Abstract
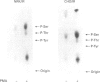
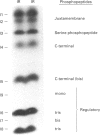
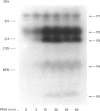
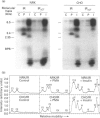
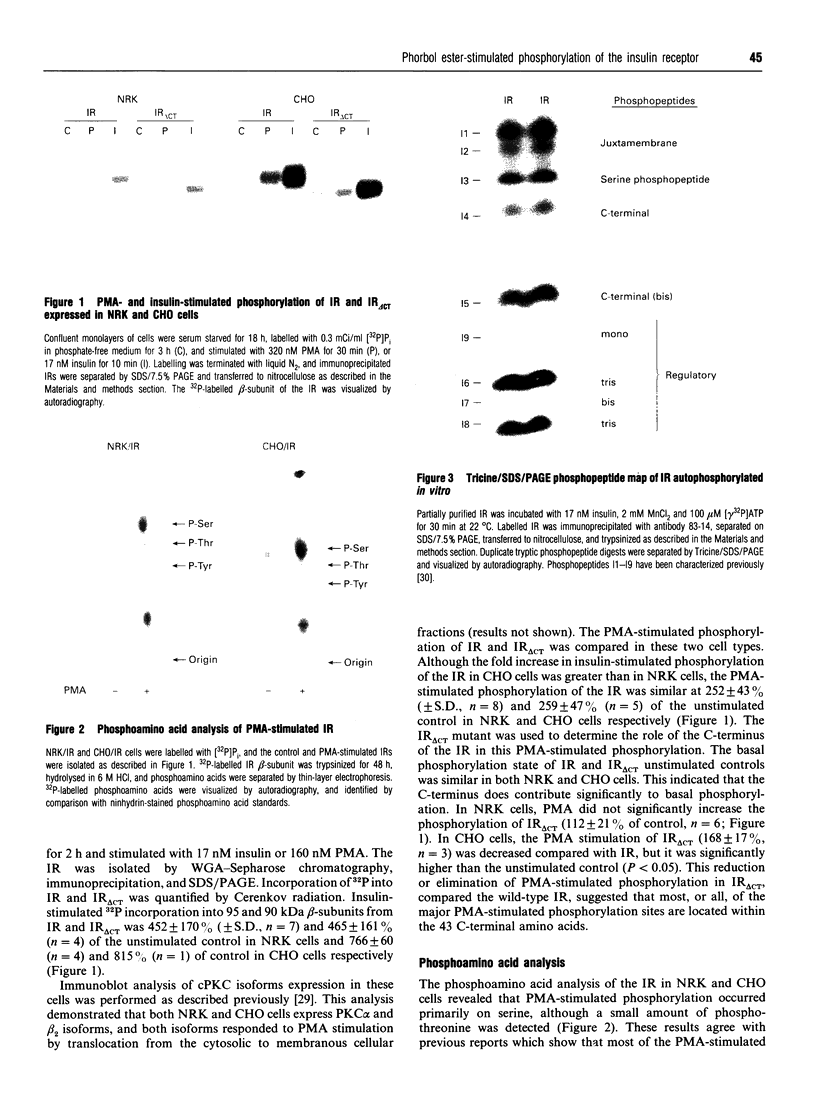
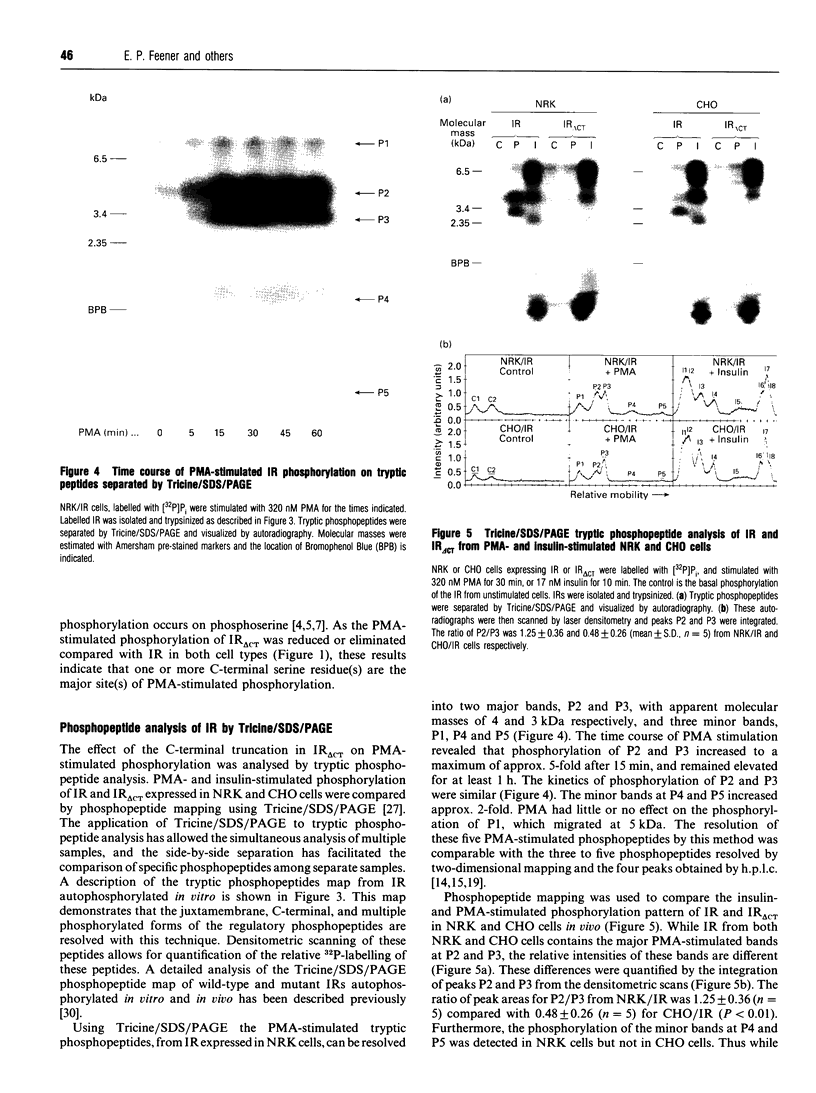
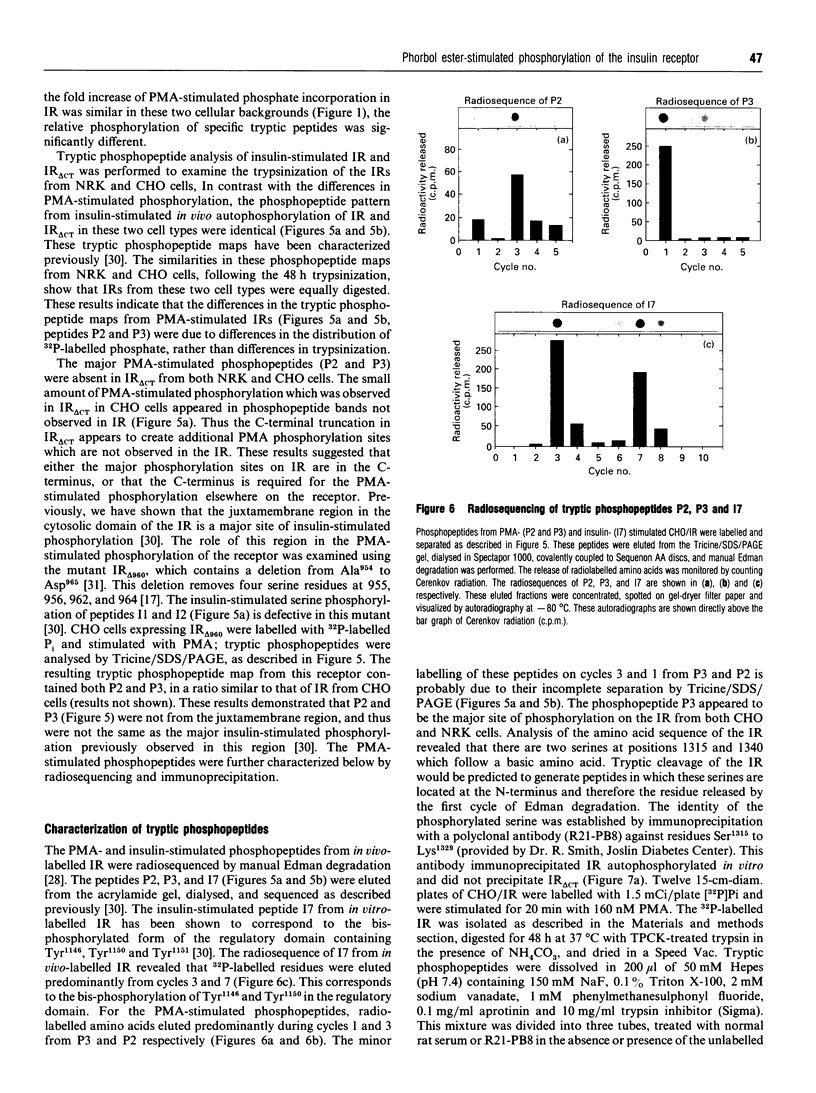
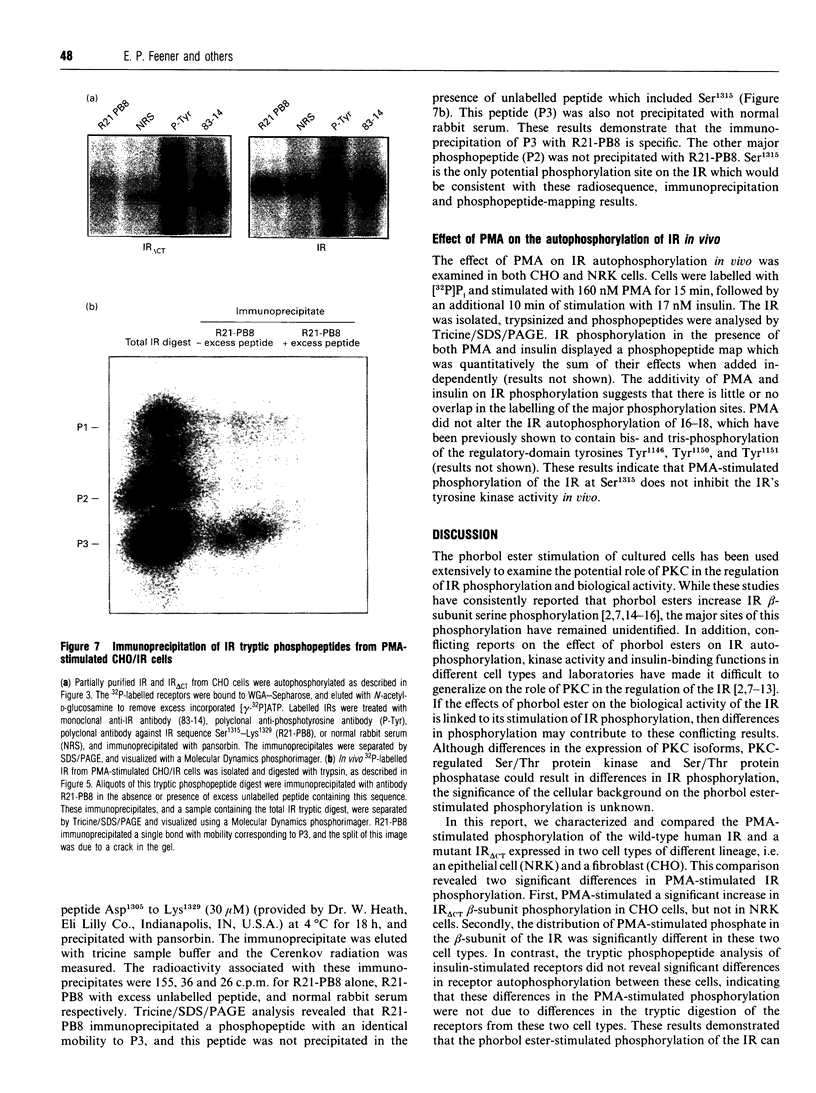
Phorbol 12-myristate 13-acetate (PMA)-stimulated phosphorylation of the human insulin receptor (IR) was characterized and compared in two cell types of different lineage: normal rat kidney epithelial (NRK) cells and Chinese hamster ovary (CHO) fibroblasts. PMA stimulation increased IR beta-subunit phosphorylation to 252 +/- 43 and 25- +/- 47% (+/- S.D.) of the unstimulated control in NRK and CHO cells respectively. Tryptic phosphopeptide analysis by Tricine/SDS/PAGE revealed significant differences in the PMA-stimulated phosphorylation of the IR in these two cell types. This phosphorylation of the IR was predominantly located in two tryptic phosphopeptides, and these phosphopeptides were absent in an IR mutant truncated by 43 C-terminal amino acids. The major PMA-stimulated tryptic phosphopeptide from in vivo-labelled CHO/IR was immunoprecipitated with an antibody against residues Ser1315 to Lys1329, and this precipitation was blocked with excess unlabelled peptide containing this sequence. Radiosequencing by manual Edman degradation revealed that this tryptic phosphopeptide was phosphorylated at Ser1315. This PMA-stimulated phosphorylation did not inhibit autophosphorylation of the IR in vivo. These results demonstrate that PMA-stimulated phosphorylation of the IR can exhibit significant differences when expressed in different cell types, and that Ser1315 is a major PMA-stimulated phosphorylation site on the human IR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Olefsky J. M. Phorbol ester-mediated protein kinase C interaction with wild-type and COOH-terminal truncated insulin receptors. J Biol Chem. 1991 Nov 15;266(32):21760–21764. [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., Cahill D. A., Ullrich A., White M. F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem. 1990 Sep 25;265(27):16450–16454. [PubMed] [Google Scholar]
- Blake A. D., Strader C. D. Potentiation of specific association of insulin with HepG2 cells by phorbol esters. Biochem J. 1986 May 15;236(1):227–234. doi: 10.1042/bj2360227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G. E., Roth R. A., Beaudoin J., Mochly-Rosen D., Koshland D. E., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro D. P., Bonner-Weir S., King G. L. Insulin receptor recycling in vascular endothelial cells. Regulation by insulin and phorbol ester. J Biol Chem. 1989 Apr 5;264(10):5916–5923. [PubMed] [Google Scholar]
- Chin J. E., Dickens M., Tavare J. M., Roth R. A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993 Mar 25;268(9):6338–6347. [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan M. P., Siddle K. Phorbol esters induce insulin receptor phosphorylation in transfected fibroblasts without affecting tyrosine kinase activity. Biochem Biophys Res Commun. 1993 May 28;193(1):371–377. doi: 10.1006/bbrc.1993.1633. [DOI] [PubMed] [Google Scholar]
- Dickens M., Tavaré J. M. Analysis of the order of autophosphorylation of human insulin receptor tyrosines 1158, 1162 and 1163. Biochem Biophys Res Commun. 1992 Jul 15;186(1):244–250. doi: 10.1016/s0006-291x(05)80799-5. [DOI] [PubMed] [Google Scholar]
- Feener E. P., Backer J. M., King G. L., Wilden P. A., Sun X. J., Kahn C. R., White M. F. Insulin stimulates serine and tyrosine phosphorylation in the juxtamembrane region of the insulin receptor. J Biol Chem. 1993 May 25;268(15):11256–11264. [PubMed] [Google Scholar]
- Grunberger G., Gorden P. Affinity alteration of insulin receptor induced by a phorbol ester. Am J Physiol. 1982 Oct;243(4):E319–E324. doi: 10.1152/ajpendo.1982.243.4.E319. [DOI] [PubMed] [Google Scholar]
- Hachiya H. L., Takayama S., White M. F., King G. L. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. J Biol Chem. 1987 May 5;262(13):6417–6424. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Tumor-promoting phorbol esters increase the Km of the ATP-binding site of the insulin receptor kinase from rat adipocytes. J Biol Chem. 1986 Mar 15;261(8):3869–3875. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Koshio O., Akanuma Y., Kasuga M. Identification of a phosphorylation site of the rat insulin receptor catalyzed by protein kinase C in an intact cell. FEBS Lett. 1989 Aug 28;254(1-2):22–24. doi: 10.1016/0014-5793(89)81001-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Cao L., Perregaux D., Czech M. P. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990 Feb 20;29(7):1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. E., Wu G. P., MacDonald R. G., Czech M. P. Insulin-sensitive phosphorylation of serine 1293/1294 on the human insulin receptor by a tightly associated serine kinase. J Biol Chem. 1990 Jan 15;265(2):947–954. [PubMed] [Google Scholar]
- Marais R. M., Nguyen O., Woodgett J. R., Parker P. J. Studies on the primary sequence requirements for PKC-alpha, -beta 1 and -gamma peptide substrates. FEBS Lett. 1990 Dec 17;277(1-2):151–155. doi: 10.1016/0014-5793(90)80831-3. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Parker P. J. Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem. 1989 Jun 1;182(1):129–137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Levy J., Huecksteadt T., Dull T. J., Lee J., Ullrich A., Olefsky J. M. Properties of a human insulin receptor with a COOH-terminal truncation. I. Insulin binding, autophosphorylation, and endocytosis. J Biol Chem. 1988 Jun 25;263(18):8904–8911. [PubMed] [Google Scholar]
- Oliver F. J., de la Rubia G., Feener E. P., Lee M. E., Loeken M. R., Shiba T., Quertermous T., King G. L. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991 Dec 5;266(34):23251–23256. [PubMed] [Google Scholar]
- Roth R. A., Beaudoin J. Phosphorylation of purified insulin receptor by cAMP kinase. Diabetes. 1987 Jan;36(1):123–126. doi: 10.2337/diab.36.1.123. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shigekawa K., Dower W. J. Electroporation of eukaryotes and prokaryotes: a general approach to the introduction of macromolecules into cells. Biotechniques. 1988 Sep;6(8):742–751. [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Increasing the cAMP content of IM-9 cells alters the phosphorylation state and protein kinase activity of the insulin receptor. J Biol Chem. 1986 Mar 5;261(7):3402–3407. [PubMed] [Google Scholar]
- Sullivan S., Wong T. W. A manual sequencing method for identification of phosphorylated amino acids in phosphopeptides. Anal Biochem. 1991 Aug 15;197(1):65–68. doi: 10.1016/0003-2697(91)90356-x. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Kahn C. R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988 Mar 5;263(7):3440–3447. [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaré J. M., Dickens M. Changes in insulin-receptor tyrosine, serine and threonine phosphorylation as a result of substitution of tyrosine-1162 with phenylalanine. Biochem J. 1991 Feb 15;274(Pt 1):173–179. doi: 10.1042/bj2740173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos P., Testa U., Gourdin M. F., Hervy C., Titeux M., Vainchenker W. Inhibition of insulin receptor binding by phorbol esters. Eur J Biochem. 1982 Dec 15;129(2):389–393. doi: 10.1111/j.1432-1033.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Shoelson S. E., Keutmann H., Kahn C. R. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988 Feb 25;263(6):2969–2980. [PubMed] [Google Scholar]