Abstract
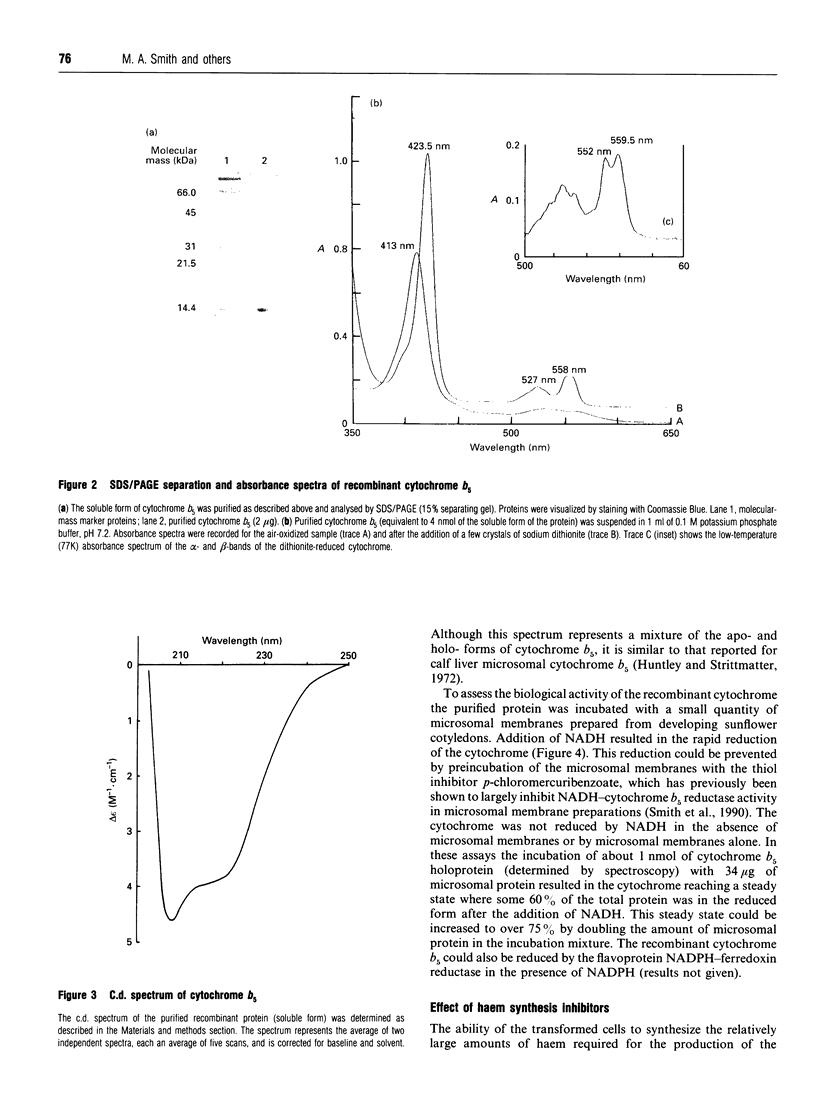
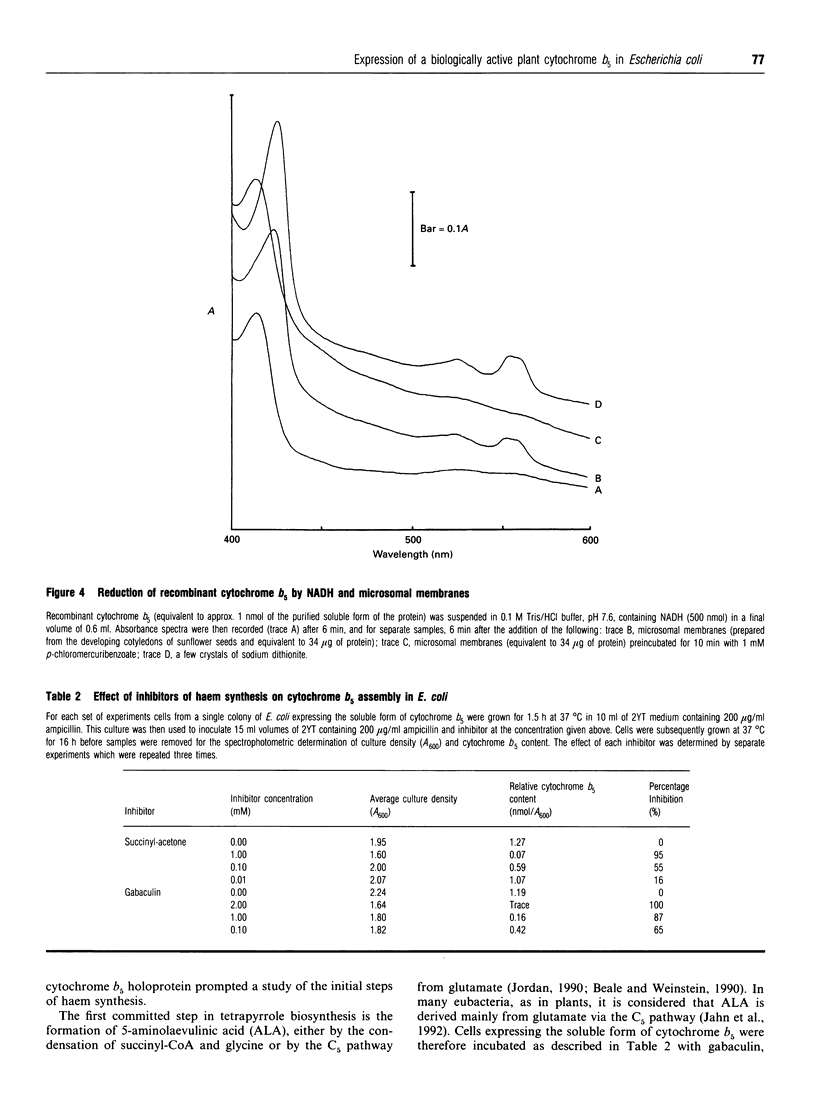
Cytochrome b5 from tobacco (Nicotiana tabacum) was expressed in Escherichia coli using a T7 polymerase/promoter system as described by Studier, Rosenberg, Dunn and Dubendorff (1990) (Methods Enzymol. 185, 60-89). Transformed cells were red in colour and accumulated cytochrome b5 to a level of around 30% of the total cell protein. The purified cytochrome had oxidized, reduced and low-temperature absorbance spectra characteristic of plant microsomal cytochrome b5, and exhibited a c.d. spectrum resembling that of a mammalian cytochrome b5. The recombinant protein appeared to be correctly assembled and biologically active, being reduced by NADH in the presence of microsomal membranes prepared from the developing seeds of sunflower (Helianthus annuus). Inhibition of haem synthesis in the transformed E. coli cells expressing cytochrome b5, by the use of gabaculin or succinylacetone, prevented the assembly of the cytochrome b5 holoprotein but had little effect on the accumulation of cytochrome apoprotein. The recombinant protein expressed in E. coli therefore has the biochemical features of the higher-plant cytochrome b5 and can be used in studies of plant microsomal oxidation/reduction reactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Gallagher J., Kaderbhai N., Kaderbhai M. A. Gene-dose-dependent expression of soluble mammalian cytochrome b5 in Escherichia coli. Appl Microbiol Biotechnol. 1992 Oct;38(1):77–83. doi: 10.1007/BF00169423. [DOI] [PubMed] [Google Scholar]
- Huntley T. E., Strittmatter P. The effect of heme binding on the tryptophan residue and the protein conformation of cytochrome b 5 . J Biol Chem. 1972 Jul 25;247(14):4641–4647. [PubMed] [Google Scholar]
- Jahn D., Verkamp E., Söll D. Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends Biochem Sci. 1992 Jun;17(6):215–218. doi: 10.1016/0968-0004(92)90380-r. [DOI] [PubMed] [Google Scholar]
- Kearns E. V., Hugly S., Somerville C. R. The role of cytochrome b5 in delta 12 desaturation of oleic acid by microsomes of safflower (Carthamus tinctorius L.). Arch Biochem Biophys. 1991 Feb 1;284(2):431–436. doi: 10.1016/0003-9861(91)90319-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Baker R. C., Stephens N., Snyder F. Evidence for participation of cytochrome b5 in microsomal delta-6 desaturation of fatty acids. Biochim Biophys Acta. 1977 Oct 24;489(1):25–31. doi: 10.1016/0005-2760(77)90228-4. [DOI] [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshiro M., Omura T. Immunochemical study on the electron pathway from NADH to cytochrome P-450 of liver microsomes. J Biochem. 1978 Jan;83(1):61–77. doi: 10.1093/oxfordjournals.jbchem.a131913. [DOI] [PubMed] [Google Scholar]
- Ozols J. Cytochrome b5 from microsomal membranes of equine, bovine, and porcine livers. Isolation and properties of preparations containing the membranous segment. Biochemistry. 1974 Jan 29;13(3):426–434. doi: 10.1021/bi00700a005. [DOI] [PubMed] [Google Scholar]
- Ozols J. Structure of cytochrome b5 and its topology in the microsomal membrane. Biochim Biophys Acta. 1989 Jul 27;997(1-2):121–130. doi: 10.1016/0167-4838(89)90143-x. [DOI] [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Reddy V. V., Kupfer D., Caspi E. Mechanism of C-5 double bond introduction in the biosynthesis of cholesterol by rat liver microsomes. J Biol Chem. 1977 May 10;252(9):2797–2801. [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. Cytochrome components of plant microsomes. Eur J Biochem. 1975 Jul 1;55(2):333–341. doi: 10.1111/j.1432-1033.1975.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Cross A. R., Jones O. T., Griffiths W. T., Stymne S., Stobart K. Electron-transport components of the 1-acyl-2-oleoyl-sn-glycero-3-phosphocholine delta 12-desaturase (delta 12-desaturase) in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons. Biochem J. 1990 Nov 15;272(1):23–29. doi: 10.1042/bj2720023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Jonsson L., Stymne S., Stobart K. Evidence for cytochrome b5 as an electron donor in ricinoleic acid biosynthesis in microsomal preparations from developing castor bean (Ricinus communis L.). Biochem J. 1992 Oct 1;287(Pt 1):141–144. doi: 10.1042/bj2870141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Stobart A. K., Shewry P. R., Napier J. A. Tobacco cytochrome b5: cDNA isolation, expression analysis and in vitro protein targeting. Plant Mol Biol. 1994 Jun;25(3):527–537. doi: 10.1007/BF00043880. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]