Extract
Pulmonary arterial hypertension (PAH) is a cardiovascular disorder characterised by pulmonary vascular remodelling resulting in increased pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), which can lead to progressive right heart failure [1, 2]. Right heart catheterisation (RHC) remains the gold standard for the diagnosis of pulmonary hypertension (PH) [1, 2]. Furthermore, several haemodynamic variables are included in the multiparametric model used at baseline to assess the risk of death [1, 2]. At follow-up, it is recommended to assess at least World Health Organization/New York Heart Association Functional Class (WHO/NYHA FC), 6-min walk distance (6MWD) and biomarkers (brain natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP)) to establish the risk status according to the non-invasive four-strata model [1, 2]. Additional variables such as haemodynamics should be considered as needed. It is left to the discretion of the physician to carry out RHC [1, 2]. However, it is not clear which patients should benefit from this examination.
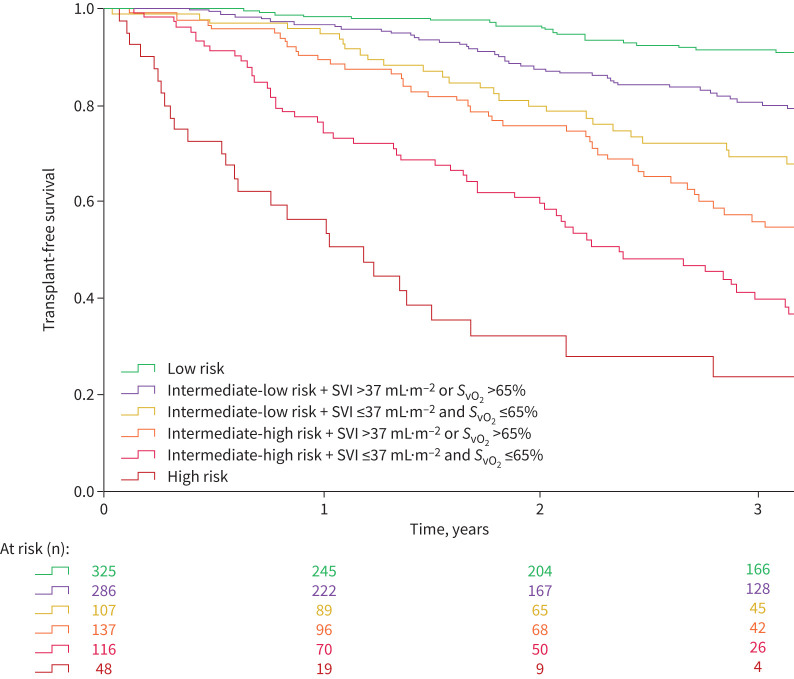
Graphical abstract
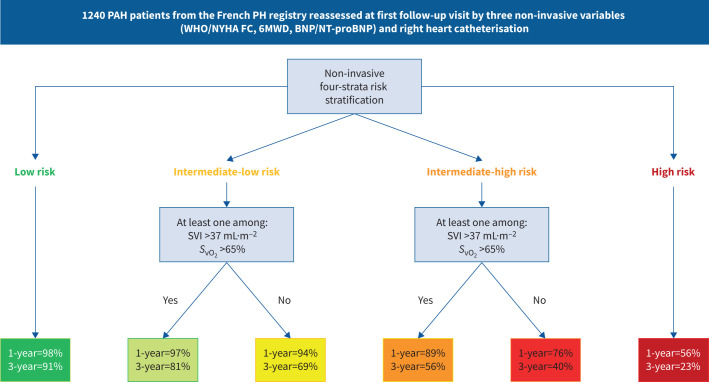
1240 pulmonary arterial hypertension (PAH) patients from the French pulmonary hypertension (PH) registry reassessed at first follow-up visit by three non-invasive variables (World Health Organization/New York Heart Association Functional Class (WHO/NYHA FC), 6-min walk distance (6MWD) and brain natriuretic peptide (BNP)/N-terminal pro-BNP) and right heart catheterisation. 1- and 3-year transplant-free survival rates. Cardiopulmonary haemodynamics improve risk stratification at follow-up in patients at intermediate risk. SVI: stroke volume index; SvO2: mixed venous oxygen saturation.
Abstract
Background
Haemodynamic variables are prognostic factors in pulmonary arterial hypertension (PAH). However, right heart catheterisation (RHC) is not systematically recommended to assess the risk status during follow-up. This study aimed to assess the added value of haemodynamic variables in prevalent patients to predict the risk of death or lung transplantation according to their risk status assessed by the non-invasive four-strata model as recommended by the European guidelines.
Methods
We evaluated incident patients with PAH enrolled in the French pulmonary hypertension registry between 2009 and 2020 who had a first follow-up RHC. Cox regression identified, in each follow-up risk status, haemodynamic variables significantly associated with transplant-free survival. Optimal thresholds were determined by time-dependent receiver operating characteristics. Several multivariable Cox regression models were performed to identify the haemodynamic variables improving the non-invasive risk stratification model.
Results
We analysed 1240 incident patients reassessed within 1 year by RHC. None of the haemodynamic variables were significantly associated with transplant-free survival among low-risk (n=386) or high-risk (n=71) patients. Among patients at intermediate (intermediate-low, n=483 and intermediate-high, n=300) risk at first follow-up, multivariable models including either stroke volume index (SVI) or mixed venous oxygen saturation (SvO2) were the best. The prognostic performance of a refined six-strata risk stratification model including the non-invasive four-strata model and SVI >37 mL·m−2 and/or SvO2 >65% for patients at intermediate risk (area under the curve (AUC) 0.81; c-index 0.74) was better than that of the four-strata model (AUC 0.79, p=0.009; c-index 0.72).
Conclusion
Cardiopulmonary haemodynamics may improve risk stratification at follow-up in patients at intermediate risk.
Shareable abstract
Cardiopulmonary haemodynamics assessed by right heart catheterisation improve risk stratification at follow-up in patients with pulmonary arterial hypertension at intermediate-low or intermediate-high risk according to the non-invasive four-strata tool https://bit.ly/4bd0ScP
Introduction
Pulmonary arterial hypertension (PAH) is a cardiovascular disorder characterised by pulmonary vascular remodelling resulting in increased pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), which can lead to progressive right heart failure [1, 2]. Right heart catheterisation (RHC) remains the gold standard for the diagnosis of pulmonary hypertension (PH) [1, 2]. Furthermore, several haemodynamic variables are included in the multiparametric model used at baseline to assess the risk of death [1, 2]. At follow-up, it is recommended to assess at least World Health Organization/New York Heart Association Functional Class (WHO/NYHA FC), 6-min walk distance (6MWD) and biomarkers (brain natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP)) to establish the risk status according to the non-invasive four-strata model [1, 2]. Additional variables such as haemodynamics should be considered as needed. It is left to the discretion of the physician to carry out RHC [1, 2]. However, it is not clear which patients should benefit from this examination.
In the first publication from the National Institutes of Health in 1991, haemodynamic variables such as right atrial pressure (RAP), mean PAP (mPAP) and cardiac index (CI) were shown to be strongly associated with survival [3]. Since then, studies have assessed the prognostic value of haemodynamic measurements at the time of PAH diagnosis [4–6]. However, it became obvious that haemodynamic evaluation at follow-up was much more reliable for assessing prognosis [7–10].
A previous study showed that stroke volume index (SVI) and RAP at first follow-up reassessment were the strongest haemodynamic prognostic variables independently of age, sex, aetiology of PAH, WHO/NYHA FC and 6MWD [11]. In a multivariable Cox regression analysis including WHO/NYHA FC, 6MWD, BNP/NT-proBNP, RAP and CI, only the three non-invasive variables were independently associated with transplant-free survival, whereas the haemodynamic variables were no longer significant [8]. The results of these studies on the contribution of haemodynamic variables to risk stratification at follow-up are therefore inconsistent. In the era of non-invasive risk stratification tools development, the prognostic value of haemodynamic parameters investigated by RHC during follow-up needs to be reassessed.
This study aimed to assess the added value of haemodynamic variables of patients with PAH to predict the risk of death or lung transplantation according to their risk status assessed at first follow-up by the non-invasive four-strata model.
Methods
This study complied with the Declaration of Helsinki. Although French law does not require ethics committee approval or informed consent for retrospective data collection, the data collected in the French Pulmonary Hypertension Network registry were anonymised and complied with the requirements of the Commission Nationale Informatique et Liberté (CNIL). CNIL, which is the organisation dedicated to privacy, information technology and civil rights in France, approved the methods used to collect and analyse the data on 24 May 2003 (approval number 842063).
Study population
Data were collected from the web-based French PH registry (https://registre-htap.aphp.fr; PAHTool; Inovultus, Santa Maria de Feira, Portugal). We reviewed data from all adult patients with newly diagnosed PAH who were enrolled between 1 January 2009 and 31 December 2020. PAH was diagnosed according to the current guidelines and defined as resting mPAP ≥25 mmHg, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and PVR >3 WU on baseline RHC [12, 13]. Patients were included if they had PAH diagnosed by RHC, a calculable follow-up time and at least one follow-up RHC after diagnosis. Patients were excluded if they had known pulmonary veno-occlusive disease, unrepaired congenital heart disease, portal hypertension, positive response to acute vasoreactivity test or missing data for WHO/NYHA FC, 6MWD and/or BNP/NT-proBNP at first follow-up.
Measurements
Clinical measurements at first follow-up included WHO/NYHA FC and 6MWD. All patients were sampled for BNP/NT-proBNP. Haemodynamic measurements included RAP, systolic, diastolic and mPAP, and PAWP. Cardiac output (CO) was measured by thermodilution. CI was calculated as CO divided by body surface area. PVR was calculated as mPAP−PAWP divided by CO. SVI was calculated as CI divided by heart rate. Pulmonary artery blood samples were collected to measure mixed venous oxygen saturation (SvO2).
Statistical analysis
Continuous variables were expressed as mean±sd or median (interquartile range (IQR)) according to data distribution. Categorical variables were expressed as absolute frequencies (n) and percentage (%). Data were randomly divided into training (50%) and replication (50%) cohorts.
The paired t-test, Wilcoxon matched-pairs signed-rank test and Chi-squared test were used to compare changes from baseline to follow-up. The primary outcome for survival analysis was defined as all-cause mortality or lung transplantation. Transplant-free survival time was calculated from the date of first reassessment RHC to date of death or lung transplantation. The cut-off date was 31 December 2021. In each non-invasive risk status, we examined the relationship between haemodynamic variables at the time of first follow-up RHC using Cox proportional hazards regression adjusted for age. Time-dependent receiver operating characteristics (ROC) analysis was used to determine the area under the curve (AUC) for continuous variables identified from the Cox analysis, and optimal thresholds of haemodynamic variables were determined by the value maximising the sum of sensitivity and specificity (Youden index) in both training and replication cohorts. Cox proportional hazards regression models for transplant-free survival of haemodynamic variables expressed as dichotomous variables according to previously identified thresholds were performed at baseline and follow-up.
Akaike Information Criteria (AIC) of different multivariable models including non-invasive risk score and haemodynamic dichotomous variables according to the thresholds previously identified were compared in the replication cohort and the whole cohort. A refined risk stratification model based on non-invasive variables and haemodynamic variables having the lowest AIC was developed. Survival analyses were performed using the Kaplan–Meier method and transplant-free survival according to risk status was compared using the log-rank test. Time-dependent ROC analyses of the actual non-invasive four-strata model and the refined risk stratification model including haemodynamic variables were performed and compared using the DeLong test. Harrell's c-statistic was used to compare accuracy and discrimination of the different risk stratification methods [1, 2, 14, 15].
All comparisons were two-sided and a p-value <0.05 was considered statistically significant.
Statistical analysis was performed in R version 4.3.1 (www.r-project.org) and SPSS version 29 (IBM, Armonk, NY, USA).
Results
Study population
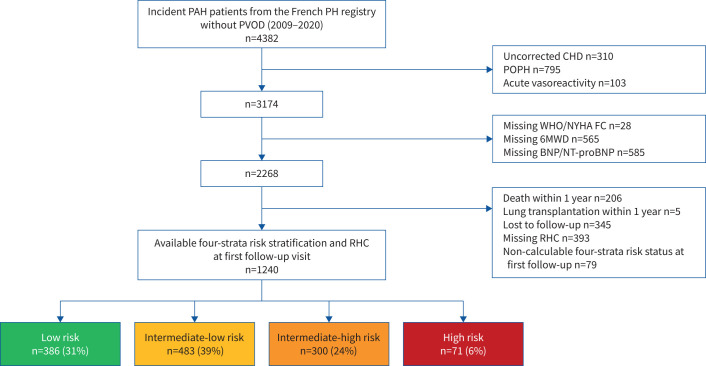
Patient selection is shown in figure 1. The analysis cohort included 1240 incident PAH patients who had been reassessed by RHC within 1 year. The characteristics of patients excluded from the analysis are presented in supplementary table S1. Baseline characteristics of our study population are shown in tables 1 and 2. 64% were female and mean±sd age was 60±15 years. The majority of patients had idiopathic PAH (45%) or connective tissue disease-associated PAH (32%). Mean±sd baseline 6MWD was 301±152 m and 68% of patients presented in WHO/NYHA FC III or IV. The most frequently used initial treatment strategy within the first 3 months after PAH diagnosis was monotherapy (52%), followed by dual oral combination therapy (39%) and upfront triple combination therapy (5%).
FIGURE 1.
Patient flowchart. PAH: pulmonary arterial hypertension; PVOD: pulmonary veno-occlusive disease; CHD: congenital heart disease; POPH: portopulmonary hypertension; WHO/NYHA FC: World Health Organization/New York Heart Association Functional Class; 6MWD: 6-min walk distance; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP; RHC: right heart catheterisation.
TABLE 1.
Demographic characteristics and initial therapy of the study population (n=1240)
| Sex | |
| Female | 799 (64) |
| Male | 441 (36) |
| Age, years | 60±15 |
| BMI, kg·m−2 | 27±6 |
| Comorbidities | |
| Obesity | 361 (29) |
| Hypertension | 537 (43) |
| Diabetes | 277 (22) |
| Coronary heart disease | 122 (10) |
| DLCO <45% predicted | 378 (30) |
| Atrial fibrillation | 115 (9) |
| Renal insufficiency | 88 (7) |
| H2FPEF score >5 | 25 (2) |
| PAH aetiology | |
| Idiopathic | 561 (45) |
| Heritable | 104 (8) |
| Drugs | 123 (10) |
| Connective tissue disease | 398 (32) |
| HIV | 45 (4) |
| Corrected congenital heart disease | 9 (1) |
| No therapy initiated within first 3 months | 53 (4) |
| Monotherapy | 649 (52) |
| ERA | 367 (29.5) |
| PDE5i | 272 (22) |
| Inhaled PGI2 | 1 (0) |
| i.v./s.c. PGI2 | 9 (0.5) |
| Dual therapy | 480 (39) |
| ERA+PDE5i | 460 (37) |
| PDE5i+selexipag | 2 (0) |
| Oral therapy+inhaled PGI2 | 3 (0.5) |
| Oral therapy+i.v./s.c. PGI2 | 15 (1.5) |
| Triple therapy | 58 (5) |
| ERA+PDE5i+selexipag | 6 (1) |
| ERA+PDE5i+inhaled PGI2 | 1 (0) |
| ERA+PDE5i+i.v./s.c. PGI2 | 51 (4) |
Data are presented as n (%) or mean±sd. BMI: body mass index; DLCO: diffusing capacity of the lung for carbon monoxide; H2FPEF: scoring system for diagnosing heart failure with preserved ejection fraction; PAH: pulmonary arterial hypertension; ERA: endothelin receptor antagonist; PDE5i: phosphodiesterase type 5 inhibitor; PGI2: prostacyclin; i.v.: intravenous; s.c.: subcutaneous.
TABLE 2.
Clinical and haemodynamic characteristics at baseline and first follow-up reassessment
| Baseline | First re-evaluation | p-value | |
|---|---|---|---|
| WHO/NYHA FC | <0.001 | ||
| I–II | 393 (32) | 754 (61) | |
| III | 687 (55) | 403 (32.5) | |
| IV | 160 (13) | 83 (6.5) | |
| 6MWD, m | 301±152 | 338±153 | <0.001 |
| Haemodynamics | |||
| RAP, mmHg | 8±5 | 7±5 | <0.001 |
| mPAP, mmHg | 46±13 | 40±12 | <0.001 |
| PAWP, mmHg | 9±4 | 10±4 | <0.001 |
| CO, L·min−1 | 4.4±1.4 | 5.5±1.6 | <0.001 |
| CI, L·min−1·m−2 | 2.5±0.7 | 3.1±0.8 | <0.001 |
| SVI, mL·m−2 (n=867) | 32±.11 | 41±11 | <0.001 |
| PVR, WU | 9±.5 | 6±3 | <0.001 |
| SvO2, % (n=754) | 63±10 | 67±8 | <0.001 |
| BNP, ng·L−1 (n=627) | 223 (74–528) | 82 (34–230) | <0.001 |
| NT-proBNP, ng·L−1 (n=613) | 981 (334–2362) | 385 (146–1184) | <0.001 |
Data are presented as n (%), mean±sd or median (interquartile range), unless otherwise stated. WHO/NYHA FC: World Health Organization/New York Heart Association Functional Class; 6MWD: 6-min walk distance; RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; SVI: stroke volume index; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP.
After a median (IQR) follow-up of 3.1 (1.5–5.5) years, 376 patients (30%) had died and 39 (3%) underwent lung transplantation. Overall survival at 1, 2, 3 and 5 years from diagnosis was 97%, 90%, 81% and 65%, respectively (supplementary figure S1). Transplant-free survival at 1, 2, 3 and 5 years was 97%, 89%, 79% and 63%, respectively (supplementary figure S2).
First follow-up reassessment
Median (IQR) interval between diagnosis and first re-evaluation was 5.1 (4.0–8.4) months. At the time of first follow-up reassessment, there were significant improvements in WHO/NYHA FC, 6MWD, BNP/NT-proBNP and haemodynamic variables (table 2). 754 patients (61%) were in WHO/FC I or II at first follow-up, 6MWD improved from 301±152 to 338±153 m (p<0.001) and PVR decreased from 9±5 to 6±3 WU (p<0.001).
386 patients (31%) were classified as low risk, 483 (39%) as intermediate-low risk, 300 (24%) as intermediate-high risk and 71 (6%) as high risk. Clinical and haemodynamic characteristics according to the non-invasive risk status at the time of first follow-up reassessment are shown in table 3.
TABLE 3.
Clinical and haemodynamic characteristics according to the risk status at the time of first follow-up reassessment
| Low risk (n=386) |
Intermediate-low risk (n=483) |
Intermediate-high risk (n=300) |
High risk (n=71) |
|
|---|---|---|---|---|
| WHO/NYHA FC | ||||
| I–II | 386 (100) | 325 (67) | 43 (14.5) | 0 (0) |
| III | 0 (0) | 155 (32) | 226 (75) | 22 (31) |
| IV | 0 (0) | 3 (1) | 31 (10.5) | 49 (69) |
| 6MWD, m | 473±77 | 346±105 | 218±114 | 0 (0–109) |
| Haemodynamics | ||||
| RAP, mmHg | 6±4 | 7±4 | 9±5 | 12±8 |
| mPAP, mmHg | 36±12 | 40±12 | 43±10 | 47±12 |
| PAWP, mmHg | 9±4 | 10±4 | 11±5 | 10±5 |
| CO, L·min−1 | 5.9±1.6 | 5.5±1.5 | 5.1±1.6 | 4.4±1.1 |
| CI, L·min−1·m−2 | 3.3±0.8 | 3.1±0.7 | 2.8±0.8 | 2.5±0.6 |
| SVI, mL·m−2 | 44±11 | 40±10 | 38±11 | 32±10 |
| PVR, WU | 5±2 | 6±3 | 7±4 | 9±4 |
| SvO2, % | 71±6 | 67±8 | 63±8 | 58±12 |
| BNP, ng·L−1 | 28 (17–49) | 88 (47–190) | 242 (117–430) | 820 (395–1019) |
| NT-proBNP, ng·L−1 | 130 (75–210) | 456 (196–857) | 1211 (690–2537) | 2395 (1427–4066) |
Data are presented as n (%), mean±sd or median (interquartile range). WHO/NYHA FC: World Health Organization/New York Heart Association Functional Class; 6MWD: 6-min walk distance; RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; SVI: stroke volume index; SvO2: mixed venous oxygen saturation; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP.
Prognostic haemodynamic variables according to follow-up risk status
Age-adjusted Cox proportional hazards regression models of haemodynamic variables according to risk status at first follow-up are presented in supplementary table S2 and table 4. None of the haemodynamic variables were significantly associated with transplant-free survival among low-risk or high-risk patients (supplementary table S2).
TABLE 4.
Age-adjusted Cox proportional hazards regression for transplant-free survival according to risk status at first follow-up in patients at intermediate-low (n=483) or intermediate-high (n=300) risk
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| Intermediate-low risk | ||
| RAP, per mmHg | 1.028 (0.993–1.064) | 0.124 |
| mPAP, per mmHg | 1.014 (1.002–1.026) | 0.023 |
| PAWP, per mmHg | 1.011 (0.974–1.049) | 0.567 |
| CO, per L·min−1 | 0.915 (0.816–1.025) | 0.126 |
| CI, per L·min−1·m−2 | 0.887 (0.710–1.109) | 0.292 |
| PVR, per WU | 1.042 (0.995–1.091) | 0.079 |
| HR, per beats·min−1 | 1.009 (0.999–1.020) | 0.088 |
| SVI, per mL·m−2 | 0.973 (0.954–0.993) | 0.007 |
| SvO2, per % | 0.970 (0.948–0.992) | 0.007 |
| Intermediate-high risk | ||
| RAP, per mmHg | 0.990 (0.959–1.022) | 0.546 |
| mPAP, per mmHg | 0.997 (0.981–1.012) | 0.661 |
| PAWP, per mmHg | 0.985 (0.954–1.018) | 0.372 |
| CO, per L·min−1 | 0.880 (0.796–0.974) | 0.014 |
| CI, per L·min−1·m−2 | 0.774 (0.622–0.694) | 0.022 |
| PVR, per WU | 1.034 (0.989–1.082) | 0.140 |
| HR, per beats·min−1 | 0.994 (0.980–1.008) | 0.399 |
| SVI, per mL·m−2 | 0.987 (0.970–1.004) | 0.126 |
| SvO2, per % | 0.968 (0.944–0.993) | 0.011 |
RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; HR: heart rate; SVI: stroke volume index; SvO2: mixed venous oxygen saturation. p<0.05 is indicated in bold.
In order to identify dichotomous thresholds for haemodynamic variables, we pooled all patients at intermediate risk. In the 783 intermediate-risk patients, RAP, mPAP, CO, CI, PVR, SVI and SvO2 were associated with transplant-free survival (table 5). Time-dependant ROC analysis in the training cohort identified optimal thresholds of haemodynamic variables maximising the sum of sensitivity and specificity (table 6): 8 mmHg for RAP, 47 mmHg for mPAP, 4.6 L·min−1 for CO, 2.56 L·min−1·m−2 for CI, 5.7 WU for PVR, 37 mL·m−2 for SVI and 65% for SvO2. The same thresholds were identified in the training and replication cohorts. Cox proportional hazards regression models for transplant-free survival of haemodynamic variables expressed as dichotomous variables are presented in supplementary table S3 (at follow-up) and supplementary table S4 (at baseline).
TABLE 5.
Cox proportional hazards regression adjusted for age for transplant-free survival of haemodynamic variables at follow-up in intermediate-risk patients (n=783)
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| RAP, per mmHg | 1.027 (1.004–1.051) | 0.019 |
| mPAP, per mmHg | 1.014 (1.005–1.023) | 0.002 |
| PAWP, per mmHg | 1.004 (0.979–1.029) | 0.761 |
| CO, per L·min−1 | 0.888 (0.821–0.960) | 0.003 |
| CI, per L·min−1·m−2 | 0.787 (0.671–0.922) | 0.003 |
| PVR, per WU | 1.060 (1.027–1.093) | <0.001 |
| HR, per beats·min−1 | 1.005 (0.996–1.013) | 0.283 |
| SVI, per mL·m−2 | 0.975 (0.962–0.988) | <0.001 |
| SvO2, per % | 0.964 (0.950–0.979) | <0.001 |
RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; HR: heart rate; SVI: stroke volume index; SvO2: mixed venous oxygen saturation.
TABLE 6.
Thresholds of haemodynamic variables identified by time-dependent receiver operating characteristics in the training cohort
| Threshold | |
|---|---|
| RAP, mmHg | 8 |
| mPAP, mmHg | 47 |
| CO, L·min−1 | 4.6 |
| CI, L·min−1·m−2 | 2.56 |
| PVR, WU | 5.7 |
| SVI, mL·m−2 | 37 |
| SvO2, % | 65 |
RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; SVI: stroke volume index; SvO2: mixed venous oxygen saturation.
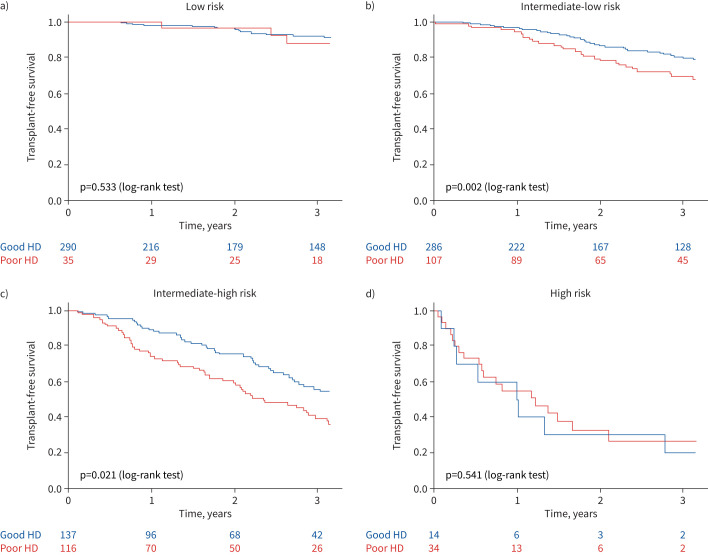
Among patients at intermediate-risk status at first follow-up, we compared multivariable Cox regression models including non-invasive risk score and haemodynamic dichotomous variables according to the thresholds previously identified (in the replication cohort (supplementary table S5) and the whole cohort (table 7)). When adjusted for non-invasive four-strata risk score, CO, PVR, SVI and SvO2 at follow-up were associated with the risk of death or lung transplantation. Adjusted models including either SVI (model F) or SvO2 (model G) had the lowest AIC values, indicating they were the best variables to predict transplant-free survival in patients at intermediate (-low or -high) risk at follow-up (supplementary table S6). The transplant-free survival of each risk status according to SVI and SvO2 is presented in figure 2. A good haemodynamic profile was defined by at least one criterion among SVI >37 mL·m−2 and SvO2 >65%. Kaplan–Meier survival curves according to haemodynamics at follow-up were not significantly different among patients at low risk (figure 2a; log-rank test p=0.533) or high risk (figure 2d; log-rank test, p=0.541). On the other hand, SVI and SvO2 discriminated patients at intermediate-low risk (figure 2b; log-rank test, p=0.002) and intermediate-high risk of death (figure 2c; log-rank test, p=0.021).
TABLE 7.
Comparisons of multivariable Cox regression models for haemodynamic variables in intermediate-risk patients at first follow-up (n=783)
| Hazard ratio (95% CI) | p-value | AIC | |
|---|---|---|---|
| Reference | 3579 | ||
| Non-invasive risk | 2.326 (1.866–2.900) | <0.001 | |
| Model A | 3476 | ||
| Non-invasive risk | 2.381 (1.896–2.990) | <0.001 | |
| RAP <8 mmHg | 0.942 (0.750–1.182) | 0.604 | |
| Model B | 3579 | ||
| Non-invasive risk | 2.337 (1.870–2.921) | <0.001 | |
| mPAP <47 mmHg | 1.058 (0.842–1.331) | 0.628 | |
| Model C | 3528 | ||
| Non-invasive risk | 2.294 (1.837–2.865) | <0.001 | |
| CO >4.6 L·min−1 | 0.727 (0.581–0.909) | 0.005 | |
| Model D | 3508 | ||
| Non-invasive risk | 2.278 (1.820–2.852) | <0.001 | |
| CI >2.5 L·min−1·m−2 | 0.826 (0.651–1.049) | 0.117 | |
| Model E | 3447 | ||
| Non-invasive risk | 2.283 (1.822–2.862) | <0.001 | |
| PVR >5 WU | 1.262 (1.002–1.591) | 0.048 | |
| Model F | 2427 | ||
| Non-invasive risk | 2.310 (1.779–2.999) | <0.001 | |
| SVI >37 mL·m−2 | 0.739 (0.570–0.958) | 0.022 | |
| Model G | 1990 | ||
| Non-invasive risk | 2.301 (1.715–3.087) | <0.001 | |
| SvO2 >65% | 0.694 (0.518–0.931) | 0.015 |
AIC: Akaike Information Criteria; RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; SVI: stroke volume index; SvO2: mixed venous oxygen saturation.
FIGURE 2.
Kaplan–Meier survival curves in each at first follow-up risk status according to haemodynamic variables in 1019 pulmonary arterial hypertension patients with at least stroke volume index (SVI) or mixed venous oxygen saturation (SvO2) available at first follow-up reassessment: a) low risk, b) intermediate-low risk, c) intermediate-high risk and d) high risk. A good haemodynamic profile (Good HD) was defined by at least one criterion among SVI >37 mL·m−2 and SvO2 >65%. A poor haemodynamic profile (Poor HD) was defined as neither SVI >37 mL·m−2 nor SvO2 >65%.
Refined risk score
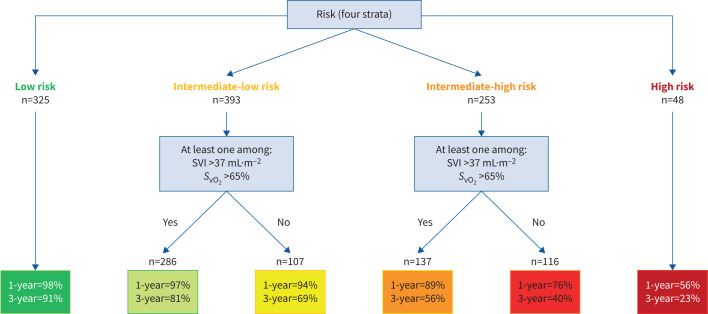
On the 1240 patients of the study population, 1019 had at least SVI or SvO2 calculated/measured at the first follow-up RHC. An algorithm based on the non-invasive four-strata model and SVI and/or SvO2 is proposed in figure 3. It comprises two stages: only the three non-invasive variables are needed for patients at low or high risk. A second stage based on haemodynamics classified patients at intermediate (-low or -high) risk into four subgroups according to two criteria (SVI and SvO2). Among patients at intermediate risk, a good haemodynamic profile was defined by at least one of the following criteria: SVI >37 mL·m−2 and/or SvO2 >65%, whereas patients having neither SVI >37 mL·m−2 nor SvO2 >65% had a poor haemodynamic profile. There was a good granularity between the Kaplan–Meier survival curves for the six strata (figure 4; log-rank test p<0.001). Transplant-free survival of patients at intermediate-low risk with a good haemodynamic profile was similar at 1 year to that of low-risk patients (97% and 98%, respectively), whereas it was worse at 3 years (81% and 91%, respectively). Among patients at intermediate-high risk, haemodynamics enabled us to identify those with a risk of death or lung transplantation of ∼10% in the following year. The survival of patients with neither SVI >37 mL·m−2 nor SvO2 >65% was poorer (76% at 1 year and 40% at 3 years).
FIGURE 3.
Refined algorithm based on the non-invasive four-strata model and right heart catheterisation variables developed in 1019 pulmonary arterial hypertension patients with at least stroke volume index (SVI) or mixed venous oxygen saturation (SvO2) available at first follow-up reassessment. 1- and 3-year transplant-free survival rates.
FIGURE 4.
Kaplan–Meier survival curves according to risk status calculated from the three non-invasive variables (World Health Organization/New York Heart Association Functional Class, 6-min walk distance and brain natriuretic peptide (BNP)/N-terminal pro-BNP) and stroke volume index (SVI) and/or mixed venous oxygen saturation (SvO2).
The prognostic performance of the refined six-strata model (AUC 0.81 (95% CI 0.76–0.86)) was significantly better than that of the non-invasive four-strata model (AUC 0.79 (95% CI 0.74–0.84); DeLong test p=0.009) (supplementary figure S3). Similar results were obtained with bootstrap resampling (1000 replicates): AUC six-strata model 0.81 (95% CI 0.78–0.83) and AUC four-strata model 0.79 (95% CI 0.76–0.81) (p<0.001). All risk scores were accurate at follow-up: c-statistic between 0.72 and 0.74 (supplementary table S7). Discrimination between the different risk stratification methods, as measured by the c-statistic, was slightly greater for the refined six-strata model than for other risk assessment strategies, at both baseline (c-statistic 0.64) and follow-up (c-statistic 0.74) (supplementary table S7).
Discussion
The main findings from this large multicentre cohort study of incident PAH patients were: 1) haemodynamic variables (assessed by RHC) did not improve risk stratification at follow-up of patients with PAH classified at low or high risk of death according to the non-invasive four-strata model; 2) among patients at intermediate-low or intermediate-high risk at follow-up, SVI and SvO2 were the best haemodynamic variables to predict transplant-free survival; 3) the prognostic performance of a refined six-strata risk stratification model including the non-invasive four-strata model (as a first step) and haemodynamics as a second step (at least one criterion among SVI >37 mL·m−2 and SvO2 >65%) for patients at intermediate risk was better than that of the non-invasive four-strata model.
This study confirms that risk stratification based on three non-invasive variables (WHO/NYHA FC, 6MWD and BNP or NT-proBNP) is sufficient in patients at low or high risk of death at follow-up to predict transplant-free survival. Our analysis completes the findings of a previous analysis of patients with idiopathic, heritable or drug-induced PAH showing that the three non-invasive variables assessed at follow-up were able to predict the risk of death or lung transplantation [8]. A multivariable Cox regression analysis from this study, including WHO/NYHA FC, 6MWD, BNP/NT-proBNP, RAP and CI, indeed showed that only the three non-invasive variables were independently associated with transplant-free survival, whereas the haemodynamic variables were no longer significant [8]. This non-invasive risk stratification model based on low-risk values for NYHA/WHO FC, 6MWD and BNP/NT-proBNP identified patients at very low risk of death or lung transplantation (5-year transplant-free survival of 97%), making it unnecessary to consider haemodynamic variables in these patients. This approach has been cross-validated in the COMPERA registry [16]. Similarly, patients classified as high risk by the non-invasive approach had a very high mortality rate at 1 year, with no added value for prognosis from haemodynamic variables (supplementary figure S3d). A rapid decision of treatment escalation with parenteral prostacyclin and/or a rapid listing for lung transplantation in eligible patients is recommended in this subset of patients.
In our study, the best haemodynamic variables to predict transplant-free survival were SVI and SvO2 in patients at intermediate-low or intermediate-high risk at follow-up. SVI has been identified in previous studies as one of the strongest haemodynamic prognostic variables at follow-up, independent of age, sex, aetiology of PAH, WHO/NYHA FC and 6MWD [11, 17]. Interestingly, we found that the optimal cut-point of SVI was 37 mL·m−2, which is strikingly similar to that found in these studies [11, 17] and recommended by the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines [1, 2]. Our study is consistent with a recent study evaluating whether cardiac magnetic resonance could be utilised to improve risk stratification at follow-up [18]. Percentage predicted right ventricular end-systolic volume index significantly improved risk stratification [18] when used in conjunction with current risk stratification models (REVEAL 2.0 [15, 19] or French PH registry approach [8]). However, the added value of haemodynamic variables assessed by cardiac magnetic resonance on top of the non-invasive four-strata model has not yet been evaluated. SvO2 is a well-known prognostic factor in PAH [3, 20, 21]. A study performed in idiopathic PAH patients identified SvO2 as a prognostic factor, both at baseline and during follow-up, independently of WHO/NYHA FC, 6MWD and NT-proBNP [7]. Despite its undeniable prognostic value, the extent of missing data for SvO2 in our study but also in other registries is regrettable [9, 10]. SvO2 closely correlates with superior vena cava oxygen saturation, with a correlation coefficient of 0.91 (p<0.0001), and it also has a significant relationship with haemodynamics [22]. SvO2 reflects the balance between oxygen delivery (related to CO, haemoglobin concentration and oxygen saturation) and oxygen consumption. In PAH, assuming that patients are neither hypoxaemic nor anaemic and that oxygen consumption is stable, SvO2 mainly reflects CO.
According to the 2022 ESC/ERS guidelines, achieving a low-risk status is considered as the treatment goal of patients with PAH [1, 2]. In our study, less than a third of the study population achieved a low-risk status at first follow-up. The algorithm we propose comprises two stages: assessment of the three non-invasive variables for all patients, then consideration of SVI and/or SvO2 for patients at intermediate (-low or -high) risk of death. Although significantly better, the discrimination of the six-strata model is very close to that of the four-strata model. However, the refined six-strata algorithm discriminates better between patients at intermediate risk, since each intermediate stratum is subdivided into two strata. The survival curves separated earlier in the intermediate-high risk group, as early as 4 months, than in the intermediate-low-risk group, where the difference appeared after 1 year. It is important to emphasise that the AUC and Harrell's c-statistic of the different scores (ESC/ERS four-strata model, REVEAL 2.0 and refined six-strata model) are approximately identical. Although significant, the p-value of the analysis only represents the high precision of the estimate, but not the clinical importance of the difference.
Interestingly, we showed that transplant-free survival of patients at intermediate-low risk with a good haemodynamic profile (SVI >37 mL·m−2 and/or SvO2 >65%) was similar at 1 year to that of low-risk patients (97% and 98%, respectively). This suggests that such patients might be treated as low-risk patients, i.e. without treatment escalation. However, after 1 year, the survival of patients at intermediate-low risk with a good haemodynamic profile was poorer than that of true low-risk patients, suggesting that in any case, regular reassessment of patients is essential [1, 2]. Among patients at intermediate-high risk, haemodynamics enabled us to identify those with a risk of death or lung transplantation of ∼10% in the following year (when SVI >37 mL·m−2 and/or SvO2 >65%). Conversely, in patients with a poor haemodynamic profile (neither SVI >37 mL·m−2 nor SvO2 >65%) it was much higher (24% at 1 year and 60% at 3 years), close to that of high-risk patients. This result suggests that a similar therapeutic approach should be applied in high-risk patients and intermediate-high-risk patients with a poor haemodynamic profile: treatment escalation with parenteral prostacyclin or listing for lung transplantation in eligible patients on maximal therapy.
This study was carried out in the era of therapies targeting the three pathways of endothelial dysfunction. It is important to note that randomised control trials are currently underway with promising new therapies such as sotatercept [23, 24] or tyrosine kinase inhibitors [25]. In the phase 3 STELLAR trial, patients receiving sotatercept as add-on therapy (on top of current drugs targeting the endothelial dysfunctional pathways) demonstrated significant improvements in RAP, SvO2, mPAP and therefore PVR, without substantial changes in CO or SVI [26]. It would therefore be interesting to carry out a similar analysis in the future, in a population of patients treated with such therapies, in order to check whether other haemodynamic variables could improve risk stratification.
We acknowledge that other risk scores including haemodynamic variables, such as REVEAL 2.0, are already available. This score predicts survival in both incident and prevalent patients with PAH and can be used even if some variables (including RHC) are missing. However, the objective of our study was more pragmatic. We sought to identify the patients who might benefit from RHC reassessment at follow-up. We showed that haemodynamic variables did not improve risk stratification in either low-risk or high-risk patients. In patients at intermediate-low or intermediate-high risk of death, RHC improved risk stratification. We therefore propose a two-step algorithm: first, assessment of the three non-invasive variables for all patients, then consideration of SVI and/or SvO2 for patients at intermediate risk.
The major strengths of our multicentre study were the large cohort size of patients having been reassessed by RHC and the inclusion of a large cohort PAH from all causes, which means that our results could be applied to all patients with PAH. Additionally, a notable strength was the high proportion (44%) of patients receiving initial combination therapy compared with other studies, which reflects PAH management in the modern era. We excluded patients with portopulmonary hypertension or uncorrected congenital heart disease since these two types of PAH have specific prognostic factors as well as different management and outcomes [1, 2, 27–36].
We recognise some limitations given the retrospective nature of the study, along with the notable absence of certain haemodynamic data, including SVI or SvO2. However, the baseline clinical and haemodynamic characteristics of patients with a missing value were broadly similar to those with complete datasets, suggesting that our sample broadly represents the overall population. Selection bias could have been introduced because some patients died or underwent lung transplantation before having reassessment by RHC and were therefore not included in the analysis. While this is an inherent challenge in studies involving follow-up variables, it does underscore the severe and often rapidly progressing nature of PAH in real-world clinical settings. In addition, this refined score has only been assessed at the first follow-up visit. The robustness and applicability of this score would be further enhanced by validating it in subsequent follow-up visits, thereby providing a more comprehensive understanding of its utility over the course of the disease.
Conclusions
In summary, we found that haemodynamic variables during follow-up did not improve risk stratification of patients with PAH classified at low or high risk of death according to the non-invasive four-strata model. The refined six-strata model, which is an approach in two steps (assessment of the three non-invasive variables for all patients, then consideration of SVI and/or SvO2 for patients at intermediate risk), enhanced the granularity of risk assessment, which will likely help clinicians identify patients who require a more aggressive approach.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00197-2024.SUPPLEMENT (499.4KB, pdf)
Shareable PDF
Footnotes
Members of the PulmoTension Network: Emmanuel Bergot (Caen), Laurent Bertoletti (Saint Etienne), Hélène Bouvaist (Grenoble), Arnaud Bourdin (Montpellier), Céline Chabanne (Rennes), Bruno Degano (Grenoble), Claire Dauphin (Clermont-Ferrand), Nicolas Favrolt (Dijon), Delphine Horeau Langlard (Nantes), Etienne-Marie Jutant (Poitiers), Bouchra Lamia (Le Havre), Pascal Magro (Tours), Pierre Mauran (Reims), Pamela Moceri (Nice), Sylvain Palat (Limoges), Patrice Poubeau (Saint Pierre de la Réunion), Sébastien Renard (Marseille), Martine Reynaud Gobert (Marseille), Marianne Riou (Strasbourg), Olivier Sanchez (Paris), Cécile Tromeur (Brest).
Ethics statement: This study complied with the Declaration of Helsinki. Although French law does not require ethics committee approval or informed consent for retrospective data collection, the data collected in the French Pulmonary Hypertension Network registry were anonymised and complied with the requirements of the Commission Nationale Informatique et Liberté (CNIL). CNIL approved the methods used to collect and analyse the data on 24 May 2003 (approval number 842063).
This article has an editorial commentary: https://doi.org/10.1183/13993003.00943-2024
Conflict of interest: A. Boucly reports grants from Acceleron, Janssen and MSD, and lecture honoraria and travel support from Janssen, Merck, AOP Orphan and Ferrer, outside the submitted work. S. Turquier reports consulting fees from Ferrer, lecture honoraria from MSD, and travel support from Ferrer, MSD and Janssen, outside the submitted work. P. de Groote reports consulting fees from Boehringer Ingelheim, Servier, Bayer and MSD, and lecture honoraria from BMS, Novartis, Vifor and Pfizer, outside the submitted work. A. Chaouat reports travel support from MSD and Janssen, outside the submitted work. C. Cheron reports travel support from MSD, outside the submitted work. X. Jaïs reports grants from Acceleron, Janssen, MSD and Bayer HealthCare, and lecture honoraria from Janssen and MSD, outside the submitted work. F. Picard reports lecture honoraria from Bayer, Boehringer Ingelheim/Lilly, Novartis, MSD, AstraZeneca and Novo Nordisk, and travel support from Bayer and Novartis, outside the submitted work. G. Prévot reports lecture honoraria and travel support from Janssen and MSD, outside the submitted work. V. Cottin reports consulting fees from Ferrer/United Therapeutics and Liquidia, and lecture honoraria from Ferrer/United Therapeutics, outside the submitted work. D. Montani reports grants from Acceleron, Merck MSD and Janssen, consulting fees from Acceleron, Merck MSD, Janssen and Ferrer; and lecture honoraria from Bayer, Janssen, Boehringer, Chiesi, GSK, Ferrer and Merck MSD, outside the submitted work. M. Humbert reports grants from Acceleron, AOP Orphan, Janssen, Merck and Shou Ti, consulting fees from 35 Pharma, Aerovate, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Keros, Merck, MorphogenIX, Shou Ti and United Therapeutics, lecture honoraria from Janssen and Merck, and advisory board participation with Acceleron, Altavant, Janssen, Merck and United Therapeutics, outside the submitted work. L. Savale reports lecture honoraria and travel support from MSD and Janssen and Janssen, outside the submitted work. O. Sitbon reports grants from Aerovate, AOP Orphan, Ferrer, Janssen and MSD, consulting fees from Altavant/Enzyvant, AOP Orphan, Ferrer, Gossamer Bio, Janssen, Liquidia, MSD and Respira Therapeutics, lecture honoraria from AOP Orphan, Janssen, Ferrer and MSD, and advisory board participation with Altavant/Enzyvant, Gossamer Bio and Janssen, outside the submitted work. The remaining authors have no potential conflicts of interest to disclose.
Support statement: A. Boucly is supported by ERS/EU RESPIRE4 Marie Skłodowska-Curie Postdoctoral Research Fellowship (R4202205-00947).
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 3.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. doi: 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 4.Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 2010; 35: 1079–1087. doi: 10.1183/09031936.00072709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. doi: 10.1161/CIRCULATIONAHA.109.911818 [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. doi: 10.1183/09031936.00057010 [DOI] [PubMed] [Google Scholar]
- 7.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. doi: 10.1183/09031936.00092311 [DOI] [PubMed] [Google Scholar]
- 8.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. doi: 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 10.Kylhammar D, Kjellström B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. doi: 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 11.Weatherald J, Boucly A, Chemla D, et al. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation 2018; 137: 693–704. doi: 10.1161/CIRCULATIONAHA.117.029254 [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 14.Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined four-strata risk assessment model for pulmonary arterial hypertension. Eur Respir J 2021; 60: 2102311. doi: 10.1183/13993003.02311-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benza RL, Kanwar MK, Raina A, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021; 159: 337–346. doi: 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeper MM, Pittrow D, Opitz C, et al. Risk assessment in pulmonary arterial hypertension. Eur Respir J 2018; 51: 1702606. doi: 10.1183/13993003.02606-2017 [DOI] [PubMed] [Google Scholar]
- 17.Weatherald J, Boucly A, Launay D, et al. Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J 2018; 52: 1800678. doi: 10.1183/13993003.00678-2018 [DOI] [PubMed] [Google Scholar]
- 18.Lewis RA, Johns CS, Cogliano M, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 201: 458–468. doi: 10.1164/rccm.201909-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Marini C, Formichi B, Bauleo C, et al. Improved survival in limited scleroderma-related pulmonary artery hypertension. Intern Emerg Med 2014; 9: 385–396. doi: 10.1007/s11739-013-0900-7 [DOI] [PubMed] [Google Scholar]
- 21.Benza RL, Gomberg-Maitland M, Naeije R, et al. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011; 30: 982–989. doi: 10.1016/j.healun.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 22.Chin KM, Channick RN, Kim NH, et al. Central venous blood oxygen saturation monitoring in patients with chronic pulmonary arterial hypertension treated with continuous IV epoprostenol: correlation with measurements of hemodynamics and plasma brain natriuretic peptide levels. Chest 2007; 132: 786–792. doi: 10.1378/chest.07-0694 [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, McLaughlin V, Gibbs JSR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Badesch DB, Ghofrani HA, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023; 388: 1478–1490. doi: 10.1056/NEJMoa2213558 [DOI] [PubMed] [Google Scholar]
- 25.Galkin A, Sitapara R, Clemons B, et al. Inhaled seralutinib exhibits potent efficacy in models of pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102356. doi: 10.1183/13993003.02356-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza R, Badesch DB, Ghofrani HA, et al. Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial. Eur Respir J 2023; 62: 2301107. doi: 10.1183/13993003.01107-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal M, Li M, Bhardwaj A, et al. Predictors of survival in portopulmonary hypertension: a 20-year experience. Eur J Gastroenterol Hepatol 2022; 34: 449–456. doi: 10.1097/MEG.0000000000002322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 2008; 178: 637–643. doi: 10.1164/rccm.200804-613OC [DOI] [PubMed] [Google Scholar]
- 29.Savale L, Sattler C, Coilly A, et al. Long-term outcome in liver transplantation candidates with portopulmonary hypertension. Hepatology 2017; 65: 1683–1692. doi: 10.1002/hep.28990 [DOI] [PubMed] [Google Scholar]
- 30.Savale L, Guimas M, Ebstein N, et al. Portopulmonary hypertension in the current era of pulmonary hypertension management. J Hepatol 2020; 73: 130–139. doi: 10.1016/j.jhep.2020.02.021 [DOI] [PubMed] [Google Scholar]
- 31.Sithamparanathan S, Nair A, Thirugnanasothy L, et al. Survival in portopulmonary hypertension: outcomes of the United Kingdom national pulmonary arterial hypertension registry. J Heart Lung Transplant 2017; 36: 770–779. doi: 10.1016/j.healun.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 32.Krowka MJ, Fallon MB, Kawut SM, et al. International Liver Transplant Society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation 2016; 100: 1440–1452. doi: 10.1097/TP.0000000000001229 [DOI] [PubMed] [Google Scholar]
- 33.Hascoët S, Baruteau A-E, Humbert M, et al. Long-term outcomes of pulmonary arterial hypertension under specific drug therapy in Eisenmenger syndrome. J Heart Lung Transplant 2017; 36: 386–398. doi: 10.1016/j.healun.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Hascoet S, Fournier E, Jaïs X, et al. Outcome of adults with Eisenmenger syndrome treated with drugs specific to pulmonary arterial hypertension: a French multicentre study. Arch Cardiovasc Dis 2017; 110: 303–316. doi: 10.1016/j.acvd.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 35.Diller G-P, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006; 27: 1737–1742. doi: 10.1093/eurheartj/ehl116 [DOI] [PubMed] [Google Scholar]
- 36.Kempny A, Hjortshøj CS, Gu H, et al. Predictors of death in contemporary adult patients with Eisenmenger syndrome: a multicenter study. Circulation 2017; 135: 1432–1440. doi: 10.1161/CIRCULATIONAHA.116.023033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00197-2024.SUPPLEMENT (499.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00197-2024.Shareable (1.6MB, pdf)