Abstract
Many toxic metals are involved in the initiation and progression of DNA damage that can result in the activation of DNA damage response machinery at double- and single-stranded DNA; this response can result in global and gene-specific DNA alteration. The toxicological profiles from the Agency for Toxic Substances and Disease Registry (ATSDR) and several other studies have demonstrated the influence of metal exposure-induced genotoxic endpoints and epigenetic modifications. Our review systematically summarizes accumulating evidence from ATSDR toxicological profiles and the available literature that demonstrate a possible induction of various genotoxic endpoints and metal exposures. We include in this article studies on chromium, arsenic, nickel, lead, mercury, and zinc.
Introduction
This article compiles information from several of the Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles to summarize genotoxic endpoints associated with exposure to metals. Humans are exposed to various toxins and environmental pollutants in our day-to-day lives. Some of these toxicants damage the genetic information within a cell and lead to genetic alterations. In general, genotoxicity is a temporary or permanent change to the genome that can be caused by exposure to specific chemicals. Genotoxicity can occur through exposure to toxicants found in air, food, water, and the workplace (Alloway & Ayres, 1993; Antonini et al., 2019; Boyce et al., 2020; Kodali et al., 2020; Langston, 1990; Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Friend, et al., 2017; Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Roberts, et al., 2017; Shoeb et al., 2020, 2021).
ATSDR (2022a, 2022b) has developed detailed toxicological profiles for many hazardous substances. The agency uses its Substance Priority List to select substances based on their toxicity and frequency of detection in the environment and then prepares toxicological profiles for highly ranked substances. Each peer-reviewed toxicological profile evaluates, summarizes, and interprets available toxicological and epidemiological information on a substance. The profiles provide insight into a substance’s occurrence, physiochemical properties, different exposures, potential health effects, mechanisms of action, and other topics (ATSDR, 2023a).
Within the toxicological profiles, evidence of genotoxicity includes evidence of very specific changes in some part of the processes of natural cell growth, division, and death. ATSDR defines genotoxicity as a specific adverse effect on the genome of living cells that, upon the duplication of affected cells, can be expressed as a mutagenic, clastogenic (i.e., chromosomal breakage-induced mutation), or carcinogenic event due to specific alteration of the molecular structure of the genome. Table 1 lists and defines some of the genotoxic terms used to analyze DNA alterations.
TABLE 1.
Genotoxic Endpoints and Definitions
| Endpoint | Definition |
|---|---|
| Chromosomal damage | Chromosomal alterations (i.e., coding properties) that occur during division of cells |
| DNA methylation | Addition of methyl group to cytosine (5’-CpG-3’) |
| DNA–protein cross-linking | Chemically activated trapping of proteins on DNA strands |
| Micronuclei formation | Fragments of chromosomal matter outside the nucleus |
| Sister-chromatid exchange | Exchange of genetic material between two identical copies of the same chromosome |
| DNA repair inhibition | Inhibition of the natural process of repairing a harmful DNA lesion |
| Gene mutation | Alteration of DNA or nucleotide sequence of one or more genes |
| Chromosomal aberrations | Changes in chromosome number or structure |
| DNA fragmentation | Rapid separation or breaking of DNA strands into pieces |
| DNA strand breaks | Single- or double-break of the DNA sequence in the genetic material |
| Telomere alteration | Change in the noncoding ends of the DNA that serves to protect the coding portion |
Note. Endpoints are listed in hierarchical order from less to more permanent gene damage.
ATSDR began to organize these varied markers and their effects across several toxic substances to 1) compare the effects, 2) create a hierarchy of genotoxic endpoints to help prioritize evidence of cancer, 3) identify data gaps, and 4) generate ideas that enable further research. For a genotoxic substance to cause an observable health effect, it must first damage genetic information within a cell and then not be repaired by the cell’s natural DNA repair processes. Genotoxic endpoints could serve as a first step in assessing the genotoxic risk of a corresponding health effect following hazardous exposures.
In this initial analysis, we examine the genotoxic-endpoint data found in the toxicological profiles and published studies for chromium (Cr), arsenic (As), nickel (Ni), lead (Pb), mercury (Hg), and zinc (Zn; Table 2). These metals are among the most frequently found contaminants at hazardous waste sites—with As, Pb, and Hg ranking as the top three substances on the Substance Priority List. Table 2 also includes the cancer classification of the National Toxicology Program (NTP, 2021) within the U.S. Department of Health and Human Services. The metals listed in Table 2 are ordered according to their NTP classification, from most to least carcinogenic (left to right, respectively) and top to bottom in order of the most generally investigated genotoxic endpoint.
TABLE 2.
Genotoxic Endpoints for Six Metals
| Chromium-6 (Cr6+) | Arsenic (As) | Nickel (Ni) | Lead (Pb) | Mercury (Hg) | Zinc (Zn) | |
|---|---|---|---|---|---|---|
| National Toxicology Program classification | 1 | 1 | 1 (Ni compounds) | 2 | 3 | 3 |
| Substance Priority List rank | 17 | 1 | 58 | 2 | 3 | 75 |
| Chromosomal damage | * | |||||
| DNA methylation | * | |||||
| DNA–protein cross-linking | ||||||
| Micronuclei formation | * | |||||
| Sister-chromatid exchange | ||||||
| DNA repair inhibition | ||||||
| Gene mutation | * | |||||
| Chromosomal aberrations | * | |||||
| DNA fragmentation | * | |||||
| DNA strand breaks | * | |||||
| Telomere alteration |
Represents some studies with preventive effects.
Note. Table 1 presents the number of published studies that identified the genotoxic endpoint from a search of toxicological profiles and literature using PubMed conducted on October 17, 2022. Search terms included: endpoint induced by X metal exposure (e.g., telomere alteration induced by arsenic metal exposure, only lead and zinc were searched as Pb and Zn). The shading is based on the number of given genotoxic endpoints studied: green = 1–10; yellow = 11–24; and red = >24. A lack of shading indicates no studies were found. For the National Toxicology Program classification: 1 = known carcinogen; 2 = reasonably anticipated to be a human carcinogen; and 3 = not classified by the National Toxicology Program. For the Substance Priority List rank: 1 represents the highest priority. The only form of chromium classified as carcinogenic to humans is hexavalent chromium (Cr6+).
The genotoxic potential of these metals is based on several factors, including oxidation state, physiochemical properties, and target cell or organ interaction. Based on their genotoxic and mutagenic potential, As, Pb, Hg, and Cr are categorized as highly toxic (Arora & Chauhan, 2021). Unlike the other metals, Ni compounds are considered weak mutagens because of their inability to efficiently induce base pair substitutions, frameshift mutations, or deletions in human cells (ATSDR, 2023b; International Agency for Research on Cancer, 2023; Stroebel et al., 1993). Studies have shown, however, a mutagenic response of Ni in vitro, mutation of the tumor suppressor p53 gene, and inhibition of DNA repair enzymes (ATSDR, 2023b; Cameron et al., 2011; Chen et al., 2010; Chiocca et al., 1991; Clemens et al., 2005; Cohen et al., 1993; Haugen et al., 1994; Klein et al., 1994; Salnikow et al., 2003).
In stark contrast are the genotoxic effects of Zn. In fact, there is an association between Zn deficiency and carcinogenicity. Genotoxicity studies failed to provide evidence for mutagenicity of Zn, but there are indications of weak clastogenic effects. Although chromosome aberrations have been observed in the lymphocytes of workers at a Zn smelting plant, those workers had elevated levels of Pb and cadmium (Bauchinger et al., 1976).
Interestingly, in many cases, exposure to mixtures of these metals and the resulting genotoxic effects can differ from the effects of exposure to a single metal. Welding, for example, can generate a complex mixture of genotoxic metals (e.g., Cr, Ni) that might result in altered DNA methylation, telomere length, and shelterin proteins; DNA damage; and DNA strand breaks (Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Friend, et al., 2017; Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Roberts, et al., 2017; Shoeb et al., 2020, 2021). Elevated Ni concentrations in welders’ blood have been found to result in significantly increased DNA damage, DNA–protein cross-linking, and DNA strand breaks (Danadevi et al., 2004; Popp et al., 1991).
Discussion and Conclusion
By profiling and comparing genotoxic endpoints reported in the toxicological profiles, we provide insight into genotoxic endpoints augmenting genome stability for various metals. We provide a guide to compare DNA damage from exposures to six different metals. Table 2 presents the most investigated endpoints associated with exposure to these metals.
The genotoxic effects of exposure to Zn clearly contrast with other genotoxic metals, as many of the effects associated with Zn exposure are beneficial. Differences among the other genotoxic metals are apparent to a lesser degree, with Cr and As (NTP classification 1, known human carcinogens) having a greater number of studies finding adverse genotoxic effects, including telomere alteration. Yet each of the highly toxic metals (including Hg) present differing genotoxic effects due to differences in their mechanism pathways.
Metals such as Ni, Cr, As, Pb, and Hg cause minor to severe DNA damage and telomere alteration by activating DNA damage response machinery (Figures 1 and 2). In some instances, the damage can be repaired by cellular repair mechanisms. If it is not repaired, or if the damage is repaired inappro priately, then genomic instability, epigenetic alterations, and several disease conditions can occur. For example, activation of DNA damage response might result in inflammatory responses and activation or mutation of p53, which could activate anti- or pro-apoptotic pathways (Figures 1 and 2). Some of these metals can also bind directly with DNA and proteins, causing direct genotoxic effects (Figure 1). Further, metal ions can catalyze reactive oxidative stress, and then form toxic lipid aldehydes, also known as by-products of lipid peroxidation (Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Friend, et al., 2017; Shoeb, Kodali, Farris, Bishop, Meighan, Salmen, Eye, Roberts, et al., 2017). Metal-induced (Ni, Pb, Hg, Cr, and As) epigenetic modifications have been reported, including inhibition of tumor suppressor genes by DNA methylation and As-induced mediation of noncoding RNAs (Cheng et al., 2012).
FIGURE 1.
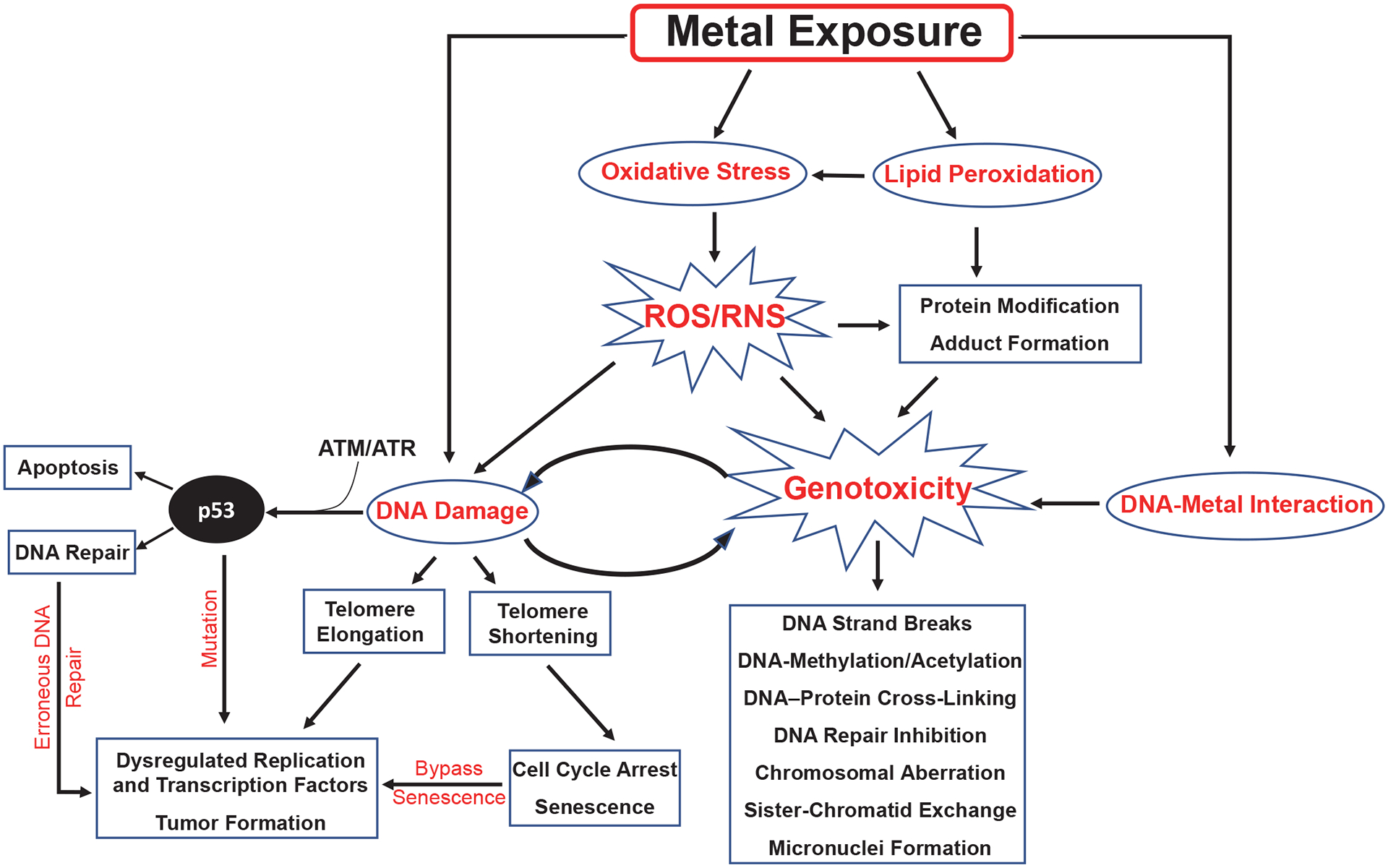
Possible Activation of Genotoxic Endpoints and DNA Damage-Induced Dysregulation of p53 Pathways After Exposure to Metals
Note. ATM = ataxia telangiectasia mutated; ATR = ATM and Rad3-related; ROS = reactive oxygen species; RNS = reactive nitrogen species.
FIGURE 2.
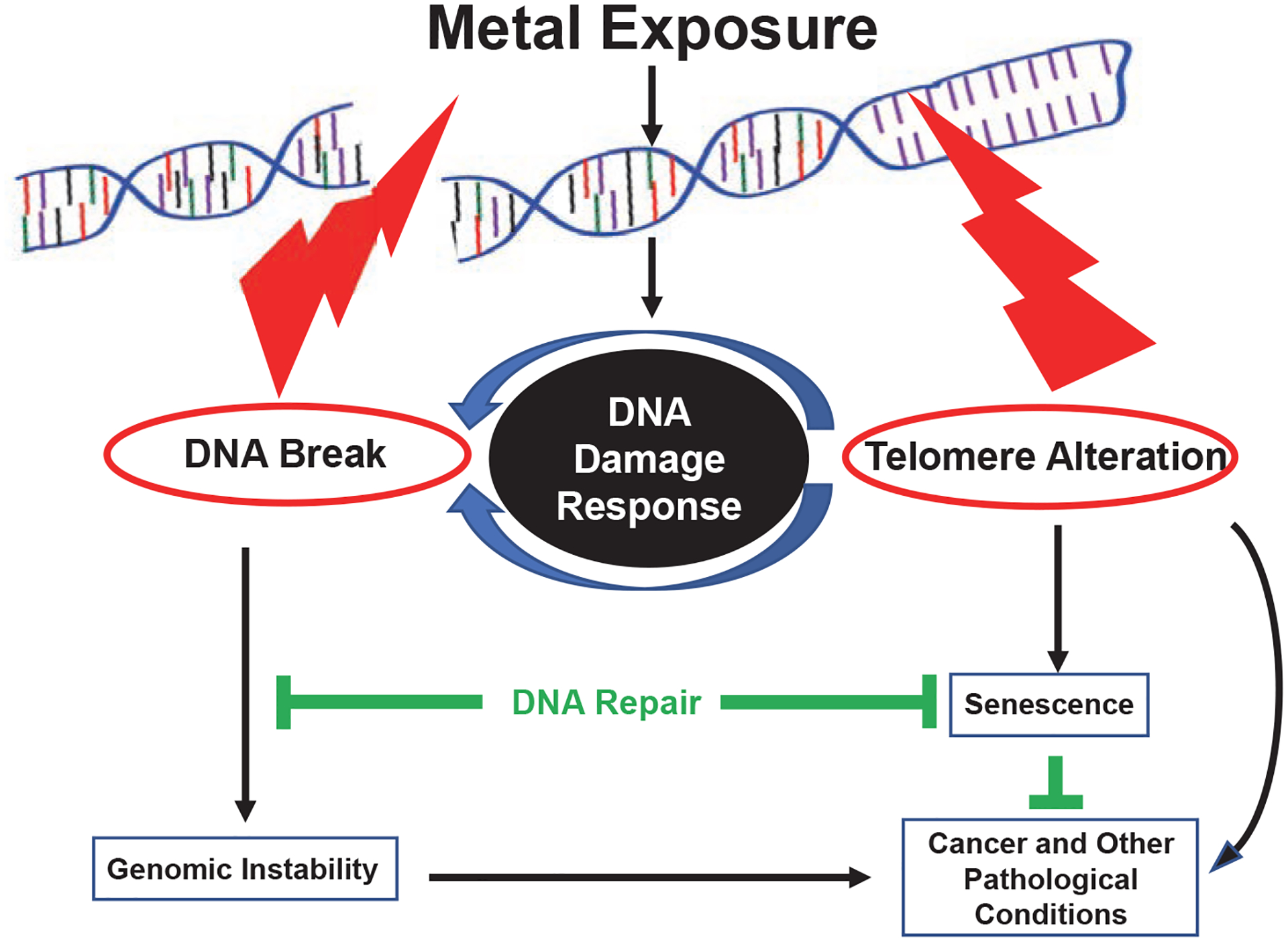
Schematic of Metal-Induced Telomere Alteration Signaling Pathway Linking DNA Damage Response and Activation of DNA Damage, Senescence, and Disease Prognosis
In this article, we summarized genotoxic endpoints that have been associated with exposure to these metals in humans and animals. More importantly, some of these metals are known human carcinogens and are recognized as common environmental hazards.
We had two primary goals for this initial analysis. The first goal was to use the information found in toxicological profiles to synthesize accumulating genotoxic endpoints that have been associated with exposure to different metals. The second goal was to provide a simple visual comparison of the endpoints shown in a systematic order for each of the metals. Activation of genotoxic endpoints can progressively alter cellular function and increase susceptibility to various disease conditions. Screening of genotoxic endpoints and genotoxic biomarkers, therefore, might help the scientific community to mitigate toxic health outcomes after exposure to these metals.
What is already known about this topic?
Metals are known to cause DNA damage.
What is added by this article?
The article summarizes varied findings of genotoxic effects into specifically defined genotoxic endpoints and provides a simple visual comparison of the endpoints in systematic order for each of the metals discussed.
What are the implications for environmental public health practice?
By first ranking metals according to the impact on genotoxic endpoints that could result in cancer and other disease pathologies, researchers can begin to consider developing a suite of biomarkers for early evaluation of various disease conditions.
Footnotes
Disclaimer:
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC, ATSDR, or the National Center for Environmental Health. The use of product names in this article does not constitute an endorsement of any manufacturer’s product.
References
- Agency for Toxic Substances and Disease Registry. (2005). Toxicological profile for zinc. U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxPr006Ffiles/tp60.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2007). Toxicological profile for arsenic. U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp2.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2012). Toxicological profile for chromium. U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp7.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2020). Toxicological profile for lead. U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2022a). ATSDR’s Substance Priority List. https://www.atsdr.cdc.gov/spl/
- Agency for Toxic Substances and Disease Registry. (2022b). Completed Exposure Pathway (CEP) Site Count Report. https://www.atsdr.cdc.gov/cep/index.html [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2022c). Toxicological profile for mercury (Draft for public comment). U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp46.pdf [Google Scholar]
- Agency for Toxic Substances and Disease Registry. (2023a). Toxicological profiles. https://www.atsdr.cdc.gov/toxprofiledocs/index.html
- Agency for Toxic Substances and Disease Registry. (2023b). Toxicological profile for nickel (Draft for public comment). U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp15.pdf [Google Scholar]
- Alloway BJ, & Ayres DC (1993). Chemical principles of environmental pollution. Blackie Academic & Professional. [Google Scholar]
- Antonini JM, Kodali V, Meighan TG, Roach KA, Roberts JR, Salmen R, Boyce GR, Zeidler-Erdely PC, Kashon M, Erdely A, & Shoeb M (2019). Effect of age, high-fat diet, and rat strain on serum biomarkers and telomere length and global DNA methylation in peripheral blood mononuclear cells. Scientific Reports, 9(1), Article 1996. 10.1038/s41598-018-38192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora NK, & Chauhan R (2021). Heavy metal toxicity and sustainable interventions for their decontamination. Environmental Sustainability, 4, 1–3. 10.1007/s42398-021-00164-y [DOI] [Google Scholar]
- Bauchinger M, Schmid E, Einbrodt HJ, & Dresp J (1976). Chromosome aberrations in lymphocytes after occupational exposure to lead and cadmium. Mutation Research/Genetic Toxicology, 40(1), 57–62. 10.1016/0165-1218(76)90023-9 [DOI] [PubMed] [Google Scholar]
- Boyce GR, Shoeb M, Kodali V, Meighan TG, Roach KA, McKinney W, Stone S, Powell MJ, Roberts JR, Zeidler-Erdely PC, Erdely A, & Antonini JM (2020). Welding fume inhalation exposure and high-fat diet change lipid homeostasis in rat liver. Toxicology Reports, 7, 1350–1355. 10.1016/j.toxrep.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KS, Buchner V, & Tchounwou PB (2011). Exploring the molecular mechanisms of nickel-induced genotoxicity and carcinogenicity: A literature review. Reviews on Environmental Health, 26(2), 81–92. 10.1515/reveh.2011.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Giri NC, Zhang R, Yamane K, Zhang Y, Maroney M, & Costa M (2010). Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. Journal of Biological Chemistry, 285(10), 7374–7383. 10.1074/jbc.A109.058503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T-F, Choudhuri S, & Muldoon-Jacobs K (2012). Epigenetic targets of some toxicologically relevant metals: A review of the literature. Journal of Applied Toxicology, 32(9), 643–653. 10.1002/jat.2717 [DOI] [PubMed] [Google Scholar]
- Chiocca SM, Sterner DA, Biggart NW, & Murphy EC Jr. (1991). Nickel mutagenesis: Alteration of the MuSVts110 thermosensitive splicing phenotype by a nickel-induced duplication of the 3’ splice site. Molecular Carcinogenesis, 4(1), 61–71. 10.1002/mc.2940040110 [DOI] [PubMed] [Google Scholar]
- Clemens F, Verma R, Ramnath J, & Landolph JR (2005). Amplification of the Ect2 proto-oncogene and over-expression of Ect2 mRNA and protein in nickel compound and methylcholanthrene-transformed 10T1/2 mouse fibroblast cell lines. Toxicology and Applied Pharmacology, 206(2), 138–149. 10.1016/j.taap.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, & Costa M (1993). Mechanisms of chromium carcinogenicity and toxicity. Critical Reviews in Toxicology, 23(3), 255–281. 10.3109/10408449309105012 [DOI] [PubMed] [Google Scholar]
- Danadevi K, Rozati R, Banu BS, & Grover P (2004). Genotoxic evaluation of welders occupationally exposed to chromium and nickel using the Comet and micronucleus assays. Mutagenesis, 19(1), 35–41. 10.1093/mutage/geh001 [DOI] [PubMed] [Google Scholar]
- Haugen A, Maehle L, Mollerup S, Rivedal E, & Ryberg D (1994). Nickel-induced alterations in human renal epithelial cells. Environmental Health Perspectives, 102(Suppl. 3), 117–118. 10.1289/ehp.94102s3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. (2023). Agents classified by the IARC Monographs, volumes 1–134. https://monographs.iarc.who.int/agents-classified-by-the-iarc/2019/
- Klein CB, Kargacin B, Su L, Cosentino S, Snow ET, & Costa M (1994). Metal mutagenesis in transgenic Chinese hamster cell lines. Environmental Health Perspectives, 102(Suppl. 3), 63–67. 10.1289/ehp.94102s363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali V, Shoeb M, Meighan TG, Eye T, Friend SA, Hubczak J, Kashon ML, Zeidler-Erdely PC, Antonini JM, & Erdely A (2020). Bioactivity of circulatory factors after pulmonary exposure to mild or stainless steel welding fumes. Toxicological Sciences, 177(1), 108–120. 10.1093/toxsci/kfaa084 [DOI] [PubMed] [Google Scholar]
- Langston WJ (1990). Toxic effects of metals and the incidence of metal pollution in marine ecosystems. In Furness RW (Ed.), Heavy metals in the marine environment (1st ed., pp. 101–120). CRC Press. 10.1201/9781351073158-7 [DOI] [Google Scholar]
- National Toxicology Program. (2021). 15th report on carcinogens. U.S. Department of Health and Human Services. https://ntp.niehs.nih.gov/go/roc14 [Google Scholar]
- Popp W, Vahrenholz C, Schmieding W, Krewet E, & Norpoth K (1991). Investigations of the frequency of DNA strand break-age and cross-linking and of sister chromatid exchange in the lymphocytes of electric welders exposed to chromium- and nickel-containing fumes. International Archives of Occupational and Environmental Health, 63(2), 115–120. 10.1007/BF00379074 [DOI] [PubMed] [Google Scholar]
- Salnikow K, Davidson T, Kluz T, Chen H, Zhou D, & Costa M (2003). GeneChip analysis of signaling pathways effected by nickel. Journal of Environmental Monitoring, 5(2), 206–209. 10.1039/b210262p [DOI] [PubMed] [Google Scholar]
- Shoeb M, Kodali VK, Farris BY, Bishop LM, Meighan TG, Salmen R, Eye T, Friend S, Schwegler-Berry D, Roberts JR, Zeidler-Erdely PC, Erdely A, & Antonini JM (2017). Oxidative stress, DNA methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact, 5, 61–69. 10.1016/j.impact.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Kodali V, Farris B, Bishop LM, Meighan T, Salmen R, Eye T, Roberts JR, Zeidler-Erdely P, Erdely A, & Antonini JM (2017). Evaluation of the molecular mechanisms associated with cytotoxicity and inflammation after pulmonary exposure to different metal-rich welding particles. Nanotoxicology, 11(6), 725–736. 10.1080/17435390.2017.1349200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Meier HCS, & Antonini JM (2021). Telomeres in toxicology: Occupational health. Pharmacology & Therapeutics, 220, Article 107742. 10.1016/j.pharmthera.2020.107742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Mustafa GM, Kodali VK, Smith K, Roach KA, Boyce G, Meighan T, Roberts JR, Erdely A, & Antonini JM (2020). A possible relationship between telomere length and markers of neurodegeneration in rat brain after welding fume inhalation exposure. Environmental Research, 180, Article 108900. 10.1016/j.envres.2019.108900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel CF (1993). Mutagenic potential of nickel compounds in human lymphoblasts in vitro [Master’s thesis, University of North Carolina at Chapel Hill]. UNC University Libraries: Carolina Digital Repository. 10.17615/kwpv-j614 [DOI] [Google Scholar]


