholar]
Lopes 1998
- Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor‐quality semen samples and correlates with failed fertilisation in intracytoplasmic sperm injection. Fertility and Sterility 1998;69:528‐32. [DOI] [PubMed] [Google Scholar]
Mahfouz 2010
- Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, Agarwal A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertility and Sterility 2010;94:2141‐6. [DOI] [PubMed] [Google Scholar]
Nasr‐Esfahani 1990
- Nasr‐Esfahani MH, Aitken RJ, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990;109:501‐7. [DOI] [PubMed] [Google Scholar]
Parinaud 1993
- Parinaud J, Labal B, Mieusset R, Richoilley G, Vieitez G. Influence of sperm parameters on embryo quality. Fertility and Sterility 1993;60:888‐92. [DOI] [PubMed] [Google Scholar]
Saleh 2003
- Saleh RA, Agarwal A, Nada EA, El‐Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertility and Sterility 2003;79:1597‐605. [DOI] [PubMed] [Google Scholar]
Simon 2011
- Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertility and Sterility 2011;95:652‐7. [DOI] [PubMed] [Google Scholar]
Song 2002
- Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta‐analyses of diagnostic accuracy. International Journal of Epidemiolology 2002;31:88‐95. [DOI] [PubMed] [Google Scholar]
Twigg 1998
- Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Human Reproduction 1998;13:1429‐36. [DOI] [PubMed] [Google Scholar]
van der Ven 1985
- Ven HH, Al‐Hasani S, Diedrich K, Hamerich U, Lehmann F, Krebs D. Polyspermy in in vitro fertilization of human oocytes: frequency and possible causes. Annals of the New York Academy of Sciences 1985;442:88‐95. [DOI] [PubMed] [Google Scholar]
van Loendersloot 2010
- Loendersloot LL, Wely M, Limpens J, Bossuyt PM, Repping S, Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta‐analysis. Human Reproduction Update 2010;16:577‐89. [DOI] [PubMed] [Google Scholar]
Venkatesh 2011
- Venkatesh S, Shamsi MB, Dudeja S, Kumar R, Dada R. Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Archives of Gynecology and Obstetrics 2011;283:121‐6. [DOI] [PubMed] [Google Scholar]
Virroet 2004
- Virro MR, Larson‐Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilisation, blastocyst development,and ongoing pregnancy in in vitro fertilisation and intracytoplasmic sperm injection cycles. Fertility and Sterility 2004;81:1289‐95. [DOI] [PubMed] [Google Scholar]
Wang 2003
- Wang WH, Day BN, Wu GM. How does polyspermy happen in mammalian oocytes?. Microscopy Research and Technique 2003;61:335‐41. [DOI] [PubMed] [Google Scholar]
Zorn 2003
- Zorn B, Vidmar G, Meden‐Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. International Journal of Andrology 2003;26:279‐85. [DOI] [PubMed] [Google Scholar]


