Abstract
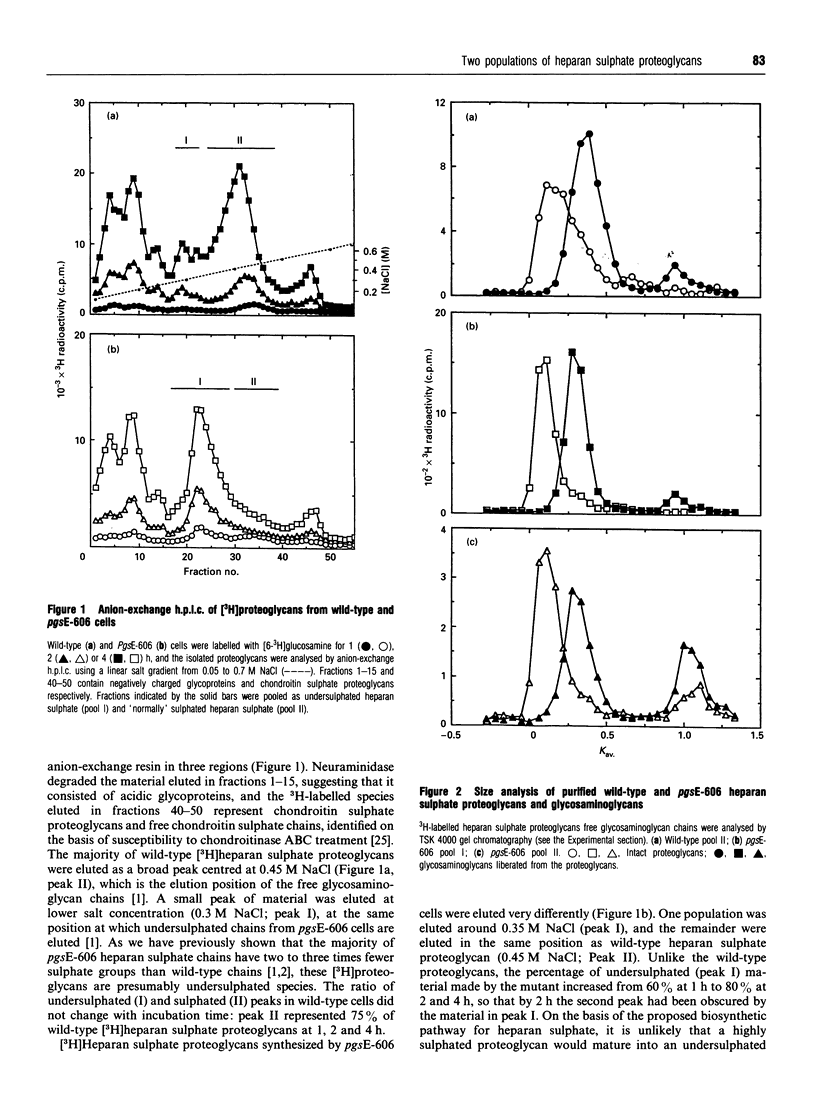
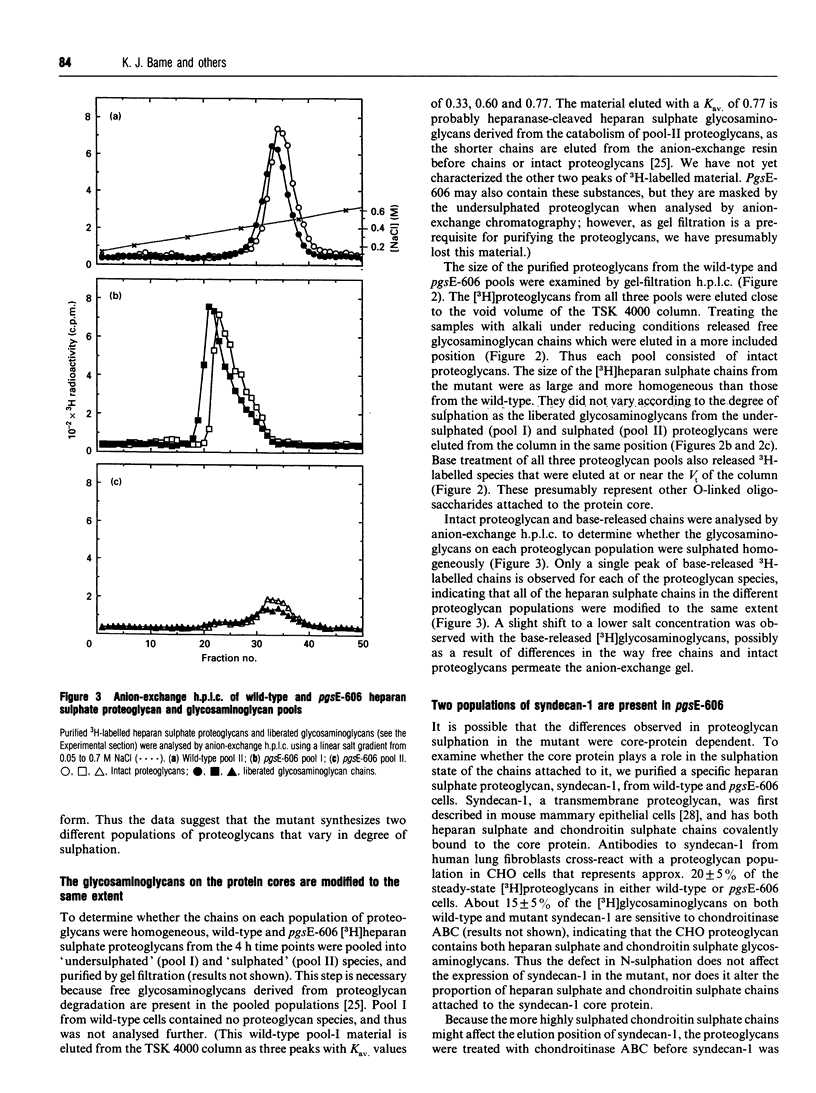
The Chinese hamster ovary cell mutant, pgsE-606, synthesizes undersulphated heparan sulphate glycosaminoglycans because of a deficiency in N-sulphotransferase activity [Bame and Esko (1989) J. Biol. Chem. 264, 8059-8065]. We compared the heparan sulphate proteoglycans synthesized by mutant and wild-type cells to determine what effect the undersulphation defect had on proteoglycan structure. The majority of heparan sulphate proteoglycans synthesized by pgsE-606 were undersulphated, but the mutant also synthesized a population of proteoglycans that were sulphated to the same extent as wild-type molecules. Anion-exchange analysis of the glycosaminoglycans in each proteoglycan population showed that they were all modified in the same way. The length of the glycosaminoglycans in each proteoglycan population were similar, suggesting that N-sulphation does not affect chain polymerization. To examine whether the sulphation state of the attached heparan sulphate glycosaminoglycans was dependent on the protein core, we purified syndecan-1 from mutant and wild-type cells using antibodies against the core protein. As with the unfractionated heparan sulphate proteoglycans, pgsE-606 synthesized both undersulphated and sulphated syndecan-1. Each pool contained either undersulphated or sulphated glycosaminoglycan chains respectively. Thus the modification of all heparan sulphate chains on a core protein occurs on a proteoglycan-wide basis (i.e. to the same extent).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bame K. J., Esko J. D. Undersulfated heparan sulfate in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J Biol Chem. 1989 May 15;264(14):8059–8065. [PubMed] [Google Scholar]
- Bame K. J., Reddy R. V., Esko J. D. Coupling of N-deacetylation and N-sulfation in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J Biol Chem. 1991 Jul 5;266(19):12461–12468. [PubMed] [Google Scholar]
- Bame K. J. Release of heparan sulfate glycosaminoglycans from proteoglycans in Chinese hamster ovary cells does not require proteolysis of the core protein. J Biol Chem. 1993 Sep 25;268(27):19956–19964. [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bienkowski M. J., Conrad H. E. Kinetics of proteoheparan sulfate synthesis, secretion, endocytosis, and catabolism by a hepatocyte cell line. J Biol Chem. 1984 Nov 10;259(21):12989–12996. [PubMed] [Google Scholar]
- David G. Biology and pathology of the pericellular heparan sulphate proteoglycans. Biochem Soc Trans. 1991 Nov;19(4):816–820. doi: 10.1042/bst0190816. [DOI] [PubMed] [Google Scholar]
- David G., Van den Berghe H. Cell-surface heparan sulfate and heparan-sulfate/chondroitin-sulfate hybrid proteoglycans of mouse mammary epithelial cells. Eur J Biochem. 1989 Jan 2;178(3):609–617. doi: 10.1111/j.1432-1033.1989.tb14489.x. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Elgavish A., Prasthofer T., Taylor W. H., Weinke J. L. Sulfate transport-deficient mutants of Chinese hamster ovary cells. Sulfation of glycosaminoglycans dependent on cysteine. J Biol Chem. 1986 Nov 25;261(33):15725–15733. [PubMed] [Google Scholar]
- Esko J. D., Stewart T. E., Taylor W. H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Weinke J. L., Taylor W. H., Ekborg G., Rodén L., Anantharamaiah G., Gawish A. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J Biol Chem. 1987 Sep 5;262(25):12189–12195. [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Turnbull J. E., Lyon M. Patterns of sulphation in heparan sulphate: polymorphism based on a common structural theme. Int J Biochem. 1992 Apr;24(4):553–560. doi: 10.1016/0020-711x(92)90326-v. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson I. N., Kumar S., Gallagher J. T. Heterogeneity of cell-associated and secretory heparan sulphate proteoglycans produced by cultured human neuroblastoma cells. Biochim Biophys Acta. 1984 Sep 28;801(2):306–313. doi: 10.1016/0304-4165(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Heterogeneity of rat skin heparin chains with high affinity for antithrombin. Biochem J. 1987 Jun 15;244(3):693–698. doi: 10.1042/bj2440693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V. Presence of unsulfated heparan chains on the heparan sulfate proteoglycan of human colon carcinoma cells. Implications for heparan sulfate proteoglycan biosynthesis. J Biol Chem. 1989 Feb 15;264(5):2690–2699. [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Structural characterization of heparan sulfate proteoglycan subclasses isolated from bovine aortic endothelial cell cultures. Biochemistry. 1988 Mar 22;27(6):2136–2144. doi: 10.1021/bi00406a048. [DOI] [PubMed] [Google Scholar]
- Lidholt K., Lindahl U. Biosynthesis of heparin. The D-glucuronosyl- and N-acetyl-D-glucosaminyltransferase reactions and their relation to polymer modification. Biochem J. 1992 Oct 1;287(Pt 1):21–29. doi: 10.1042/bj2870021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidholt K., Weinke J. L., Kiser C. S., Lugemwa F. N., Bame K. J., Cheifetz S., Massagué J., Lindahl U., Esko J. D. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom A., Bengtsson-Olivecrona G., Fransson L. A. Domain structure of endothelial heparan sulphate. Biochem J. 1991 Nov 1;279(Pt 3):821–829. doi: 10.1042/bj2790821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7009–7016. [PubMed] [Google Scholar]
- Schmidtchen A., Fransson L. A. Analysis of glycosaminoglycan chains from different proteoglycan populations in human embryonic skin fibroblasts. Eur J Biochem. 1992 Sep 1;208(2):537–546. doi: 10.1111/j.1432-1033.1992.tb17218.x. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Gallagher J. T. Distribution of iduronate 2-sulphate residues in heparan sulphate. Evidence for an ordered polymeric structure. Biochem J. 1991 Feb 1;273(Pt 3):553–559. doi: 10.1042/bj2730553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Swiedler S. J., Ishihara M., Orellana A., Hirschberg C. B. A single protein catalyzes both N-deacetylation and N-sulfation during the biosynthesis of heparan sulfate. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3885–3888. doi: 10.1073/pnas.90.9.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992 May 15;267(14):9451–9454. [PubMed] [Google Scholar]


