Abstract
Parkinson’s disease (PD) represents a challenging condition where different therapeutic options have evolved over the last 50 years. The potential use of cell transplantation for cell replacement or gene delivery of neurotrophic factors has received a great deal of attention. Currently, all available treatment options are directed towards the amelioration of symptoms. A greater understanding of the distinctive pathology underlying PD might offer some novel therapeutic approaches. Transplantation of embryonic ventral mesencephalon (VM) dopaminergic neurons has shown promise in animal studies, but similar transplant procedures have shown limited success in clinical trials. One important issue may be the site of transplantation. Previous studies have transplanted VM into the striatum, which is the target of these neurons. With increased understanding of growth and guidance molecule effecting dopaminergic neurons, it may be feasible to place transplants in the damaged substantia nigra and direct the growth of axons into target regions to reconstruction midbrain dopamine circuitry. In this review, we discuss transplantation therapy and how selective guidance molecules could be used to reconstruction of nigrostriatal circuit.
Keywords: Neurotrophins, Parkinson’s disease, dopamine, transplants, netrin
2. INTRODUCTION
PD is typically characterized as a disease of the basal ganglia, with a progressive degeneration of dopaminergic neurons located in the substantia nigra (SN) and projecting to the striatum with subsequently loss of the nigrostriatal circuit. The loss of dopamine in striatum results in motor dysfunction, including resting tremor, muscular rigidity, bradykinesia and postural instability. Many strategies have attempted to reconstruct this circuit but failed to satisfy clinical trials. No complete therapies are yet available that reverses or slows down the progression of this illness. The inhibitory environment of the adult brain and the long distance between the SN and the striatum make the reconstruction of nigrostriatal circuits difficult. The concept of restoring or repairing damaged neural pathways can be broadly divided into techniques that involve neurotrophic factors for dopaminergic neurons survival and the transplantation of dopaminergic cells for cell replacement. Transplantation mechanisms for repair in PD have utilized human fetal graft techniques in which embryonic dopaminergic cells are harvested from the developing midbrain. The clinical outcomes of these studies have been mixed but show very good transplant survival in the striatum and integration of grafted dopaminergic neurons (1, 2, 3, 4). Some trials evaluating human fetal grafting raised significant concerns about this technique with a number of patients developing ‘off’-medication dyskinesia (5, 6). The other exciting novel therapy that has gained interest in recent decades is the use of neurotrophic factors (NFTs) to enhance neuronal survival. NTFs are secreted proteins that regulate multiple aspects of neuronal development including neuronal maintenance, survival, axonal growth, axonal guidance and synaptic plasticity. These properties of NTFs make them likely candidates for preventing neurodegeneration and promoting neuroregeneration. Thus fine tuning of transplantation therapy with neurotrophins may lead to novel therapeutic strategies for Parkinson’s disease.
3. BRIEF BACKGROUND ABOUT PD
The symptoms of PD were first described in the year 1817 by James Parkinson. He named the disease Paralysis agitans (shaking palsy). The French neurologist Jean-Martin Charcot in 1886 renamed this disorder to PD after James Parkinson. The cardinal motor symptoms of PD are resting tremor (tremors while at rest), rigidity (resistance to passive movement), bradykinesia (slowness of movement) and postural instability (poor balance).
The pathology of the disease is characterized by the accumulation of a protein called alpha-synuclein into inclusions called Lewy bodies in neurons, and the selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The selective loss of dopaminergic neurons in SNpc subsequently causes a profound reduction of dopamine in the striatum. When 50–60% of the DA neurons degenerated and 70–80% of DA terminals in the brain have been depleted, the motor symptoms become evident (7). Overtime the level of dopamine will continue to decrease and the motor symptoms will continue to worsen.
Approximately 1% of the population at age 65 has PD and the incidence increases with age, reaching 5% at age 85 (8). Young-onset PD (below 40 years old) affects 5–10% of patients. The ratio of men to women is about 2:1. Ethnicity does not affect the risk of PD (9).
Several factors are involved including aging, genetic mutation and environmental factors (10). Aging is one of the major factors in the development of PD. After age 50, normally up to 6% of DA neurons in the substantia nigra are lost (11) and this number increases with age. Another important factor for PD development is genetic mutation. There are two major types of PD. One is the heritable PD, which accounts for about 10% of PD cases. Heritable PD is associated with mutations in a number of specific genes, including alpha-synuclein, LRRK2, Parkin, PINK1, DJ1, ATP13A2 and FBXO7. Mutations in these genes potentially lead to autosomal dominant or autosomal recessive forms of PD (12, 13, 14, 15, 16, 17, 18, 19, 20). Another type of PD is sporadic, which accounts for 90% of all PD cases. The sporadic PD is related to environmental factors. Exposure to pesticides, industrial wastes and neurotoxins are thought to be involved in disease progression (21). Interactions of all these factors likely drive the progressive loss of DA neurons within the SN. Studies have shown that some common factors causing DA cell death are impaired energy metabolism produced by mitochondrial dysfunction, selective oxidative stress in the SN and accumulation of toxic proteins due to inefficiency of the ubiquitin-proteasome pathway (22, 23, 24, 25, 26).
4. PHARMA THERAPY
With advances in the understanding of the etiology and molecular pathophysiology of PD, a variety of pharmacologic therapies have been developed, aiming to replace lost dopamine to alleviate motor symptoms. The most widely used type of medication is the dopamine precursor called L-3, 4-dihydroxyphenylalanine (levodopa, L-dopa). Levodopa helps to alleviate the tremors and muscle stiffness that come with the disease. However, long-term treatment with levodopa eventually leads to reduced clinical benefits and troubling motor complications known as “levodopa–induced dyskinesia”. Long-term use can also cause hallucinations (27, 28, 29, 30, 31). Other medications have also been used to treat Parkinsonian symptoms as alternative or complementary medications, such as DA agonists, monoamine oxidase (MAO) inhibitors cathecol-O-methyltransferase (COMT) inhibitors, 5-HT1A agonist sarotizan, adenosine A2A antagonists, anticholinergics, amantadine and opioids. These medications act through different mechanisms (25, 32, 33, 34). These pharmacologic treatments, although having benefits at early stage of PD, are not always effective in treating PD over time.
5. NEUROTROPHIN THERAPY
GDNF and neurturin (NTN) are the two main members of the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) widely tested in animal models of PD and clinically tested in PD patients. GDNF has neuroprotective and neurorestorative effects on dopaminergic neurons demonstrated using adeno associated virus (AAV) in rodents (35, 36, 37, 38), AAV in non-human primates (39, 40, 41, 42), Lentivirus (LV) in rodents (43, 44, 45, 46), LV in non-human primates (38, 47), Adeno virus in rodents (48, 49, 50, 51) and Herpes Simplex Virus in rodents (52, 53). A recent study using gene delivery of GDNF in an aged rhesus monkey model of PD suggests that the degree of neuroprotection depends on GDNF levels (54). Likewise, Neurturin (NTN), the homolog of GDNF has recently also show effectiveness in promoting neuronal survival. NTN shares 42% protein homology with GDNF, uses similar signaling pathways (55), and promotes survival of dopaminergic neurons (56). AAV-mediated delivery of NTN in a rodent model of PD shows bioactive NTN is stably expressed, is neuroprotective, and shows no adverse effects (57, 58). Injections directly into the striatum have growth-promoting effects on dopaminergic neurons within the substantia nigra (59). Studies using AAV-NTN in a primate model show long-term expression and neuroprotection with no adverse side effects with a wide safety margin of dosages (59, 60, 61, 62).
6. CLINICAL TRIALS: BENEFITS AND TRIBULATIONS
Based on the encouraging results from animal studies, the first clinical trial using GDNF in PD patients was initiated in 1996. Direct bolus injections of GDNF into the lateral ventricles were initiated for trophic factor delivery. The study was unblinded after the first 8 months and the results were disappointing. Patients showed no statistically significant recovery and several adverse events including nausea, vomiting and anorexia occurred for several days after GDNF administration. Patients who received higher doses of GDNF also experienced weight loss and symptoms of depression. Postmortem analysis of one patient from this study indicated that GDNF did not efficiently diffuse out of the lateral ventricles and, thus, was unable to elicit any effect in the striatum or the substantia nigra (63). The trial initiated by AMGEN was halted in September 2004 (64).
Amgen optimistically again conducted an initial Phase I open-labeled trial for intra-putamen injection of GDNF in five patients (65). No adverse side effects were reported after 1 year of treatment, and in fact significant decreases were reported in both “on” and “off” Unified Parkinson’s Disease Rating Scale (UPDRS) scores. Postmortem analyses of one of the patient’s brain tissue indicated that there was a more than five-fold increase in tyrosine hydroxylase immunoreactivity in the right putamen (infused side) compared to left putamen (66). Patients received these unilateral infusions also showed increased growth-associated protein 43 (GAP43) staining in the right putamen. The increased TH staining in the putamen might either be a result of sprouting of fibers or an upregulation of the enzyme in spared fibers.
The intra-putamen delivery of AAV-NTN (CERE-120) was also performed in humans for dosing, tolerability and safety (67, 68). Phase II testing failed because there was no change in the primary UPDRS motor off score at 12 months (Ceregene, Press Release 11/26/2008). Unfortunately, Phase II clinical trials have failed for both GDNF and neurturin (CERE-120) in PD patients (69). Briefly, initial results of intra-putamen GDNF administration in PD patients were promising but a Phase II double-blinded study was halted prematurely, mainly because of two safety issues (64). One concern was the detection of antibodies to GDNF in blood samples of some patients. The other safety concern was the formation of lesions in the cerebellum of rhesus monkeys receiving high doses of GDNF within the putamen for a 6 month-period.
7.1. Transplantation therapy
Transplantation therapy for PD can be divided into two types i.e intrastriatal transplantation and nigral transplantation. Transplantation of neuronal tissue has been extensively studied both in animal models and in clinical trials, in an effort to regain lost function in PD. The most commonly transplanted cell type in the rodent models of PD is dopaminergic neurons from the ventral mesencephalon (VM) of developing embryos (70, 71, 72, 73). Rodent striatal transplantation was done by direct injection of these cells into the striatum (5, 74, 75, 76). In an open clinical trial, 40 patients between 34 to 75 years of age having severe Parkinson’s disease (mean duration, 14 years) received striatal transplants of these cells (5). In transplant recipients, cultured mesencephalic tissue from four embryos was implanted into the putamen bilaterally. In summary, human embryonic dopamine-neuron transplants survive well in patients with severe Parkinson’s disease resulting in some clinical benefit for younger but not for older patients. The occurrence of late dystonia and dyskinesia in five of the patients with transplants indicates that the surgical technique needs further refinement. Another double blind, placebo-controlled clinical trial also failed to show significant clinical benefit after fetal nigral transplantation (77). In this study, transplantation was associated with modest improvement in more traditional motor outcomes, particularly in patients younger than 60 years, but approximately 15% developed a disabling form of dyskinesia. Thus, fetal nigral transplantation failed to provide significant clinical benefits despite having evidence of survival of high numbers of implanted cells. The role of nigral transplantation as a treatment for PD thus has not been established.
The use of human fetal tissue as a source for transplantation has always posed a number of ethical and practical concerns and it is possible that these could be avoided by the use of human embryonic stem (ES) cells or induced pluripotent stem (iPS) cells. ES cells could potentially provide an unlimited source of human midbrain dopaminergic neurons with appropriate neural precursor cell selection and culture techniques (78). Transplantation of these cells into animal models of PD induced partial functional recovery, although evidence of striatal reinnervation or in vivo dopamine release was not demonstrated. One major concern regarding ES cells is their underlying potential to produce tumors (79). Unlike ES cells, iPS cells are a potential autologous source of dopaminergic cells with reduced concerns for both ethical and immunological difficulties. They are derived by exposing a patient’s own somatic cells, such as fibroblasts (80) to genetic manipulation. The functionality has only been demonstrated using mouse fibroblasts in a rat model of PD (81). Evaluation of the potentiality of stem cell therapy has been gained through a number of clinical trials (82, 83, 84). The long-term success of these grafted cells is difficult to predict. Although they have the ability to form synaptic connections within the host, it remains unclear if grafted stem cells will be able to match similar levels of refinement as observed with fetal VM transplants. It may be possible to improve treatment efficacy by generating stem cells with dopaminergic characteristics that also release trophic factors like GDNF for therapeutic value of PD patients.
7.2. Axonal targeting in transplantation therapy of Parkinson’s disease
A few studies have attempted to reconstruct the nigrostriatal pathway by placing the transplants into the substantia nigra (85, 86, 87). Several studies have been done using “bridging” techniques to create a growth supportive conduit extending from the substantia nigra to the target striatum. Bridges have been created by injecting GDNF (88), dissociated striatal tissue (89), fibroblast growth factor (FGF)-4-secreting schwannoma cells (90), GDNF-secreting Schwann cells (91) and kidney tissue (92). Although these techniques increased the growth of tyrosine hydroxylase (TH+) fibers from the SN to the striatum and resulted in some improvement in motor recovery, the amount of striatal reinnervation was still too low to reach clinical relevant behavioral improvement such as spontaneous motor behavior changes.
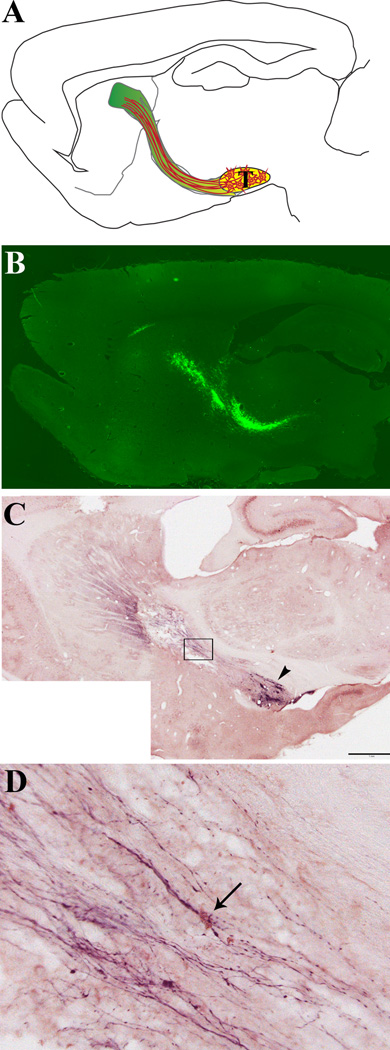
Further improvements are still needed to reconstruct of nigrostriatal circuit after placing the transplant into the substantia nigra. We have established long distance directional growth of dopaminergic axons from VM transplant along pathways of netrin-1 and GDNF in the rat brain (93). The goal of this research is to reconstruct nigrostriatal circuit by targeting dopaminergic axons to the striatum along the nigrostriatal pathway prepared by lentiviral expression of axon growth supporting molecules (GDNF, GFRα1, Netrin-1) in a 6-hydroxy dopamine (6-OHDA) induced rat model (Figure 1). Identifying guidance molecules involved in directing the growth of grafted neurons could be useful for cellular therapy in Parkinson’s patients, as these molecules may help direct axon growth over the long distance they have to travel from the substantia nigra to the striatum. We are presently using this method to identify dopaminergic axons growth and guidance factors for improving transplantation therapy.
Figure 1.
Reconstruction of the nigrostriatal pathway in adult rats: A) Schematic diagram showing the location of transplant (T), the preformed growth supportive pathway (green), and axons growing along the pathway (orange lines). B) A sample sagittal section from a brain injected with lenti-GFP, showing GFP expression (fluorescence) all along the pathway between the midbrain (lower right) and the striatum (upper left), with some spread of expression within the corpus callosum (top). C & D) Double immunostaining showing TH+ fibers and netrin-1 expression along the nigrostriatal pathway. Eight weeks after transplantation, brains were harvested, sliced in parasagittal sections and stained with antibodies to both tyrosine hydroxylase (TH) and netrin-1, then developed with DAB + Nickel enhancement (purple color, TH) and NovaRed (pink color, netrin-1). C) Low magnification image showing TH+ axon outgrowth along the preformed growth pathway extending from transplant (arrowhead) to striatum, scale bar = 1mm. D) Higher magnification of boxed area in A, arrow points to a cell with high expression of netrin-1 with a thick bundle of TH+ axons growing over it.
7.3. Factors affecting growth and targeting of dopaminergic axon
Previous studies show GDNF increases survival of dopamine neurons, but was not known to act as a guidance factor for these neurons. It has been widely used in animal models and in clinical trials for Parkinson’s disease (94, 95, 96, 97). It has also been shown to increase differentiation, fiber outgrowth and dopamine release of fetal midbrain dopaminergic neurons both in vitro and in vivo (98, 99). GDNF has been used successfully to increase the survival of fetal dopaminergic cell transplants in the 6-OHDA-lesioned rat striatum (100,101). Viral mediated over-expression of GDNF within the striatum establishes a gradient that induces axon growth into the striatum from dopaminergic neurons within nigral transplant in both mouse and non-human primate models, thus acting to guide axon towards their target. Both studies were performed in partially lesioned animals. Complete lesions in the mouse model failed to show growth of transplanted axons toward the striatum, indicating surviving endogenous dopaminergic might act as a growth supportive scaffold. Although, there is little evidence supporting the role of GDNF as a chemo attractant for dopaminergic axons, when combined with the GPI-linked glial cell line-derived neurotrophic factor receptor α1 (GFRα1) there is an attractive guidance effect on other populations of GDNF-responsive neurons (sensory and sympathetic) (102). The signaling of GDNF has been shown to be dependent of binding to its co-receptor GFRα1. This dimer complex binds to the transmembrane receptor tyrosine kinase RET, causing auto phosphorylation and activation of downstream signaling events (103). In another signaling pathway independent of cRET, NCAM acts an alternative receptor for GDNF/GFRα1. Signaling through this pathway activates the cytoplasmic protein tyrosine kinases Fyn and FAK in cells, thus stimulates axonal growth (104, 105). GDNF signaling can occur by forming dimmers with GFR-α1 on neurons themselves (cis mechanism), or between the neurons and substrate or target cells (trans) (106). In the trans configuration GFRα1 has been shown to bind and increase the local concentration of GDNF to enhance short or long range directional guidance of axons even when in a uniform concentration (102).
Another molecule which acts as a chemo attractant guidance cue is netrin-1, which has recently been shown to have positive directional effects on neurite outgrowth from cultured dopaminergic neurons (107). Netrin-1 is well-characterized member of the netrin family of guidance cues. It has been identified as a bi-functional guidance factor, either attracting or repelling extending axons during the development of the central nervous system (108, 109, 110, 111). The attractive or repulsion actions of netrin-1 are known to depend on the activation of specific receptors or receptor complexes on the cell surface: the deleted in colorectal cancer (DCC) or the UNC5 homologues (UNC5H, A-D), respectively (112, 113). Down syndrome cell adhesion molecule (DSCAM) has been identified as a novel netrin-1 receptor involved in signaling axon guidance but the signal transduction has not been well understood (114, 115). Netrin-1 binds to DCC receptor and signals through downstream proteins that regulate cytoskeletal reorganization, thus causes growth cone extension and neurite growth (107, 116, 117). Netrin-1 directs the assembly and disassembly of actin filaments by activation of the Rho family of small GTPases (118, 119) and promotes actin polymerization in lamellipodia and filopodia by activation of Rac and Cdc42 (120, 121). Deleted in colorectal cancer (DCC) receptor is widely expressed in developing brain. However, in adult brain DCC is only expressed in limited populations of neurons, the majority of them being ventral A9 dopamine neurons in the substantia nigra (122). In addition, there is a dramatically decrease in the ratio of DCC: UNC5H receptors expression in the DA neurons after puberty with UNC5H receptors predominance (123). When UNC5H receptors signaling predominate in the dopaminergic neurons in our adult animals, no endogenous dopaminergic axons growth were seen.
8. SUMMARY AND PERSPECTIVE
The original restoration of nigrostriatal dopaminergic system in PD focused predominantly on the transplantation therapy. Most strategies graft embryonic dopaminergic neurons into the striatum, with only a few studies attempt to reconstruct the entire nigrostriatal pathway by grafting into the substantia nigra (124, 125, 126, 127). In this review, we discussed transplantation studies performed over the past 10 years and summarize the current knowledge of cellular and molecular signals involved in mediating survival, growth and guidance of dopaminergic neurons. A major challenge in transplant therapies in the SN is to identify potential dopaminergic growth factors and assay their ability to direct axon growth along an appropriate pathway to reach their targets.
A definitive therapy for PD is reliant upon a complete understanding of the underlying neurodegenerative process, and future strategies need to be developed that are able to stop or reverse progression of the disease. Clarification of the molecular mechanisms of interaction between transplants and neurotropic factors will provide the optimal therapeutic target for PD and perhaps represents the only real hope for a potential cure for the disease in its entirety. It might be worthwhile in future studies to introduce combined manipulation of both the intrinsic growth properties of neurons while providing extrinsic factors to create a favorable local environment for axonal growth and connection. For example, a recent study induced denervating lesions to the striatum and used AAV encoding Akt/Rheb to transduce surviving dopaminergic neurons in the SN to promote recovery (128). They demonstrate that expression of Rheb expression promoted axons growth and reinnervation of the striatum. It will be easier for transplanted neurons with a higher intrinsic growth capacity to extend axons within the inhibitory environment of the adult brain and reinnvervate the denervated striatum, especially with the help of growth factors.
Acknowledgments
This work was funded by a grant from the National Institute of Neurological Disorders and Stroke R01 NS060784 and the Shriners Hospital for Pediatric Research grants SHC 84050 and SHC 85200 (GMS).
REFERENCES
- 1.Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 3.Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson’s disease. Mov Disord. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- 4.Piccini P, Lindvall O, Bjorklund A, Brundin P, Hagell P, Ceravolo R. Delayed recovery of movement-related cortical function in Parkinson’s disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–695. [PubMed] [Google Scholar]
- 5.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–729. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 6.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF. A doubleblind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 7.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 8.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 9.Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord. 2010;25:2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCulloch CC, Kay DM, Factor SA, Sami A, Nutt JG, Higgins DS, Griffith A, Roberts JW, Leis BC, Montimurro JS, Zabetian CP, Payami H. Exploring gene-environment interactions in Parkinson's disease. Hum Genet. 2008;123:257–265. doi: 10.1007/s00439-008-0466-z. [DOI] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991;54:388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, Sanges G, Stenroos ES, Pho LT, Schaffer AA, Lazzarini AM, Nussbaum RL, Duvoisin RC. Mapping of a gene for Parkinson's disease to chromosome 4q21-q23. Science. 1996;274:1197–1199. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- 13.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 14.Valente EM, Bentivoglio AR, Dixon PH, Ferraris A, Ialongo T, Frontali M, Albanese A, Wood NW. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am J Hum Genet. 2001;68:895–900. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duijn CM, Dekker MC, Bonifati V, Galjaard RJ, Houwing-Duistermaat JJ, Snijders PJ, Testers L, Breedveld GJ, Horstink M, Sandkuijl LA, van Swieten JC, Oostra BA, Heutink P. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K .alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 17.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 18.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens M, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 20.Fonzo AD, Dekker MC, Montagna P, Baruzzi A, Yonova EH, Correia L, Guedes A, Szczerbinska T, Zhao LO, Dubbel-Hulsman CH, de Graaff E, Oyen WJ, Simons EJ, Breedveld GJ, Oostra BA, Horstink MW, Bonifati V. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology. 2009;72:240–245. doi: 10.1212/01.wnl.0000338144.10967.2b. [DOI] [PubMed] [Google Scholar]
- 21.Thomas B. Parkinson's disease: from molecular pathways in disease to therapeutic approaches. Antioxid Redox Signal. 2009;11:2077–2082. doi: 10.1089/ars.2009.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez TN, Greenamyre JT. Toxin Models of Mitochondrial Dysfunction in Parkinson's Disease. Antioxid Redox Signal. 2012;16:920–934. doi: 10.1089/ars.2011.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung KK, Dawson VL, Dawson TM. The role of the ubiquitin-proteasomal pathway in Parkinson's disease and other neurodegenerative disorders. Trends Neurosci. 2001;24:S7–S14. doi: 10.1016/s0166-2236(00)01998-6. [DOI] [PubMed] [Google Scholar]
- 24.Owen AD, Schapira AH, Jenner P, Marsden CD. Indices of oxidative stress in Parkinson's disease, Alzheimer's disease and dementia with Lewy bodies. J Neural Transm Suppl. 1997;51:167–173. doi: 10.1007/978-3-7091-6846-2_14. [DOI] [PubMed] [Google Scholar]
- 25.Olanow CW, Jenner P, Brooks D. Dopamine agonists and neuroprotection in Parkinson's disease. Ann Neurol. 1998;44:S167–S174. doi: 10.1002/ana.410440725. [DOI] [PubMed] [Google Scholar]
- 26.Jenner P. Oxidative stress and Parkinson's disease. Handb Clin Neurol. 2007;83:507–520. doi: 10.1016/S0072-9752(07)83024-7. [DOI] [PubMed] [Google Scholar]
- 27.Miyawaki E, Lyons K, Pahwa R, Tröster AI, Hubble J, Smith D, Busenbark K, McGuire D, Michalek D, Koller WC. Motor complications of chronic levodopa therapy in Parkinson's disease. Clin Neuropharmacol. 1997;20:523–530. doi: 10.1097/00002826-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci. 2000;23:S2–S7. doi: 10.1016/s1471-1931(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 29.Kostić VS, Marinković J, Svetel M, Stefanova E, Przedborski S. The effect of stage of Parkinson's disease at the onset of levodopa therapy on development of motor complications. Eur J Neurol. 2002;9:9–14. doi: 10.1046/j.1468-1331.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 30.Deogaonkar M, Subramanian T. Pathophysiological basis of drug-induced dyskinesias in Parkinson's disease. Brain Res Rev. 2005;50:156–168. doi: 10.1016/j.brainresrev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov Disord. 2008;23:S521–S533. doi: 10.1002/mds.22049. [DOI] [PubMed] [Google Scholar]
- 32.Tolosa E, Martí MJ, Valldeoriola F, Molinuevo JL. History of levodopa and dopamine agonists in Parkinson's disease treatment. Neurology. 1998;50:S2–S10. doi: 10.1212/wnl.50.6_suppl_6.s2. discussion S44–S18. [DOI] [PubMed] [Google Scholar]
- 33.Schapira AH. Dopamine agonists and neuroprotection in Parkinson's disease. Eur J Neurol. 2002;9:7–14. doi: 10.1046/j.1468-1331.9.s3.9.x. [DOI] [PubMed] [Google Scholar]
- 34.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Mov Disord. 2008;23:S497–S508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Harvey BK, Hoffman AF, Wang Y, Chiang YH, Lupica CR. MPTP-induced deficits in striatal synaptic plasticity are prevented by glial cell line-derived neurotrophic factor expressed via an adeno-associated viral vector. FASEB J. 2008;22:261–275. doi: 10.1096/fj.07-8797com. [DOI] [PubMed] [Google Scholar]
- 36.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Hanazono Y, Kume A, Urano F, Ichinose H, Nagatsu T, Nakano I, Ozawa K. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease. Gene therapy. 2002;9:381–389. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
- 38.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu T, Leventhal L, McBride J, Chen EY, Palf S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 39.Eberling JL, Kells AP, Pivirotto P, Beyer J, Bringas J, Federoff HJ, Forsayeth J, Bankiewicz KS. Functional Effects of AAV2-GDNF on the Dopaminergic Nigrostriatal Pathway in Parkinsonian Rhesus Monkeys. Hum Gene Ther. 2009;20:511–518. doi: 10.1089/hum.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsworth JD, Redmond DE, Jr, Leranth C, Bjugstad KB, Sladek JR, Collier TJ, Jr, Foti SB, Samulski RJ, Vives KP, Roth RH. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211:252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J. Clinically relevant effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged Rhesus monkeys. Hum Gene Ther. 2009;20:497–510. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P. Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson's disease using GDNF. Exp Neurol. 2000;164:15–24. doi: 10.1006/exnr.2000.7409. [DOI] [PubMed] [Google Scholar]
- 44.Brizard M, Carcenac C, Bemelmans AP, Feuerstein C, Mallet J, Savasta M. Functional reinnervation from remaining DA terminals induced by GDNF lentivirus in a rat model of early Parkinson's disease. Neurobiol Dis. 2006;21:90–101. doi: 10.1016/j.nbd.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Dowd E, Monville C, Torres EM, Wong LF, Azzouz M, Mazarakis ND, Dunnett SB. Lentivector mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. Eur J Neurosci. 2005;22:2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. [DOI] [PubMed] [Google Scholar]
- 46.Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- 47.Palfi S, Leventhal L, Chu Y, Ma SY, Emborg M, Bakay R, Deglon N, Hantraye P, Aebischer P, Kordower JH. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic ne2002urons in primate models of nigrostriatal degeneration. J Neurosci. 2002;22:4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Liu W, Guoyuan Y, Liu Z, Smith S, Calne DB, Chen S. Protective effects of intracerebral adenoviral-mediated GDNF gene transfer in a rat model of Parkinson's disease. Parkinsonism Relat Disord. 2003;10:1–7. doi: 10.1016/s1353-8020(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 50.Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, Chiang YL, Qian J, Bardwaj L, Bohn MC. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- 51.Smith AD, Kozlowski AD, Bohn MC, Zigmond MJ. Effect of AdGDNF on dopaminergic neurotransmission in the striatum of 6-OHDA-treated rats. Exp Neurol. 2005;193:420–426. doi: 10.1016/j.expneurol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Natsume A, Mata M, Goss J, Huang S, Wolfe D, Oligino T, Glorioso J, Fink DJ. Bcl-2 and GDNF delivered by HSV-mediated gene transfer act additively to protect dopaminergic neurons from 6-OHDA-induced degeneration. Exp Neurol. 2001;169:231–238. doi: 10.1006/exnr.2001.7671. [DOI] [PubMed] [Google Scholar]
- 53.Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson's disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emborg ME, Moirano J, Raschke J, Bondarenko V, Zufferey R, Peng S, Ebert AD, Joers V, Roitberg B, Holden JE, Koprich J, Lipton J, Kordower JH, Aebischer P. Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol. Dis. 2009;36:303–311. doi: 10.1016/j.nbd.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci U S A. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E, Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson's disease. Neurobiol Dis. 2007;2:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Gasmi M, Herzog CD, Brandon EF, Cunningham JJ, Ramirez GA, Ketchum ET, Bartus RT. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson's disease. Mol Ther. 2007;15:62–68. doi: 10.1038/sj.mt.6300010. [DOI] [PubMed] [Google Scholar]
- 59.Herzog CD, Brown L, Gammon D, Kruegel B, Lin R, Wilson A, Bolton A, Printz M, Gasmi M, Bishop KM, Kordower JH, Bartus RT. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson’s disease. Neurosurgery. 2009;64:602–612. doi: 10.1227/01.NEU.0000340682.06068.01. [DOI] [PubMed] [Google Scholar]
- 60.Herzog CD, Dass B, Gasmi M, Bakay R, Stansell JE, Tuszynski M, Bankiewicz K, Chen EY, Chu Y, Bishop K, Kordower JH, Bartus RT. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol Ther. 2008;16:1737–1744. doi: 10.1038/mt.2008.170. [DOI] [PubMed] [Google Scholar]
- 61.Herzog CD, Dass B, Holden JE, Stansell J, 3rd, Gasmi M, Tuszynski MH, Bartus RT, Kordower JH. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord. 2007;22:1124–1132. doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- 62.Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 63.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 64.Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg. 2007;106:614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- 65.Gill SS, Patel NK, Hotton GR, Sullivan KO, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 66.Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- 67.Bartus RT, Herzog CD, Bishop K, Ostrove JM, Tuszynski M, Kordower JH, Gasmi M. Issues regarding gene therapy products for Parkinson's disease: the development of CERE-120 (AAVNTN) as one reference point. Parkinsonism Relat Disord. 2007;3:S469–S477. doi: 10.1016/S1353-8020(08)70052-X. [DOI] [PubMed] [Google Scholar]
- 68.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 69.Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson's disease. Prog. Brain Res. 2009;175:201–216. doi: 10.1016/S0079-6123(09)17514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikkhah G, Cunningham MG, McKay R, Björklund A. Dopaminergic Microtransplants into the Substantia-Nigra of Neonatal Rats with Bilateral 6-Ohda Lesions.1. Evidence for Anatomical Reconstruction of the Nigrostriatal Pathway. J Neurosci. 1995;15:3548–3561. doi: 10.1523/JNEUROSCI.15-05-03548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braak H, Del Tredici K. Assessing fetal nerve cell grafts in Parkinson's disease. Nat Med. 2008;14:483–485. doi: 10.1038/nm0508-483. [DOI] [PubMed] [Google Scholar]
- 72.Thompson LH, Grealish S, Kirik D, Björklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 73.Blanchard BC, Pelka BM, Sladek JR., Jr A strategy for nigrostriatal tract reconstruction in response to striatal trophic factors. Cell Transplantation. 2010;19:332–332. [Google Scholar]
- 74.Backlund EO, Granberg PO, Hamberger B, Knutsson E, Mårtensson A, Sedvall G, Seiger A, Olson l. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg. 1985;62:169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- 75.Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain. 2000;123:1380–1390. doi: 10.1093/brain/123.7.1380. [DOI] [PubMed] [Google Scholar]
- 76.Agrawal AK, Chaturved RK, Shukla S, Seth K, Chauhan S, Ahmad A, Seth PK. Restorative potential of dopaminergic grafts in presence of antioxidants in rat model of Parkinson's disease. J Chem Neuroanat. 2004;28:253–264. doi: 10.1016/j.jchemneu.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 77.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A Double-blind Controlled Trial of Bilateral Fetal Nigral Transplantation in Parkinson’s Disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 78.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and largescale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 81.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli M, Constantine-Paton, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin. Neurol. 2007;20:688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- 83.Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol. Sci. 2009;30:260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J. Intern. Med. 2009;266:358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 85.Nikkhah G, Bentlage C, Cunningham MG, Björklund A. Intranigral fetal dopamine grafts induce behavioral compensation in the rat Parkinson model. J Neurosci. 1994;14:3449–3461. doi: 10.1523/JNEUROSCI.14-06-03449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nikkhah G, Cunningham MG, Jödicke A, Knapp U, Björklund A. Improved graft survival and striatal reinnervation by microtransplantation of fetal nigral cell suspensions in the rat Parkinson model. Brain Res. 1994;633:133–143. doi: 10.1016/0006-8993(94)91532-6. [DOI] [PubMed] [Google Scholar]
- 87.Gaillard A, Decressac M, Frappe I, Fernagut PO, Prestoz L, Besnard S, Jaber M. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol Dis. 2009;35:477–488. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Tien LT, Lapchak PA, Hoffer BJ. GDNF triggers fiber outgrowth of fetal ventral mesencephalic grafts from nigra to striatum in 6-OHDA-lesioned rats. Cell Tissue Res. 1996;286:225–233. doi: 10.1007/s004410050691. [DOI] [PubMed] [Google Scholar]
- 89.Dunnett SB, Rogers DC, Richards SJ. Nigrostriatal reconstruction after 6-OHDA lesions in rats: combination of dopamine-rich nigral grafts and nigrostriatal "bridge" grafts. Exp Brain Res. 1989;75:523–535. doi: 10.1007/BF00249903. [DOI] [PubMed] [Google Scholar]
- 90.Brecknell JE, Du JS, Muir E, Fidler PS, Hlavin ML, Dunnett SB, Fawcett JW. Bridge grafts of fibroblast growthfactor-4-secreting schwannoma cells promote functional axonal regeneration in the nigrostriatal pathway of the adult rat. Neuroscience. 1996;74:775–784. doi: 10.1016/0306-4522(96)00167-4. [DOI] [PubMed] [Google Scholar]
- 91.Wilby MJ, Sinclair SR, Muir EM, Zietlow R, Adcock KH, Horellou P, Rogers JH, Dunnett B, Fawcett JW. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fiber outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra. J Neurosci. 1999;19:2301–2312. doi: 10.1523/JNEUROSCI.19-06-02301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiang Y, Morales M, Zhou FC, Borlongan C, Hoffer BJ, Wang Y. Fetal intra-nigral ventral mesencephalon and kidney tissue bridge transplantation restores the nigrostriatal dopamine pathway in hemi-parkinsonian rats. Brain Res. 2001;889:200–207. doi: 10.1016/s0006-8993(00)03133-4. [DOI] [PubMed] [Google Scholar]
- 93.Zhang C, Jin Y, Ziemba KS, Fletcher AM, Ghosh B, Truit E, Yurek DM, Smith GM. Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF. Exp Neurol. 2013;250C:156–164. doi: 10.1016/j.expneurol.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 95.Grondin R, Gash DM. Glial cell line-derived neurotrophic factor (GDNF): a drug candidate for the treatment of Parkinson's disease. 1998;245:35–42. doi: 10.1007/pl00007744. [DOI] [PubMed] [Google Scholar]
- 96.Kirik D, Georgievska B, Björklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 97.Kells AP, Eberling J, Su X, Pivirotto P, Bringas J, Hadaczek P, Narrow WC, Bowers WJ, Federoff HJ, Forsayeth J, Bankiewicz KS. Regeneration of the MPTP-Lesioned Dopaminergic System after Convection-Enhanced Delivery of AAV2-GDNF. J Neurosci. 2010;30:9567–9577. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF - a Glial-Cell Line Derived Neurotrophic Factor for Midbrain Dopaminergic-Neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 99.Johansson M, Friedemann M, Hoffer B, Strömberg I. Effects of glial cell line-derived neurotrophic factor on developing and mature ventral mesencephalic grafts in oculo. Exp Neurol. 1995;134:25-3. doi: 10.1006/exnr.1995.1033. [DOI] [PubMed] [Google Scholar]
- 100.Rosenblad C, Kirik D, Björklund A. Neurturin enhances the survival of intrastriatal fetal dopaminergic transplants. Neuroreport. 1999;10:1783–1787. doi: 10.1097/00001756-199906030-00029. [DOI] [PubMed] [Google Scholar]
- 101.Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153:195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]
- 102.Ledda F, Paratcha G, Ibanez CF. Target-derived GFRalpha1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- 103.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the Ret proteintyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 104.Paratcha G, Ledda F. GDNF and GFR-alpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Nielsen J, Gotfryd K, Li S, Kulahin N, Soroka V, Rasmussen KK, Bock E, Berezin V. Role of Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Neural Cell Adhesion Molecule (NCAM) Interactions in Induction of Neurite Outgrowth and Identification of a Binding Site for NCAM in the Heel Region of GDNF. J Neurosci. 2009;29:11360–11376. doi: 10.1523/JNEUROSCI.3239-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 107.Lin L, Rao Y, Isacson O. Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol Cell Neurosci. 2005;28:547–555. doi: 10.1016/j.mcn.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 108.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 109.Livesey FJ, Hunt SP. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci. 1997;8:417–429. doi: 10.1006/mcne.1997.0598. [DOI] [PubMed] [Google Scholar]
- 110.Yee KT, Simon HH, Tessier-Lavigne M, O'Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24:607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- 111.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guan KL, Rao Y. Signalling mechanisms mediating neuronal responses to Guidance cues. Nat Rev Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- 113.Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Axon Growth and Guidance. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- 114.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon path finding. Proc Nat Acad Sci USA. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 117.Xu B, Goldman JS, Rymar VV, Forget C, Lo PS, Bull SJ, Vereker E, Barker PA, Trudeau LE, Sadikot AF, Kennedy TE. Critical roles for the netrin receptor deleted in colorectal cancer in dopaminergic neuronal precursor migration, axon guidance, and axon arborization. Neuroscience. 2010;169:932–949. doi: 10.1016/j.neuroscience.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 118.Govek EF, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes & Development. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 119.Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Luo LQ. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annual Review of Cell and. Developmental Biology. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- 121.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annual Review of Neuroscience. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 122.Osborne PB, Halliday GM, Cooper HM, Keast JR. Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience. 2005;131:671–681. doi: 10.1016/j.neuroscience.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 123.Manitt C, Labelle-Dumais C, Eng C, Grant A, Mimee A, Stroh T, Flores C. Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS One. 2010;5:e11463. doi: 10.1371/journal.pone.0011463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindvall O, Bjorklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lindvall O, Bjorklund A. Cell therapeutics in Parkinson’s dis-ease. Aerotherapeutics. 2011;8:539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gaillard A, Jaber M. Rewiring the brain with cell transplantation in Parkinson’s disease. Trends Neurosci. 2011;34:124–133. doi: 10.1016/j.tins.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Thompson LH, Grealish S, Kirik D, Björklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:25–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 128.Kim SR, Chen X, Oo TF, Kareva T, arygina OY, Wang C, During M, Kholodilov N, Burke RE. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70:110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]