Abstract
Objective.
To evaluate mortality risk and predictors among recently diagnosed systemic lupus erythematosus (SLE) patients.
Methods.
The vital status of 265 SLE patients and 355 controls enrolled in the Carolina Lupus Study (median time since diagnosis 13 months) was determined ~5 years after enrollment. We also assessed the utility of an 8-item quality of life instrument, derived from the standard 36-item Medical Outcomes Study Short Form 36, as an additional measure of disease impact.
Results.
Five years after diagnosis, 9.7% of patients compared with 0.3% of controls had died (P < 0.0001). Increased mortality risk was seen among older patients (adjusted hazard ratio [HR] 1.03, 95% confidence interval [95% CI] 1.01–1.06 per 1-year increment in age) and among men, African Americans, patients with lupus nephritis, and patients with anti–double-stranded DNA antibodies (adjusted HR ~2.0 for each of these factors). In addition, patients who did not provide a blood sample at study enrollment experienced increased mortality risk (age-, sex-, and race-adjusted HR 3.7, 95% CI 1.5–9.1). Similar results were seen in analyses limited to time from study enrollment. Physical component scores of the quality of life measure were 7.7 points lower (P < 0.0001) and mental component scores were 1.8 points lower (P = 0.07) in patients compared with controls.
Conclusion.
The mortality risk among SLE patients is significant, particularly among African Americans, even early in the disease process and even with currently available treatments. Differences between cases and controls in health-related quality of life using the Short Form 8 also demonstrate the multidimensional burden of SLE.
INTRODUCTION
Survival rates for patients with systemic lupus erythematosus (SLE) have increased significantly since the mid-1950s, when a 5-year survival rate of 51% in 99 clinic-based patients with SLE in the US was reported (1). In a recent review by Trager and Ward (2) of inception cohorts and near-inception cohorts (i.e., studies identifying patients within 2–3 years of diagnosis), and in studies published subsequent to this review, 5-year survival rates of 90% or higher were generally seen. Likely contributions to this improvement include earlier diagnosis, improved treatment of comorbidities, and the use of immunosuppressant medications.
Limited data are currently available regarding the association between demographic and clinical factors and mortality risk early in the disease course, because many of the inception cohorts are fairly small (<100 patients) and present analyses of factors without adjustment for potential confounders. We analyzed 5-year survival rates and demographic and clinical predictors of mortality risk in a cohort of 265 recently diagnosed patients enrolled in the Carolina Lupus Study (CLU). In addition to assessing the clinical burden of patients measured as 5-year mortality, we took a multidimensional approach to disease burden by including a humanistic analysis of quality of life using the recently developed and validated Short Form 8 (SF-8) (3). There are no previous reports measuring the quality of life of patients with SLE and age- and sex-matched controls using this scale.
PATIENTS AND METHODS
Study population and data collection.
The CLU is a population-based case–control study of SLE based in 60 contiguous counties in eastern and central North Carolina and South Carolina. Eligible patients were recruited from community-based rheumatologists and university-based rheumatology practices in the study area, with ~50% coming from each source. Lupus diagnosis was based on fulfillment of the revised American College of Rheumatology classification criteria (4) diagnosed between January 1, 1995 and July 31, 1999; age ≥18 years at study enrollment; residence within the study area during at least 6 months of the year prior to diagnosis; and the ability to speak and understand English. Controls were identified through driver’s license records and were frequency matched to cases by age, sex, and state. In total, 265 cases and 355 controls were enrolled in the study. Details of the recruitment process and study design have been presented previously (5). The study protocol was approved by the review boards at all participating institutions.
The study included the baseline assessment at enrollment (1997–1999), followup assessment conducted in 2001, and continued assessment of vital status through 2004. In the baseline study, data were collected using a structured 60-minute in-person interview. Demographic information (date of birth, education level, race) was obtained at this time. The 2001 followup interview obtained information from 198 cases and 299 controls via a 45-minute and 15-minute telephone interview, respectively. Control interviews were shorter because some sections specific to the clinical course of SLE were not included.
Participation rates in the 2001 followup interview were similar in cases and controls who were alive at the time of contact (82% and 84%, respectively). Similar proportions of cases and controls could not be located (9% and 10%, respectively) or were located but did not participate (9% and 6%, respectively). The median time since diagnosis was 4 years at the time of followup interview. Tracing of the study participants occurred in 2001 and 2004 as part of the steps taken for followup studies. The most recent information on vital status (known alive, known died, or lost contact) was used for the survival analysis.
In 2001, we performed a systematic review of CLU patients’ kidney biopsy records from a network of nephropathologists in the study area. Sixty-three patients were confirmed to have lupus nephritis with 35 classified as proliferative, 16 as membranous, 8 as mesangial, and 4 as unclear classification type.
At the time of the baseline interview, 244 cases (92%) provided a serum sample. We used this sample to determine the presence of antinuclear antibodies by immunofluorescence using HEp-2 cells; anti–native DNA antibody using antibody to native DNA by fixed Crithidia luciliae immunofluorescence; antibodies against Sm, RNP, and La/SSB using a saline nuclear extract derived from rabbit thymus acetone powder (Pel-Freez, Rogers, AR); and antibody to Ro/SSA using a human spleen cell nuclear extract as antigen, as described previously (6).
Analysis of mortality risks.
For the preliminary mortality analysis, we computed the Kaplan-Meier survival probabilities and curves for cases and controls and for different strata (e.g., males and females) among cases using the nonparametric Lifetest procedure in SAS 9.1 via the ODS Statistical Graphics (SAS Institute, Cary, NC). For survival curves, the log rank test and associated probability were used to test for stratum differences and survival times at 60 months. The more formal analyses utilized proportional hazards modeling to assess the covariates associated with mortality risk. Schoenfeld residuals and graphic plots of relationships with time were used to assess the proportional hazards assumption (7,8). Because only 3 deaths were observed among controls, we did not conduct statistical modeling, adjusting for covariates, of mortality risk in cases compared with controls.
Based on the review of information about all referred patients, there were few known losses between diagnosis and study enrollment. In one set of analyses, followup time was calculated from the date of diagnosis for cases (and the corresponding referent date for controls) to date of death or last known contact. Seventeen patients (6%) and 38 controls (6%) were treated as censored at the time of study enrollment in this analysis because no information confirming their vital status, from either the 2001 or 2004 followup effort, was found. We also repeated the analyses, basing followup time from the date of enrollment (study interview) for cases and controls. Individuals who were not found during the 2001 or the 2004 tracing efforts did not contribute any time in this analysis.
Analysis of quality of life differences between cases and controls.
The 2001 followup included the SF-8 Health-Related Quality of Life instrument (3), administered to cases and controls. The 8 items asked about general health, physical function, role physical, bodily pain, vitality, social function, mental health, and role emotion. Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were calculated applying a summated, algebraic algorithm using a norm-based scoring method, as described by Ware et al (3). Mean Short Form 36 (SF-36), version 2 scale scores from the 2000 general population sample were assigned to each SF-8 item response category. Summary scores were based on the summation of the weighted scores (physical weights for the PCS and mental weights for the MCS) for each of the 8 items. Using norm-based scoring, the general population norm is built into the scoring algorithm, with scores above or below 50 interpreted as above or below the mean in the general population. Standard deviation scores for each scale are equalized at 10.
We compared the summary scores for the 2 subscales (physical health and mental health components) by case–control status using box plots of the scores, Student’s t-test to assess the crude (unadjusted) difference in means of the summary scores between groups, and linear regression to adjust for the matching factors used in sample selection (age, sex, and state) and other demographic factors (ethnicity, education).
RESULTS
Survival analysis.
The median time from diagnosis to study interview was 13 months, and 75% of patients were interviewed within 20 months of diagnosis. A total of 32 deaths among cases and 3 deaths among controls were identified. The primary causes of death among cases were cardiovascular disease (cardiovascular collapse, cardiorespiratory arrest, and congestive heart failure) and infection (sepsis) and are indicated in Table 1. The mean ± SD age of the 233 surviving patients was 38.0 ± 14.2 years (range 15–76 years). Among the 32 cases who died, the mean ± SD age was 45.3 ± 17.6 years (range 16–81 years). At 60 months (5 years) postdiagnosis, 9.7% of cases had died compared with 0.3% of controls (P < 0.0001), yielding 5-year survival rates of 90.3% (standard error [SE] 0.02) and 99.7% (SE 0.003) for cases and controls, respectively (Figure 1). Similar results were seen in the analysis beginning at study enrollment: 10.5% of cases and 1% of controls died within 5 years of study enrollment (P < 0.0001), which resulted in 5-year postenrollment survival rates of 89.5% (SE 0.02) and 99.0% (SE 0.01) for cases and controls, respectively.
Table 1.
Causes of early mortality (5 years) in 32 systemic lupus erythematosus patients and 3 controls*
| Primary cause of death | Patients | Controls |
|---|---|---|
|
| ||
| Cardiovascular disease | 13 (41) | 2 (67) |
| Infection | 6 (19) | |
| Pulmonary embolism | 2 (6) | |
| Cancer | 1 (3) | |
| Other | 7 (22) | |
| Unknown | 3 (9) | 1 (33) |
| Total | 32 (100) | 3 (100) |
Values are the number (percentage).
Figure 1.
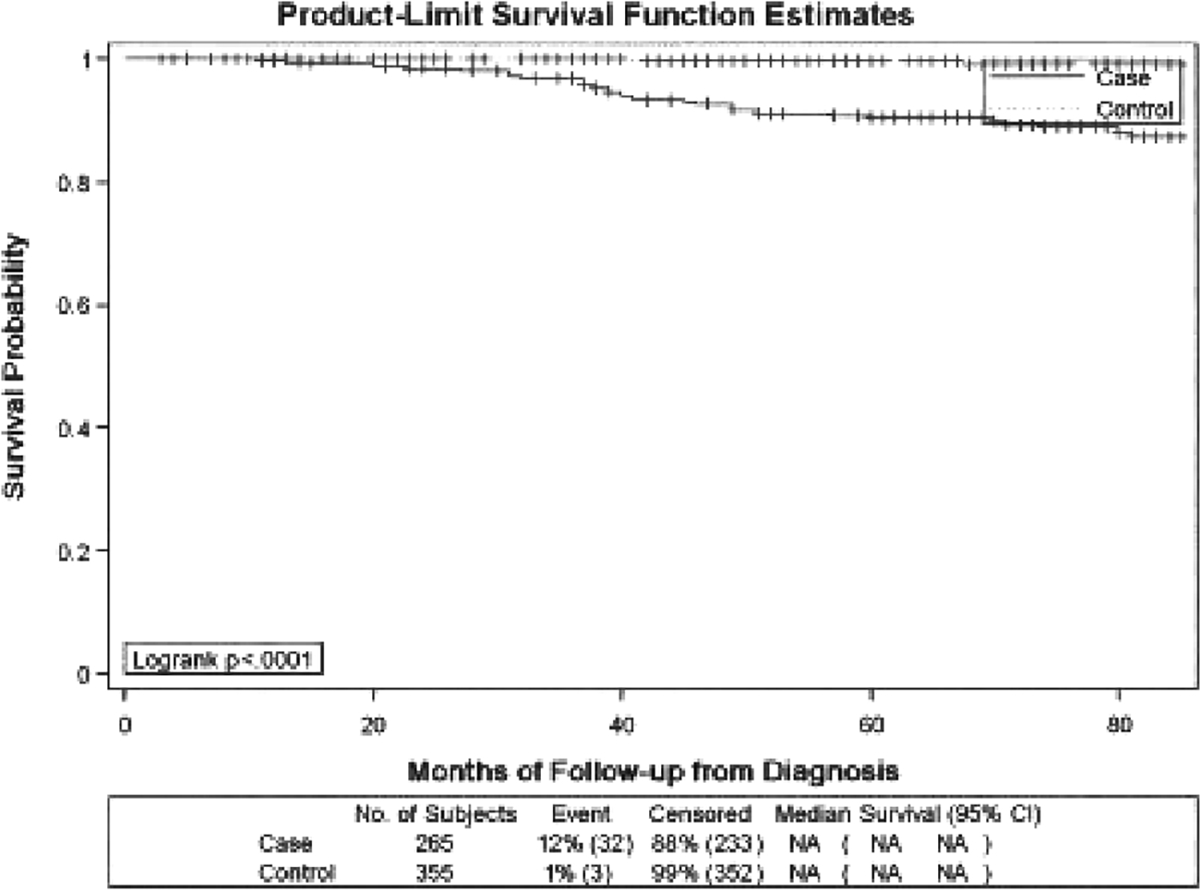
Survival probability from diagnosis between cases and controls. NA = not applicable; 95% CI = 95% confidence interval.
Among cases, mortality risk increased with age and was higher in males (Table 2). There was little difference in the results of the analyses from diagnosis compared with the analyses from enrollment, except for a small attenuation in the association observed between sex and mortality risk (from an adjusted hazard ratio [HR] of 2.7 to 2.2) (Tables 2 and 3). An increased mortality risk was seen in African Americans, with adjusted HRs of ~2.0. There was little association between education level and mortality. Using a dichotomous variable (high school education or less compared with more than high school), the HR associated with lower education was 1.2 (95% confidence interval [95% CI] 0.59–2.3), and there was no evidence of a trend across 4 levels of education (less than high school, completed high school, some college, or completed college and more; P for trend 0.77). Additional adjustment for education level did not attenuate the observed associations with other variables (data not shown).
Table 2.
Associations between demographic variables and mortality risk among systemic lupus erythematosus cases from diagnosis*
| Univariate analysis | Adjusting for age, sex, race, and blood sample | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | Died, % | HR | 95% CI | HR | 95% CI | |
|
| ||||||
| Age (per year) | 265 | 1.03 | 1.01–1.05 | 1.03 | 1.01–1.06 | |
| Sex | ||||||
| Male | 25 | 28 | 2.8 | 1.2–6.4 | 2.7 | 1.1–6.3 |
| Female | 240 | 10 | 1.0 | Referent | 1.0 | Referent |
| Race† | ||||||
| African American | 160 | 14 | 1.4 | 0.64–3.0 | 2.1 | 0.9–4.7 |
| White | 89 | 10 | 1.0 | Referent | 1.0 | Referent |
| Lupus nephritis | ||||||
| Present | 63 | 17 | 1.6 | 0.78–3.4 | 2.5 | 1.1–5.5 |
| Absent | 202 | 10 | 1.0 | Referent | 1.0 | Referent |
| Anti-dsDNA antibodies‡ | ||||||
| Present | 62 | 18 | 1.9 | 0.88–4.2 | 2.5 | 1.1–5.5 |
| Absent | 176 | 9 | 1.0 | Referent | 1.0 | Referent |
| Provided blood sample | ||||||
| No | 21 | 29 | 3.3 | 1.3–7.9 | 3.7 | 1.5–9.1 |
| Yes | 244 | 11 | 1.0 | Referent | 1.0 | Referent |
HR = hazard ratio; 95% CI = 95% confidence interval; anti-dsDNA = anti-double-stranded DNA.
Sixteen patients who were American Indian, Asian, or Hispanic were excluded from these analyses.
Among the 244 with blood samples, 6 were missing anti-dsDNA antibody data because of inadequate sample.
Table 3.
Associations between demographic variables and mortality risk among systemic lupus erythematosus cases from enrollment*
| Univariate analysis | Adjusting for age, sex, race, and blood sample | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | Died, % | HR | 95% CI | HR | 95% CI | |
|
| ||||||
| Age (per year) | 265 | 1.03 | 1.00–1.05 | 1.03 | 1.01–1.06 | |
| Sex | ||||||
| Male | 25 | 28 | 2.3 | 0.96–5.7 | 2.2 | 0.90–5.6 |
| Female | 240 | 10 | 1.0 | Referent | 1.0 | Referent |
| Race† | ||||||
| African American | 160 | 14 | 1.6 | 0.72–3.6 | 2.2 | 0.92–5.2 |
| Whites | 89 | 10 | 1.0 | Referent | 1.0 | Referent |
| Lupus nephritis | ||||||
| Present | 63 | 17 | 1.5 | 0.71–3.2 | 2.1 | 0.92–4.7 |
| Absent | 202 | 10 | 1.0 | Referent | 1.0 | Referent |
| Anti-dsDNA antibodies‡ | ||||||
| Present | 62 | 18 | 2.1 | 0.96–4.7 | 2.7 | 1.2–6.2 |
| Absent | 176 | 9 | 1.0 | Referent | 1.0 | Referent |
| Provided blood sample | ||||||
| No | 21 | 29 | 3.4 | 1.4–8.2 | 3.5 | 1.4–8.6 |
| Yes | 244 | 11 | 1.0 | Referent | 1.0 | Referent |
See Table 2 for definitions.
Sixteen patients who were American Indian, Asian, or Hispanic were excluded from these analyses.
Among the 244 with blood samples, 6 were missing anti-dsDNA antibody data because of inadequate sample.
Lupus nephritis and anti–double-stranded DNA (anti-dsDNA) antibodies were also associated with a 2–3-fold increase in mortality risk (adjusted results, Tables 2 and 3), and whether the patient had provided a blood sample at study enrollment was a strong predictor of subsequent mortality risk. Anti-dsDNA antibodies were more common among patients with lupus nephritis (39% of patients with lupus nephritis compared with 22% of patients with SLE without lupus nephritis had anti-dsDNA antibodies). However, there was no evidence of confounding because adjusting for both of these variables did not change the associations seen with each, and did not change the associations observed with ethnicity (data not shown). None of the other autoantibodies we examined (anti-Ro, anti-La, anti-Sm, and anti-RNP antibodies) were associated with mortality risk (data not shown).
Quality of life analysis.
In case and control comparisons, significant differences were seen in each of the individual 8 items except mental health. The mean ± SD PCS and MCS scores for controls were 49.5 ± 10.48 and 49.4 ± 9.06, respectively. Lower summary scores were seen among cases, with a mean of 41.4 ± 11.07 for the PCS and 47.4 ± 10.42 for the MCS. Adjusting for age (as a continuous variable), sex, state, race, and education (as a 4-level variable), a 7.7-point lower PCS score (P < 0.0001) and a 1.8-point lower MCS score (P = 0.07) were seen in cases compared with controls. Among cases, PCS scores decreased with increasing age (mean scores 44.0, 42.5, and 38.4 in the <30, 30–49, and ≥50 age groups, respectively; P = 0.02). No significant differences by any of the demographic variables were seen for the MCS scores.
DISCUSSION
As expected, patients with SLE had significantly lower survival compared with age-, sex-, and state-matched controls. The 5-year mortality risk was 9.7% in cases compared with <1% in controls. The immediate and underlying causes of death included renal failure, intracerebral hemorrhage, cerebrovascular disease, sepsis, cardiorespiratory arrest, and pulmonary embolism. However, the predominant cause of death was related to cardiovascular disease, which contributed to 41% of deaths among cases. Infection was the second leading cause of death among cases, contributing to 19% of deaths. More detailed information about the causes of death for a portion of these patients in combination with patients from the LUpus in MInorities, NAture versus nurture (LUMINA) study has been previously published (9).
The 5-year mortality risk in our cohort was somewhat higher than has been reported in some recent studies. Jonsson et al (10), Uramoto et al (11), and Peschken and Esdaile (12) reported 5-year mortality rates of ~5% among patients newly diagnosed with SLE in Sweden, Minnesota, and Canada, respectively. Our results are similar to those of the LUMINA study, which reported a mortality risk within 5 years of disease onset of 12% for the entire cohort of 288 patients and 12% in Hispanics, 15% in African Americans, and 7% in whites (9). The baseline interview included questions about the length of time between the occurrence of symptoms and diagnosis. There was no difference between African Americans and whites in the length of this onset period (14% of African Americans and 19% of whites reported >5 years between occurrence of symptoms and diagnosis; P = 0.44), therefore we cannot attribute the increased mortality risk we observed among African American patients to a delay in diagnosis. A racial difference in mortality risk was reported recently by Krishnan and Hubert, who analyzed data from 2 national data sets (13), and by Bernatsky et al (14), using data from a large, multicenter, international SLE cohort. These 2 studies included prevalent rather than incident (or recently diagnosed) patients, and therefore are not directly comparable with our results pertaining to the early disease course.
We observed an association between mortality risk and age and sex. Four previous studies have reported an increased mortality risk among older patients with SLE (15–18), and 2 of these studies (16,17), similar to the CLU study, were inception or near-inception cohorts. The increased mortality risk seen in our study in male compared with female patients with SLE was also seen in the large inception cohort study by Ward et al (16), but was not seen in the LUMINA cohort (17). SLE has long been labeled a woman’s disease, and men with SLE may only seek (or be referred to) a specialist’s services at a more advanced stage, when the disease is less likely to be misdiagnosed.
An increased mortality risk in persons of low socioeconomic status has been previously reported (16,17,19). In our study, education level was not associated with mortality, and inclusion of the education variable did not attenuate the associations seen with race or other variables. Income data were not collected in the baseline interview, and so could not be used as a measure of socioeconomic status in relation to mortality risk. In the LUMINA study, poverty, but not ethnicity, was associated with early mortality risk (17).
In our study, patients with lupus nephritis, and those positive for the presence of anti-dsDNA antibodies at enrollment, had approximately a 2.5-fold increased mortality risk, and these associations were not accounted for by the interrelationship between anti-dsDNA antibodies, lupus nephritis, and ethnicity. The autoantibody test was based on a single determination from a blood draw that was not performed as part of a clinic visit, and therefore may not reflect the most active part of the course of the disease. An increased mortality risk in patients with SLE with renal damage has been reported previously (15,16), but few studies have examined the potential confounding effects of race and age, and have been limited to the early disease period. Although we had abstracted data pertaining to other clinical features based on medical record review up to 6 months after diagnosis, we did not have updated data pertaining to anything other than kidney biopsy results, and so we did not evaluate the influence of other clinical features on mortality risk.
In our study, 21 patients (8%) did not provide a blood sample at study enrollment, and the mortality risk in this group was substantially higher (6 deaths, 29%) than that experienced by the other CLU patients. As a sensitivity test, we performed analyses assuming that all patients who declined to give blood were anti-dsDNA antibody negative. This assumption resulted in an attenuated association between anti-dsDNA antibodies and mortality risk, with an age-, sex-, and race-adjusted HR of 1.8 (95% CI 0.85–3.8). This attenuation was not unexpected, given that ethnicity was associated with failure to provide a blood sample (10% of minorities compared with 3% of whites did not provide a blood sample), and we had observed an increased mortality risk in African Americans. However, there was no association between age, sex, education level, or presence of lupus nephritis and the provision of a blood sample, and none of these factors (including ethnicity) appeared to act as a confounder of the association seen between blood sample and mortality risk. Being able and willing to provide a blood sample may be a marker for disease severity or comorbidities. For example, lack of venous access is often an indicator of multiple prior medical procedures and therefore may be associated with more severe disease. This observation from the CLU points out the potential selection (and selection biases) that may result from even seemingly basic study participation criteria.
In the development of the SF-8 quality of life scale, it was hypothesized that each SF-8 scale would substantially converge with its corresponding scale in the SF-36 and each scale was hypothesized to discriminate between its hypothesized health concept and other concepts in the SF-36. Very high correlations between the 2 physical measures (SF-8 PCS and SF-36 PCS) and the 2 mental component measures (SF-8 MCS and SF-36 MCS) and very low correlations between measures of different concepts were demonstrated in various validation studies, resulting in excellent content, convergent, and discriminate validity for the SF-8 (20). However, only 1 item for each of the 8 health concepts is used to compute a score. Because of its brevity, the SF-8 is best used to compare composite summary scores and not individual domain scores. Scores estimated from the SF-8 may be less precise and may cover a narrower range of scores compared with the SF-36, the scale from which it is derived (21). Despite these limitations, the SF-8 scales and summary measures rarely missed differences in physical or mental health status captured by the SF-36 scales and summary measures (20). Recent examples of the use of this measure include studies of persons with migraine headaches (22), patients with acute coronary syndrome (23), and patients with breast cancer (24). In the study of patients with migraines (22), correlations for the SF-8 and SF-36 version 2 were consistently high, providing additional evidence of the construct validity of the SF-8.
In our study, adjusting for age, race, and other demographic factors, the mean PCS score was 7.7 points lower (P < 0.0001) and the mean MCS score was 1.8 points lower (P = 0.07) in patients with SLE compared with controls, as assessed approximately 4 years after diagnosis. Among cases, decreasing PCS scores were seen with increasing age, but there were no differences by any of the demographic variables in the MCS scores. The PCS and MCS scores for controls in this study are similar to those reported from the general US population (mean ± SD 50 ± 10), providing some assurance of the appropriateness of the sampling and implementation process. A previously published study from Italy compared health-related quality of life scores between 126 patients with SLE (mean disease duration 9.9 years) and 96 controls (primarily hospital personnel) (25). That study used the SF-36, and patients reported a 14.7-point lower PCS score (P < 0.00001) and a 9.7-point lower MCS score (P < 0.04) compared with controls. Vu and Escalante reported that patients from the University of Texas Health Science Center at San Antonio with lupus nephritis who progressed to end-stage renal disease had improved mental well-being but reduced physical functioning and general health compared with patients with SLE with preserved renal function (26). As has been suggested by Bae et al (27) and Sutcliffe et al (28), patients with worse medical conditions may exhibit higher mental summary scores as a result of various forms of social support (emotional, instrumental, self-esteem, and companionship). Additional research is needed to better understand the contributions to the impact on the decreased physical domain and summary scores in patients with SLE, in contrast to the relative stability of the mental component scores among patients and controls.
This study elucidated the clinical and humanistic burden experienced by patients with SLE through the identification of significant differences in 5-year mortality and health-related quality of life between patients with SLE and matched controls, even early in the disease, and even with currently available treatment advances. Our study also adds to the available research on mortality risks in SLE by providing additional evidence that males have higher 5-year mortality rates compared with females and that the presence of anti-dsDNA antibodies and lupus nephritis each are also associated with increased mortality. The association between the inability (or unwillingness) to provide a blood sample and subsequent increased mortality risk has not previously been reported in studies of SLE, and we were unable to find similar analyses involving other types of chronic diseases. Our study also suggests that there is a protective psychosocial factor, which negates decline in mental health domains despite decline in physical health. The severity and heterogeneity of SLE at diagnosis and its unpredictable course highlight the need for less toxic and more effective treatments.
Acknowledgments
Dr. Campbell’s work was supported by the NIH National Research Service award (grants 1T32-AR050958 and P60-AR049459). Dr. Cooper’s work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences. Dr. Gilkeson’s work was supported by the NIH (grants 2R01-AR045476 and P60-AR049459).
Dr. Gilkeson has received speaking fees (less than $10,000) from Genentech and owns stock and/or holds stock options in Taligen.
REFERENCES
- 1.Merrell M, Shulman LE. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis 1955;1:12–32. [DOI] [PubMed] [Google Scholar]
- 2.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol 2001;13:345–51. [DOI] [PubMed] [Google Scholar]
- 3.Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8 Health Survey. Lincoln (RI): QualityMetric Inc; 2001. [Google Scholar]
- 4.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 5.Cooper GS, Dooley MA, Treadwell EL, St.Clair EW, Gilkeson GS. Smoking and use of hair treatments in relation to risk of developing systemic lupus erythematosus. J Rheumatol 2001;28:2653–6. [PubMed] [Google Scholar]
- 6.Cooper GS, Parks CG, Treadwell EL, St.Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus 2002;11:161–7. [DOI] [PubMed] [Google Scholar]
- 7.Cox DR. Regression models and life-tables. J R Stat Soc 1972;34:187–220. [Google Scholar]
- 8.Schoenfeld D Chi-squared goodness-of-fit of Cox’s regression and life model. Biometrics 1980;67:145–53. [Google Scholar]
- 9.Calvo-Alen J, Alarcon GS, Campbell R Jr, Fernandez M, Reveille JD, Cooper GS. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology (Oxford) 2005;44:1186–9. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson H, Nived O, Sturfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine (Baltimore) 1989;68:141–50. [PubMed] [Google Scholar]
- 11.Uramoto KM, Michet CJ Jr, Thumboo J, Sunku J, O’Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum 1999;42:46–50. [DOI] [PubMed] [Google Scholar]
- 12.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol 2000;27:1884–91. [PubMed] [Google Scholar]
- 13.Krishnan E, Hubert HB. Ethnicity and mortality from systemic lupus erythematosus in the US. Ann Rheum Dis 2006;65:1500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginsler E, Gladman DD. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2250–7. [DOI] [PubMed] [Google Scholar]
- 15.Reveille JD, Bartolucci A, Alarcon GS. Prognosis in systemic lupus erythematosus: negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum 1990;33:37–48. [DOI] [PubMed] [Google Scholar]
- 16.Ward MM, Pyun E, Studenski S. Long-term survival in systemic lupus erythematosus: patient characteristics associated with poorer outcomes. Arthritis Rheum 1995;38:274–83. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon GS, McGwin G Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. , for the LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. VIII. Predictors of early mortality in the LUMINA cohort [published erratum appears in Arthritis Rheum 2001;45:306]. Arthritis Rheum 2001;45:191–202. [DOI] [PubMed] [Google Scholar]
- 18.Manger K, Manger B, Repp R, Geisselbrecht M, Geiger A, Pfahlberg A, et al. Definition of risk factors for death, end stage renal disease, and thromboembolic events in a monocentric cohort of 338 patients with systemic lupus erythematosus. Ann Rheum Dis 2002;61:1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginzler EM, Diamond ES, Weiner M, Schlesinger M, Fries JF, Wasner C, et al. A multicenter study of outcome in systemic lupus erythematosus. I. Entry variables as predictors of prognosis. Arthritis Rheum 1982;25:601–11. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res 2001;10:405–13. [DOI] [PubMed] [Google Scholar]
- 21.McHorney CA, Ware JE Jr, Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts: results from the Medical Outcomes Study. Med Care 1992;30(5 Suppl): MS253–65. [DOI] [PubMed] [Google Scholar]
- 22.Turner-Bowker DM, Bayliss MS, Ware JE Jr, Kosinski M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res 2003;12:1003–12. [DOI] [PubMed] [Google Scholar]
- 23.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Perceived work performance of patients who experienced an acute coronary syndrome event. Cardiology 2005;104:120–6. [DOI] [PubMed] [Google Scholar]
- 24.Shim EJ, Mehnert A, Koyama A, Cho SJ, Inui H, Paik NS, et al. Health-related quality of life in breast cancer: a cross-cultural survey of German, Japanese, and South Korean patients. Breast Cancer Res Treat 2006;99:341–50. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi S, Doria A, Salaffi F, Ermani M, Iaccarino L, Ghirardello A, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. I. Relationship between physical and mental dimension and impact of age. Rheumatology (Oxford) 2004;43:1574–9. [DOI] [PubMed] [Google Scholar]
- 26.Vu TV, Escalante A. A comparison of the quality of life of patients with systemic lupus erythematosus with and without endstage renal disease. J Rheumatol 1999;26:2595–601. [PubMed] [Google Scholar]
- 27.Bae SC, Hashimoto H, Karlson EW, Liang MH, Daltroy LH. Variable effects of social support by race, economic status, and disease activity in systemic lupus erythematosus. J Rheumatol 2001;28:1245–51. [PubMed] [Google Scholar]
- 28.Sutcliffe N, Clarke AE, Levinton C, Frost C, Gordon C, Isenberg DA. Associates of health status in patients with systemic lupus erythematosus. J Rheumatol 1999;26:2352–6. [PubMed] [Google Scholar]


