Abstract
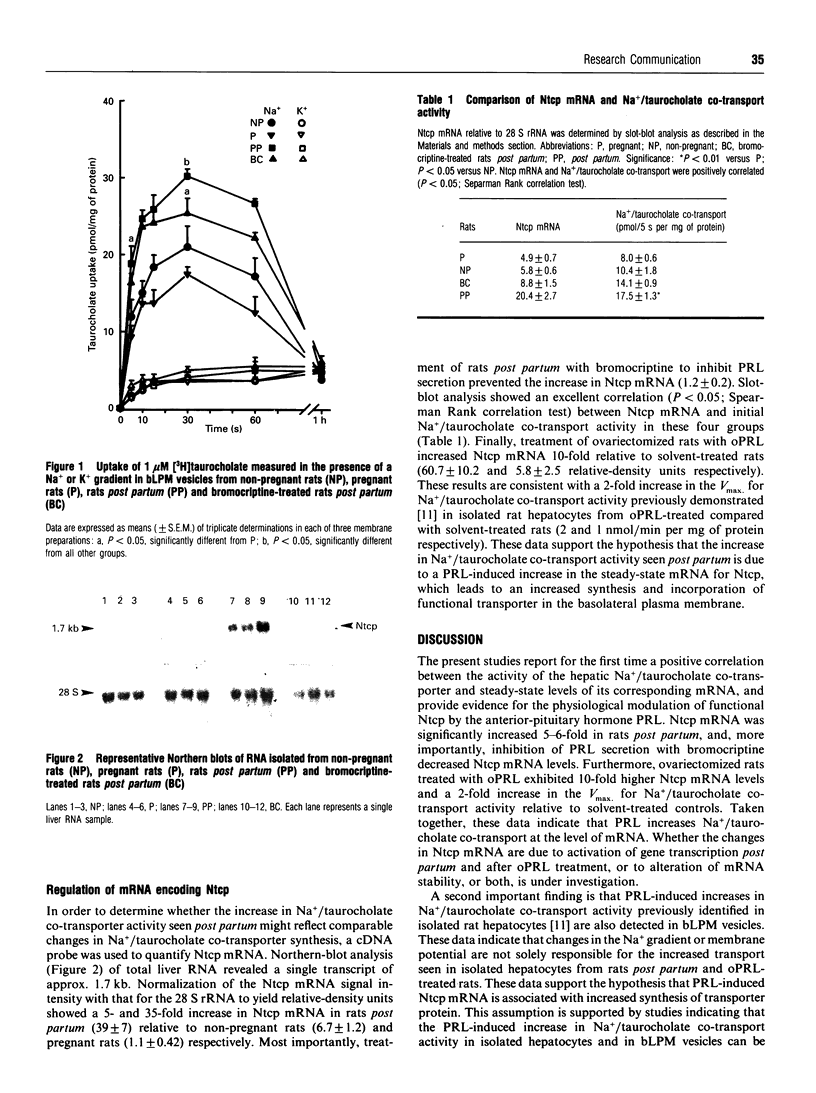
We have shown that Na+/taurocholate co-transport activity is decreased in pregnancy, but rebounds post partum relative to non-pregnant controls, and that activity can be increased by treatment with ovine prolactin [Ganguly, Hyde and Vore (1993) J. Pharmacol. Exp. Ther. 267, 82-87]. To determine the basis for these effects, Na+/taurocholate co-transport was determined in purified basolateral liver plasma-membrane (bLPM) vesicles and compared with steady-state mRNA levels encoding the Na+/taurocholate-co-transporting polypeptide (Ntcp) in non-pregnant controls, pregnant rats (19-20 days pregnant), rats post partum (48 h post partum) and rats post partum treated with bromocriptine to inhibit prolactin secretion. Na+/taurocholate co-transport activity (nmol/5 s per mg of protein) in bLPM was decreased from 10.4 +/- 1.8 in non-pregnant controls to 7.9 +/- 0.6 in bLPM in pregnant rats, but rebounded to 17.5 +/- 1.3 post partum; treatment of rats post partum with bromocriptine to inhibit prolactin secretion decreased activity to 14.1 +/- 0.9. Northern and slot-blot analyses revealed similar changes in mRNA for Ntcp, so that a positive correlation was observed between Na+/taurocholate co-transport activity and Ntcp mRNA. Furthermore, treatment of ovariectomized rats with ovine prolactin increased Ntcp mRNA 10-fold compared with solvent-treated controls, consistent with the 2-fold increase in Vmax, for Na+/taurocholate co-transport in isolated hepatocytes. These data are the first to demonstrate endogenous physiological regulation by prolactin of Ntcp mRNA in parallel with Na+/taurocholate co-transport activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer M. S., Hegner D. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):181–192. [PubMed] [Google Scholar]
- Barbu V., Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 1989 Sep 12;17(17):7115–7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berr F., Simon F. R., Reichen J. Ethynylestradiol impairs bile salt uptake and Na-K pump function of rat hepatocytes. Am J Physiol. 1984 Oct;247(4 Pt 1):G437–G443. doi: 10.1152/ajpgi.1984.247.4.G437. [DOI] [PubMed] [Google Scholar]
- Bossard R., Stieger B., O'Neill B., Fricker G., Meier P. J. Ethinylestradiol treatment induces multiple canalicular membrane transport alterations in rat liver. J Clin Invest. 1993 Jun;91(6):2714–2720. doi: 10.1172/JCI116511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Meier P. J. Characterizing mechanisms of hepatic bile acid transport utilizing isolated membrane vesicles. Methods Enzymol. 1990;192:517–533. doi: 10.1016/0076-6879(90)92091-q. [DOI] [PubMed] [Google Scholar]
- Brock W. J., Vore M. The effect of pregnancy and treatment with 17 beta-estradiol on the transport of organic anions into isolated rat hepatocytes. Drug Metab Dispos. 1984 Nov-Dec;12(6):713–716. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ganguly T., Hyde J. F., Vore M. Prolactin increases Na+/taurocholate cotransport in isolated hepatocytes from postpartum rats and ovariectomized rats. J Pharmacol Exp Ther. 1993 Oct;267(1):82–87. [PubMed] [Google Scholar]
- Hagenbuch B., Lübbert H., Stieger B., Meier P. J. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J Biol Chem. 1990 Apr 5;265(10):5357–5360. [PubMed] [Google Scholar]
- Hagenbuch B., Stieger B., Foguet M., Lübbert H., Meier P. J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10629–10633. doi: 10.1073/pnas.88.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hyde J. F., Vore M. Prolactin regulates maternal bile secretory function post partum. J Pharmacol Exp Ther. 1992 May;261(2):560–566. [PubMed] [Google Scholar]
- Meier P. J. The bile salt secretory polarity of hepatocytes. J Hepatol. 1989 Jul;9(1):124–129. doi: 10.1016/0168-8278(89)90085-8. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Scharschmidt B. F., Stephens J. E. Transport of sodium, chloride, and taurocholate by cultured rat hepatocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):986–990. doi: 10.1073/pnas.78.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman S. A., Weeks R. P. Electrogenicity of Na-coupled bile salt transport in isolated rat hepatocytes. Am J Physiol. 1993 Jul;265(1 Pt 1):G73–G80. doi: 10.1152/ajpgi.1993.265.1.G73. [DOI] [PubMed] [Google Scholar]




