Abstract
Diagnosing primary hyperparathyroidism in pregnancy is difficult due to pregnancy-related changes in parathyroid hormone (PTH); calcium; 1,25 vitamin D; and renal calcium excretion. Parathyroid hormone–related peptide (PTHrP) produced by the placenta adds additional complexity. Our case is the first to demonstrate an increased rate of PTH degradation within a pregnant individual who returned unexpectedly low PTH levels. We describe a 27-year-old female patient who presented at 25 weeks gestation with pancreatitis and hypercalcemia. Primary hyperparathyroidism was suspected but variable PTH results led to uncertainty and an assay error was considered. PTH samples were collected in both serum-separating tubes (SST) and EDTA tubes and compared to controls (5 nonpregnant and 5 pregnant individuals). Samples were retested every 2 hours for a period of 10 hours. A rapid decline in the measured PTH was noted in the index case, an observation which differed from controls. We postulated that internal and/or external factors influenced the PTH measurement obtained from our patient. From our observations, rapid PTH degradation in pregnancy, and individual variation in PTH stability and laboratory processes, can influence PTH results and impact on interpreting hypercalcemia in pregnancy.
Keywords: primary hyperparathyroidism, parathyroid hormone, pregnancy, parathyroid adenoma, hypercalcemia
Introduction
The incidence of primary hyperparathyroidism in women of childbearing age is estimated to be 0.05% (1). The most common cause in pregnancy is a benign parathyroid adenoma; however, familial and genetic syndromes should also be considered (2).
Parathyroid hormone (PTH) is an 84-amino-acid (AA) peptide hormone that binds PTH-1 receptors (PTH1R). This results in upregulation of heterotrimeric G (Gαβγ) protein coupling pathways of cyclic AMP, Gαs and Gαq, and subsequent activation of protein kinase A and C (3). Vitamin D and calcium absorption in the gastrointestinal tract is mediated via this pathway. PTH-related peptide (PTHrP) is produced predominantly in pregnancy by the placenta. It is homologous to the N-terminal 1-34 of PTH and binds to PTH1R with similar affinity (4). In pregnancy, the release of PTHrP in the second and third trimester maintains the placental-fetal calcium gradient required for fetal bone mineralization and major organ development (3, 5).
Untreated hypercalcemia can result in maternal and fetal complications in 67% and 80% of cases, respectively (6). Elevated calcium levels are associated with maternal hypertension, preeclampsia, nephrolithiasis, pancreatitis, and hypercalcemic crisis (7). Intrauterine growth restriction, neonatal hypocalcemia, and perinatal death have been described (8, 9). Compared to the baseline population, a 3.5-fold increase in the incidence of pregnancy loss in women with primary hyperparathyroidism is observed, particularly with corrected calcium (Cacorr) levels > 2.86 mmol/L (11.46 mg/dL) (normal range, 2.10-2.60 [8.40-10.4]) (7). Miscarriage typically occurs in the first or early second trimester of pregnancy, highlighting the importance of early diagnosis and treatment of this condition (10).
The diagnosis of PTH-dependent hypercalcemia is often challenging in pregnancy due to altered physiology. The rise in PTHrP is accompanied by a 50% drop in maternal PTH levels (11). In pregnancy, urinary fractional excretion of calcium above 1% is unreliable as an ancillary test in diagnosing primary hyperparathyroidism as the renal plasma flow increases by 40% to 65% (12, 13). Race, age, body mass index (BMI), and vitamin D deficiency further impact the physiological levels of PTH (14). Conventional imaging such as sestamibi [9mTc-MIBI] and four-dimensional computed tomography (4D-CT) are avoided, and ultrasonography remains the preferred investigation (15). Given these limitations, it is imperative that the PTH assay is reliable, as clinical acumen is key in the diagnosis of primary hyperparathyroidism in pregnancy.
Case Presentation
A 27-year-old female patient (G5P1M4) was transferred to our tertiary center at 25 weeks gestation with pancreatitis. She reported a history of recurrent pancreatitis of unclear etiology and symptoms of exocrine pancreatic insufficiency. Eight years prior at another health service, she had an emergency cesarean delivery at 32 weeks gestation due to pancreatitis and severe pre-eclampsia. Retrospectively, it was noted that she was hypercalcemic at the time with a Cacorr of 2.75 mmol/L (11.0 mg/dL). Unfortunately, this had not been further investigated. On admission, she had been taking a pregnancy multivitamin daily and had no other medical history.
Upon presentation to our center, she was hypercalcemic with a Cacorr of 2.85 mmol/L (11.4 mg/dL), and ionized calcium of 1.54 mmol/L (6.16 mg/dL) (normal range, 1.16-1.31 [4.65-5.25]). Her phosphate was 0.47 mmol/L (1.46 mg/dL) (normal range, 0.75-1.50 [2.32-4.65]), creatinine was 43 (0.49 mg/dL) (normal range, 44-97 [0.50-1.10]) and vitamin D was 117 nmol/L (46.8 ng/mL) (normal range, 50-150 [20-60]). The urinary fractional excretion of calcium using the Hammersmith equation was 0.0124 (1.24%). There was no known family history of hypercalcemia.
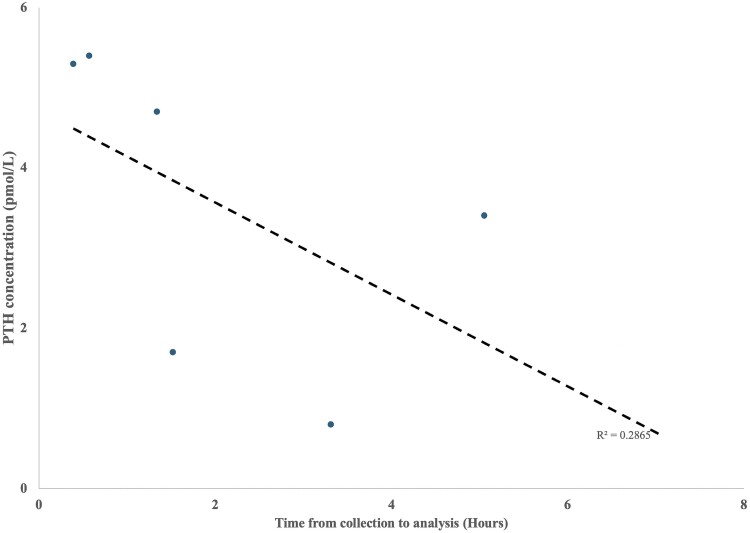
Her initial PTH was low but not completely suppressed at 1.70 pmol/L (16.03 pg/mL) (normal range, 1.00-7.00 [9.43-66.01]). Serial testing of PTH levels unexpectedly varied and ranged from 0.80-5.40 pmol/L (7.54-50.92 pg/mL). We noted that the lower PTH results were returned from samples following delayed processing times (Fig. 1). Samples processed immediately found PTH to be inappropriately normal in keeping with PTH-dependent hypercalcemia. We liaised with our biochemical pathologists to further characterize the factors influencing PTH variability in pregnancy. We hypothesized that PTH variability was impacted by the time to sample analysis.
Figure 1.
Parathyroid hormone (PTH) concentrations of blood samples observed in our index case over time from collection to analysis (Hours) using serum-separating tubes (SST). Associated linear regression is shown (R2 = 0.2865).
Diagnostic Assessment
We assessed PTH stability on the Siemens Atellica Intact PTH Assay by collecting paired blood samples in serum-separating tubes (SST) and EDTA tubes from 10 individuals (n = 5 pregnant, n = 5 nonpregnant) and compared them with paired samples collected from the index case. PTH was measured in each sample at timepoint zero (0 hours) and then every 2 hours for the next 10 hours. The absolute changes in measured PTH concentration were calculated (Tables 1 and 2).
Table 1.
Median PTH levels measured in SST tubes and the % change from baseline values of controls (pregnant n = 5, nonpregnant n = 5) and the index case
| SST collection | ||||||
|---|---|---|---|---|---|---|
| Time from initial collection | ||||||
| 0 hour | 2 hours | 4 hours | 6 hours | 8 hours | 10 hours | |
| Median PTH nonpregnant controls (IQR) n = 5 |
7.00 pmol/L (5.10-19.20); 66.01 pg/mL (48.09-181.06) |
6.90 pmol/L (4.80-18.50); 65.07 pg/mL (45.26-174.46) | 6.75 pmol/L (4.60-18.60); 63.65 pg/mL (43.38-175.40) | 6.65 pmol/L (4.60-17.90); 62.71 pg/mL (43.38-168.80) | 6.50 pmol/L (4.20-18.30); 61.30 pg/mL (39.61-172.57) |
6.30 pmol/L (4.10-18.00); 59.41 pg/mL (38.66-169.74) |
| Absolute % change | −1.43% | −3.57% | −5.00% | −7.14% | −10.00% | |
| Median PTH pregnant controls (IQR) n = 5 |
2.20 pmol/L (1.30-3.10); 20.75 pg/mL (12.26-29.23) |
2.20 pmol/L (1.20-3.00); 20.75 pg/mL (11.32-28.29) |
2.00 pmol/L (1.30-2.90); 18.86 pg/mL (12.26-27.35) | 2.00 pmol/L (1.20-2.80); 18.86 pg/mL (11.32-26.40) |
2.00 pmol/L (1.20-2.70); 18.86 pg/mL (11.32-16.03) | 1.90 pmol/L (1.30-2.60); 17.92 pg/mL (12.26 = 24.52) |
| Absolute % change | 0% | −9.09% | −9.09% | −9.09% | −13.64% | |
| PTH concentration—index case | 4.20 pmol/L; 39.61 pg/mL | 3.90 pmol/L; 36.78 pg/mL | 3.20 pmol/L; 30.18 pg/mL | 3.00 pmol/L; 28.29 pg/mL | 2.80 pmol/L; 26.40 pg/mL | 2.60 pmol/L; 24.52 pg/mL |
| Absolute % change | −7.10% | −23.80% | −28.60% | −33.30% | −38.10% | |
PTH values are expressed in Standard International (SI) units, with corresponding conventional units.
Abbreviations: IQR, interquartile range; PTH, parathyroid hormone; SST, serum-separating tube.
Table 2.
Median PTH levels measured in EDTA tubes and the % change from baseline values of controls (pregnant n = 5, nonpregnant n = 5) and the index case
| EDTA collection | ||||||
|---|---|---|---|---|---|---|
| Time from initial collection (hours) | ||||||
| 0 hour | 2 hours | 4 hours | 6 hours | 8 hours | 10 hours | |
| Median PTH nonpregnant controls (IQR) n = 5 | 8.10 pmol/L (5.20-17.70); 76.38 pg/mL (49.04-166.91) | 9.00 pmol/L (5.70-19.30); 84.87 pg/mL (53.75-182.00) | 8.90 pmol/L (5.70-19.30); 83.93 pg/mL (53.75-182.00) | 9.00 pmol/L (5.90-19.70); 84.87 pg/mL (55.64-185.77) | 8.60 pmol/L (5.70-18.60); 81.10 pg/mL (53.75-175.40) | 8.70 pmol/L (5.60-19.10); 82.04 pg/mL (52.81-180.11) |
| Absolute % change | 11.11% | 9.88% | 11.11% | 6.17% | 7.41% | |
| Median PTH pregnant controls (IQR) n = 5 |
2.10 pmol/L (1.30-3.30); 19.80 pg/mL (12.26-31.12) | 2.30 pmol/L (1.30-3.30); 21.69 pg/mL (12.26-31.12) | 2.20 pmol/L (1.0-3.30); 20.75 pg/mL (9.43-31.12) | 2.20 pmol/L (1.40-3.30); 20.75 pg/mL (13.20-31.12) | 2.30 pmol/L (1.40-3.40); 21.69 pg/mL (13.20-32.06) | 2.20 pmol/L (1.40-3.50); 20.75 pg/mL (13.20-33.01) |
| Absolute % change | 9.52% | 4.76% | 4.76% | 9.62% | 4.76% | |
| PTH concentration—index case | 4.00 pmol/L; 37.72 pg/mL | 4.00 pmol/L; 37.72 pg/mL | 4.00 pmol/L; 37.72 pg/mL | 4.00 pmol/L; 37.72 pg/mL | 3.60 pmol/L; 33.95 pg/mL | 3.50 pmol/L; 33.01 pg/mL |
| Absolute % change | 0.00% | 0.00% | 0.00% | −11.10% | −12.50% | |
PTH values are presented in Standard International (SI) units, with corresponding conventional units.
Abbreviations: IQR, interquartile range; PTH, parathyroid hormone.
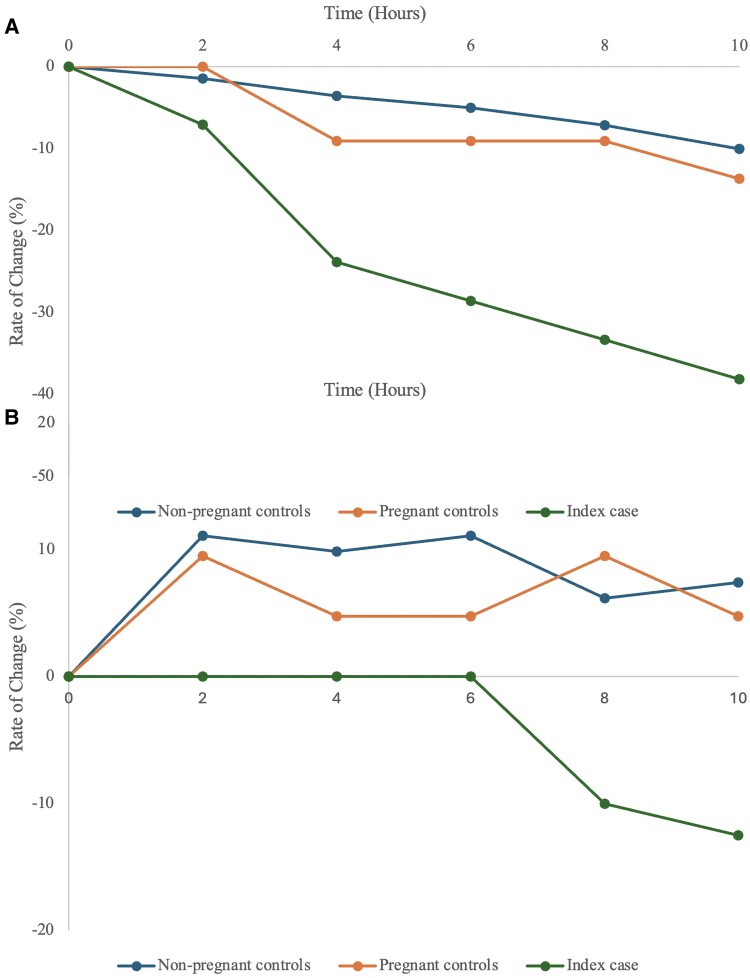
The small difference in PTH results observed in SST and EDTA samples at baseline for the controls and index case can be explained by the inherent variability of the PTH assay. However, with time, the variation in PTH levels observed between SST and EDTA samples for the controls were in opposite directions, with a 11% reduction and 5.9% increase at 10 hours, respectively (Fig. 2). The changes observed in the SST cohort started to exceed the limits of analytical variability 8 hours after baseline measurement. In our index case, PTH levels fell more rapidly by 38.1% and 12.5% in the SST and EDTA tube, respectively. The changes observed in the SST sample for our patient exceeded the analytical variability limit at 4 hours from baseline.
Figure 2.
Absolute rate of change (%) in parathyroid hormone (PTH) concentrations between nonpregnant controls (n = 5), pregnant controls (n = 5) and the index case in (A) serum-separating tube (SST) collection samples, and (B) EDTA collection samples over time (hours). A negative rate of change was more pronounced in the index case compared to the control groups in both collection tubes.
Treatment
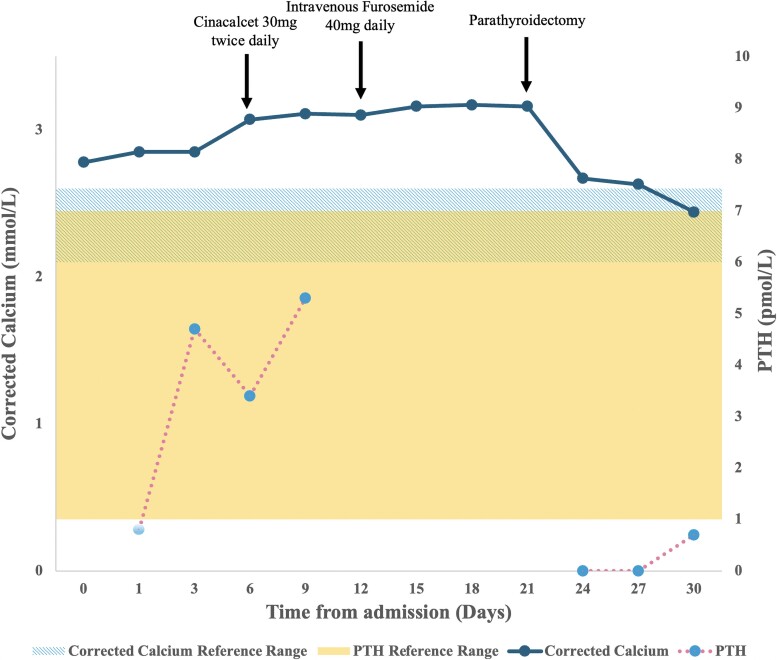
From day 6 of admission, PTH samples were sent immediately to the laboratory for analysis to improve accuracy (Fig. 3). Despite continuous intravenous fluids, cinacalcet, and furosemide, the serum calcium level continued to rise. Bisphosphonate was not given due to safety concerns in pregnancy. At 28 weeks of pregnancy, the peak Cacorr was noted to be 3.18 mmol/L [12.75 mg/dL], with an ionized calcium of 1.64 mmol/L [6.57 mg/dL]). We proceeded with ultrasonography and 4D-CT of the neck, which confirmed a 5 × 9 × 6 mm right inferior parathyroid adenoma and a parathyroidectomy was performed on day 22 of admission.
Figure 3.
Corrected calcium (mmol/L) and corresponding parathyroid hormone (PTH) results (pmol/L) for the index case over time (days from admission to hospital). Arrows indicate corresponding medical intervention during the time of hospital stay. The normal reference range for corrected calcium (2.1–2.6 mmol/L) are represented by the striped area. The normal reference range for PTH (1.0–7.0 pmol/L) are represented by the solid area. Note that after 6 days into admission, all PTH samples were run immediately post collection to improve accuracy of the test.
Outcome and Follow-Up
The biochemistry normalized post parathyroidectomy, with a PTH of 0.7 pmol/L (6.60 pg/mL), with a Cacorr of 2.44 mmol/L (9.78 mg/dL), ionized calcium 1.21 mmol/L (4.85 mg/dL), and phosphate 1.34 mmol/L (4.15 mg/dL). She experienced no surgical complications and later proceeded to an uneventful delivery at 38 weeks gestation. Six weeks postpartum, she remained normocalcemic with a Cacorr of 2.35 mmol/L (9.42 mg/dL). The histology of the surgical specimen confirmed a parathyroid adenoma and subsequent genetic testing for multiple endocrine neoplasia 1 was negative.
Discussion
Our case is the first to describe the rate of PTH degradation in pregnancy and demonstrates the importance of understanding pregnancy-related changes when interpreting biochemistry.
Currently, no standardized PTH measurement method exists, and both second- and third-generation PTH assays are freely used in commercial laboratories. These PTH assays vary in antibody specificity which results in varying degrees of sample cross-reactivity. Interlaboratory validity is therefore difficult to rely upon (16).
The second-generation “intact” PTH assay, utilizes a capture antibody against the C-terminus (AA 39-84) and a detection antibody against the N-terminus of PTH (AA 12-18, 13-24, or 26-32) (17). Besides biologically active PTH (1-84), N-terminal truncated PTH fragments which stimulate PTH1R are also detected, and account for up to 20% of the circulating PTH (17-19). In contrast, the third-generation assay is more specific for PTH (1-84) and binds to the first 4 AA molecules of PTH directly via an anti-N-terminal antibody (20). Thus, caution must be exerted when interpreting the PTH measurements, especially when comparing between laboratories.
Dai et al (21) reported that PTH samples obtained in EDTA tubes held stability for up to 72 hours when compared to heparin anticoagulant, coagulant, gel separating, and plain tubes. This was congruent with our control cohort observation, whereby the control EDTA samples were more stable over time compared to the control SST samples. In contrast, our index case exhibited a faster rate of PTH degradation compared to controls with both the SST and EDTA methods (Fig. 2). Our study highlights the issues relating to PTH variability in a real-world situation.
Interestingly, our study noted the PTH results initially incremented in the EDTA samples of our control group; a phenomenon previously noted but not evaluated (22, 23). In contrast, our individual's PTH levels in the EDTA tube did not replicate this pattern. Further statistical interpretation was limited by sample size.
This was purely an observational study, and several factors which could impact the baseline PTH in our observed individuals were not accounted for. Theoretical concerns regarding sample storage temperature and protein degradation also exist but were unable to be directly assessed in this instance (24).
Excessive consumption of biotin (found in over-the-counter supplements) can falsely lower PTH levels due to immunoassay interference (25). BMI and Black race, have also been positively correlated to PTH levels (26). Bolland et al (27) reported that normocalcemic individuals with obesity were likely to have higher PTH levels compared to their nonobese counterparts. Finally, as our nonpregnant and pregnant control samples were selected at random, we were unable to draw direct comparisons to nonpregnant individuals with primary hyperparathyroidism. In order to further delineate the impact these factors have on PTH degradation, we propose more vigorous screening, collection, and processing methods in future studies.
This case highlighted the importance of liaising with our chemical pathology colleagues when biochemical results are not in keeping with the clinical presentation, especially in pregnant individuals. In collaboration, we successfully identified the abnormality of our individual's PTH sample relative to controls, which resulted in the time-critical diagnosis and treatment of this individual's primary hyperparathyroidism.
Given the small numbers of subjects assessed, the interpretation of the study remains limited. Caution should be exercised when interpreting PTH levels in pregnancy, especially if there is a high clinical index of suspicion for primary hyperparathyroidism. In such a situation, the clinician must consider pregnancy, assay, and individual factors which may impact on the measured PTH and the synthesis of the case.
Learning Points
Hypercalcemia in pregnancy poses significant maternofetal risk—it should be identified, investigated, and treated promptly.
Physiological changes in pregnancy alter the ability to use conventional investigations to accurately diagnose primary hyperparathyroidism.
The rate of PTH degradation in pregnancy has previously not been examined and should be considered when interpreting PTH results.
Collaboration with the biochemistry department is essential to ascertain the presence of assay interference, especially when the results are not congruent with the clinical impression.
Acknowledgments
The authors would like to gratefully acknowledge our colleagues from the Chemical Pathology, General Surgery and Obstetric and Gynaecology Departments at the Royal Brisbane and Women's Hospital who provided their valuable care and expertise in the management of this case.
Abbreviations
- 4D-CT
four-dimensional computed tomography
- BMI
body mass index
- PTH
parathyroid hormone
- PTH1R
parathyroid hormone receptor
- PTHrP
parathyroid hormone–related peptide
- SST
serum-separating tubes
Contributor Information
Dianna Luong, School of Medicine, University of Queensland, Herston QLD 4006, Australia; Obstetric Medicine Department, Royal Brisbane and Women's Hospital, Herston QLD 4006, Australia.
Kate Hawke, School of Medicine, University of Queensland, Herston QLD 4006, Australia; Obstetric Medicine Department, Royal Brisbane and Women's Hospital, Herston QLD 4006, Australia.
Elzahn De Waal, Chemical Pathology Department, Pathology Queensland, Herston QLD 4029, Australia.
Madeline Duke, School of Medicine, University of Queensland, Herston QLD 4006, Australia.
Penny Wolski, School of Medicine, University of Queensland, Herston QLD 4006, Australia; Obstetric Medicine Department, Royal Brisbane and Women's Hospital, Herston QLD 4006, Australia.
Contributors
All authors made contributions to authorship. D.L. was involved in manuscript preparation and submission. E.D.W. performed analysis of laboratory data. P.W., K.H., and M.D. were responsible for the diagnosis and management of the patient. All authors reviewed and approved the final draft.
Funding
No public or commercial funding was received.
Disclosures
There are no conflicts of interest to disclose.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Hirsch D, Kopel V, Nadler V, Levy S, Toledano Y, Tsvetov G. Pregnancy outcomes in women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100(5):2115‐2122. [DOI] [PubMed] [Google Scholar]
- 2. Sharma SG, Levine SN, Yatavelli RK, Shaha MA, Nathan CAO. Parathyroidectomy in first trimester of pregnancy. J Endocr Soc. 2020;4(3):bvaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutkeviciute I, Clark LJ, White AD, Gardella TJ, Vilardaga JP. Pth/PTHrP receptor signaling, allostery, and structures. Trends Endocrinol Metab. 2019;30(11):860‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali DS, Dandurand K, Khan AA. Hypoparathyroidism in pregnancy and lactation: current approach to diagnosis and management. J Clin Med. 2021;10(7):1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malekar-Raikar S, Sinnott BP. Primary hyperparathyroidism in pregnancy-a rare cause of life-threatening hypercalcemia: case report and literature review. Case Rep Endocrinol. 2011;2011:520516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy A, Howarth S, Khoo S, et al. Management of primary hyperparathyroidism in pregnancy: a case series. Endocrinol Diabetes Metab Case Rep. 2019;2019:19-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dandurand K, Ali DS, Khan AA. Hypercalcemia in pregnancy. Endocrinol Metab Clin North Am. 2021;50(4):753‐768. [DOI] [PubMed] [Google Scholar]
- 8. Mitsiakos G, Katsaras GN, Chatziioannidis I, Gkampeta A, Mitsiakou C, Nikolaidis N. A neonate with late-onset hypocalcemia due to unrecognized maternal hyperparathyroidism and a systematic overview of similar cases. Ger Med Sci. 2021;19:Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appelman-Dijkstra NM, Ertl DA, Zillikens MC, Rjenmark L, Winter EM. Hypercalcemia during pregnancy: management and outcomes for mother and child. Endocrine. 2021;71(3):604‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norman J, Politz D, Politz L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol (Oxf). 2009;71(1):104‐109. [DOI] [PubMed] [Google Scholar]
- 11. Morton A, Teasdale S. Physiological changes in pregnancy and their influence on the endocrine investigation. Clin Endocrinol (Oxf). 2022;96(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 12. Foley KF, Boccuzzi L. Urine calcium: laboratory measurement and clinical utility. Lab Med. 2010;41(11):683‐686. [Google Scholar]
- 13. Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smit MA, van Kinschot CMJ, van der Linden J, van Noord C, Kos S. Clinical guidelines and PTH measurement: does assay generation matter?. Endocr Rev. 2019;40(6):1468‐1480. [DOI] [PubMed] [Google Scholar]
- 15. Johnson NA, Tublin ME, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007;188(6):1706‐1715. [DOI] [PubMed] [Google Scholar]
- 16. Blumsohn A, Al Hadari A. Parathyroid hormone: what are we measuring and does it matter? Ann Clin Biochem. 2002;39(Pt 3):169‐172. [DOI] [PubMed] [Google Scholar]
- 17. Kritmetapak K, Pongchaiyakul C. Parathyroid hormone measurement in chronic kidney disease: from basics to clinical implications. Int J Nephrol. 2019;2019:5496710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Amour P, Räkel A, Brossard JH, Rousseau L, Albert C, Cantor T. Acute regulation of circulating parathyroid hormone (PTH) molecular forms by calcium: utility of PTH fragments/PTH(1-84) ratios derived from three generations of PTH assays. J Clin Endocrinol Metab. 2006;91(1):283‐289. [DOI] [PubMed] [Google Scholar]
- 19. D’Amour P, Brossard JH, Rousseau L, et al. Structure of non-(1-84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int. 2005;68(3):998‐1007. [DOI] [PubMed] [Google Scholar]
- 20. Cavalier E. Determination of parathyroid hormone: from radioimmunoassay to LCMS/MS. Clin Chem Lab Med. 2023;61(5):946‐953. [DOI] [PubMed] [Google Scholar]
- 21. Dai Z, Zhang JW, Lin J, et al. The stability of intact parathyroid hormone (PTH) in different types of blood collection tubes. Clin Lab. 2022;68(3). Doi: 10.7754/Clin.Lab.2021.210421 [DOI] [PubMed] [Google Scholar]
- 22. Hanon EA, Sturgeon CM, Lamb EJ. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: a systematic review. Clin Chem Lab Med. 2013;51(10):1925‐1941. [DOI] [PubMed] [Google Scholar]
- 23. Walker KS, Seth J. Stability of parathyroid hormone in blood from renal patients on haemodialysis. Ann Clin Biochem. 2000;37(Pt 6):800‐801. [DOI] [PubMed] [Google Scholar]
- 24. Khalil H, Borai A, Dakhakhni M, et al. Stability and validity of intact parathyroid hormone levels in different sample types and storage conditions. J Clin Lab Anal. 2021;35(6):e23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waghray A, Milas M, Nyalakonda K, Siperstein AE. Falsely low parathyroid hormone secondary to biotin interference: a case series. Endocr Pract. 2013;19(3):451‐455. [DOI] [PubMed] [Google Scholar]
- 26. Haddow JE, Neveux LM, Palomaki GE, et al. The relationship between PTH and 25-hydroxy vitamin D early in pregnancy. Clin Endocrinol (Oxf). 2011;75(3):309‐314. [DOI] [PubMed] [Google Scholar]
- 27. Bolland MJ, Grey AB, Gamble GD, Reid IR. Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J Clin Endocrinol Metab. 2005;90(3):1525‐1530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.