Abstract
Telomeres are inert DNA sequences (TTAGGG) at the end of chromosomes that protect genetic information and maintain DNA integrity. Emerging evidence has demonstrated that telomere alteration can be closely related to occupational exposure and the development of various disease conditions, including cancer. However, the functions and underlying molecular mechanisms of telomere alteration and shelterin dysregulation after welding fume exposures have not been broadly defined. In this study, we analyzed telomere length and shelterin complex proteins in peripheral blood mononuclear cells (PBMCs) and in lung tissue recovered from male Sprague-Dawley rats following exposure by intratracheal instillation (ITI) to 2 mg/rat of manual metal arc-stainless steel (MMA-SS) welding fume particulate or saline (vehicle control). PBMCs and lung tissue were harvested at 30 d after instillation. Our study identified telomere elongation and shelterin dysregulation in PBMCs and lung tissue after welding fume exposure. Mechanistically, telomere elongation was independent of telomerase reverse transcriptase (TERT) activation. Collectively, our findings demonstrated that welding fume-induced telomere elongation was (a) TERT-independent and (b) associated with shelterin complex dysregulation. It is possible that an alteration of telomere length and its regulatory proteins may be utilized as predictive biomarkers for various disease conditions after welding fume exposure. This needs further investigation.
Keywords: Welding fumes, Telomere, DNA damage, Shelterin complex
1. Introduction
A recent estimate indicated that 574,000 workers were officially classified as welders in the U.S. (https://datausa.io/profile/soc/514120/). However, there are millions of workers exposed to welding fumes daily worldwide as part of their work duties. Welding employment in the U.S. is expected to increase roughly 2% over the next several years (Bureau of Labor Statistics, 2023) because of an aging workforce and the nation’s deteriorating infrastructure. Based on sufficient epidemiology and inadequate animal studies, welding fume has been classified as a Group 1 carcinogen to humans by the International Agency for Research on Cancer (IARC) (Guha et al., 2017). Welding fumes are a complex mixture of different cytotoxic and genotoxic metals (e.g., Fe, Cr, Mn, Ni) and several of these metals are known to cause genotoxic effects (Shoeb et al., 2023). Their composition is dependent on the types of electrodes/consumables used during the welding process (Antonini, 2003; Shoeb et al., 2017a, 2017b). Welding is performed at extremely high temperatures (>4000 °C), in which superheated metal vapors are oxidized on contact with the air and form easily inhaled small particulates (200–500 nm) of different complexes of metal oxides. Interestingly, a greater pneumotoxic pulmonary response has been observed when comparing freshly generated vs aged welding particles (Antonini et al., 1998, 2013; Zeidler-Erdely et al., 2012).
Telomeres (Fig. 1A) are DNA-proteins present in the nucleus of each somatic cell providing protection to 23 pairs of chromosomes from any internal and external DNA damage (de Lange, 2005; Shoeb et al., 2017c, 2019, 2021). Their appropriate length is imperative for proper cellular functioning and preventing indefinite proliferation of cells and oncogenic chromosomal rearrangements. One of the characteristics of tumorigenesis is to divert the ability of a normally dividing cell to a tumor cell through disruption of telomere length homeostasis (Shoeb et al., 2021). Telomeres control DNA damage response (DDR) and DNA repair pathways during cell division by regulating ataxia telangiectasia mutated (ATM) and ataxia telangiectasia Rad3-related (ATR) kinases (de Lange, 2005, 2018). Telomere regulatory proteins also known as shelterin complex proteins (Fig. 1A), include double stranded-telomere repeat factors 1 and 2 (Trf1 and 2), single stranded-protection of telomere 1 (Pot1), and are held together by tripeptidyl peptidase 1 (Tpp1), trf1-interacting protein 2 (Tin2), and repressor/activator protein 1 (Rap1). These six proteins perform essential functions to maintain telomere integrity and to repress DNA damage signaling by inhibiting ATM and ATR kinase activation (Shoeb et al., 2021). Other than six shelterin proteins, there are approximately 200 proteins associated with different characteristics of telomere biology, regulating telomere length and protection (Li et al., 2017).
Fig. 1.
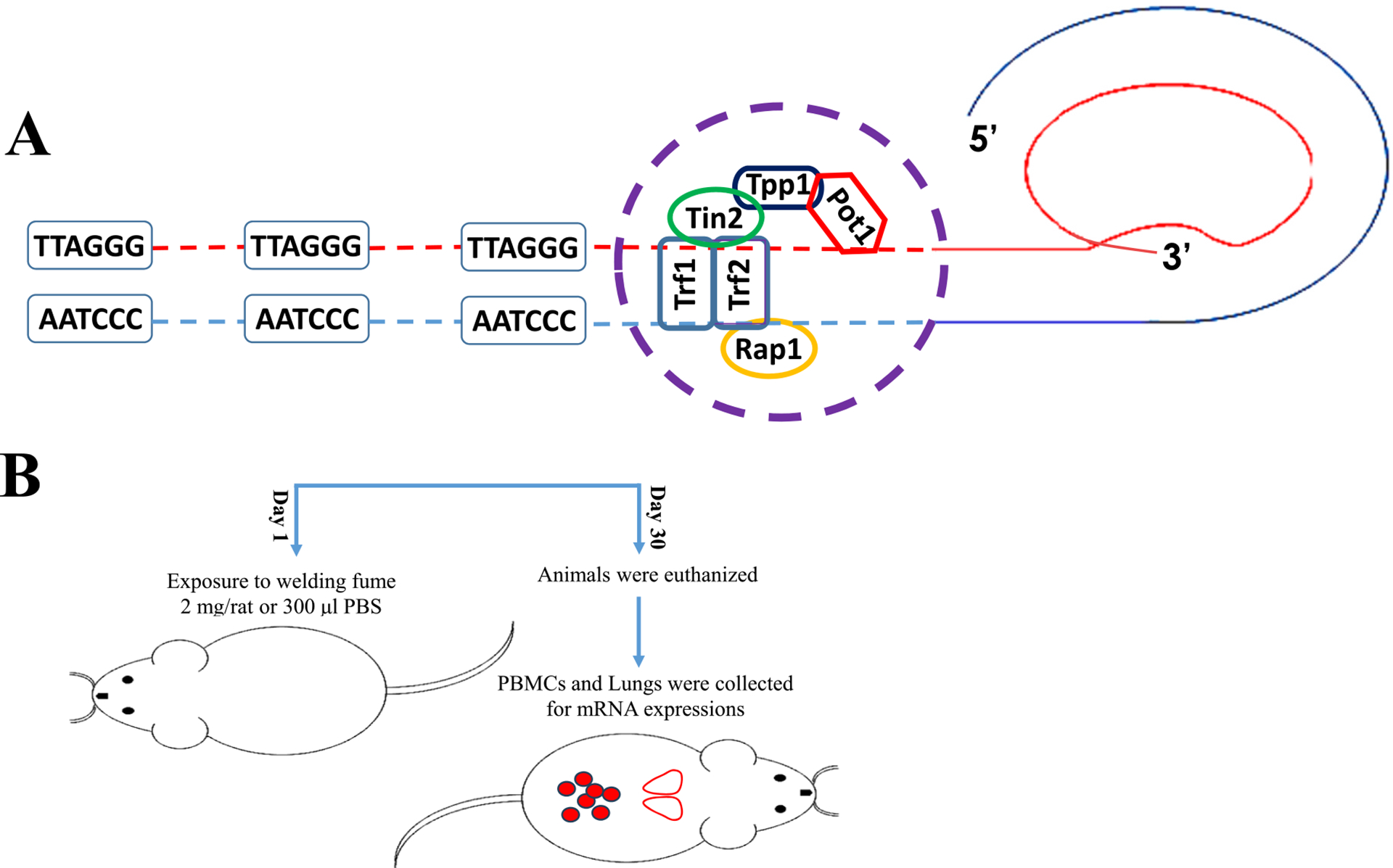
(A) Schematic diagram of the telomere in the t-loop configuration with shelterin complex protein Pot1, Tpp1, Trf1, Trf2, Tin2 and Rap1 located at single- and double-stranded DNA. (B) Graphical representation of welding fume exposure and mRNA expression analysis.
Epidemiology studies indicate that welders are likely more susceptible to developing lung cancer (Ambroise et al., 2006; Siew et al., 2008; Kjuus et al., 1986) than the general population. This may be due to exposure to various known carcinogenic metals (Toxicological_Profiles | ATSDR (cdc.gov)). For instance, Cr and Ni are classified as known carcinogens and have been reported to cause various types of cancer in welders (d’Errico et al., 2009; Hernberg et al., 1983; Deng et al., 2019). Because of the presence of Mn in most welding fumes, neurological changes have also been reported in welders (Shoeb et al., 2020). Therefore, both pulmonary and non-pulmonary health effects are of concern and have been reported in workers after welding fume exposure.
The intent of the current study was to identify possible biomarkers after welding fume exposure indicative of early potential genotoxic changes that may lead to development of chronic diseases or cancer. In the present study, we systemically investigated both the expression of shelterin complex proteins and TERT after welding fume exposure using an animal model. Our findings suggest that telomere elongation after pulmonary exposure to Cr- and Ni-containing stainless steel welding fume could be due to initiation of dysfunctional shelterin complex (Tpp1), but not TERT expression, in both PBMCs and lung tissue. Mechanistically, the main shelterin protein, Tpp1 maintains telomere stability through interacting and activating telomerase. Taken together, shelterin dysregulation may be affecting the recruitment of TERT leading to telomere elongation after welding fume exposure.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats from Hilltop Lab Animals (Scottdale, PA), weighing 250–300 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter, and CAR Bacillus, were used. The rats were acclimated for one week after arrival and were provided HEPA-filtered air, irradiated Teklad 2918 diet, and tap water ad libitum. All animal procedures used during the study were reviewed and approved by the CDC-Morgantown Institutional Animal Care and Use Committee. The animal facilities are specific pathogen free, environmentally controlled, and accredited by AAALAC, International (Frederick, MD). All methods were performed in accordance with the relevant guidelines and regulations by CDC-NIOSH Animal Care and Use Committee.
2.2. Welding fume exposure
The manual metal arc-stainless steel (MMA-SS) welding fume sample was kindly generated and provided by Lincoln Electric Co. (Cleveland, OH) as previously described (Shoeb et al., 2017b). Analysis of elements present in the welding sample was performed by inductively coupled plasma-atomic emission spectroscopy and found to be 41% Fe, 28% Cr, 17% Mn, 3% Ni, and 11% of Cu, V, and Ti combined. Estimation of ITI of welding fume particle dose and its correlation with a “real world” worker exposure was calculated and previously described (Shoeb et al., 2017a). The welding fume sample was prepared in sterile saline and sonicated to disperse the particulates. Dynamic light scattering indicated that a suspension of MMA-SS welding particles in water had an average diameter (dH) of 549 nm (Shoeb et al., 2017b). Rats were lightly anesthetized by an intraperitoneal injection of 25 mg/kg body weight of methohexital sodium (Brevital 500 mg; JHP Pharmaceuticals, LLC, Rochester, MI, USA) and exposed by ITI with 2.0 mg/rat of welding fume in 300 μl of sterile phosphate buffered saline (PBS). Vehicle control animals received 300 μl of sterile PBS by ITI (Fig. 1B). Saline solutions and anesthetics were United States Pharmacopeia (USP) grade. ITI particle dose was selected to represent a “real world” worker exposure (Antonini et al., 1996). It was found that an ITI welding particle dose of 0.2 mg/rat (regardless of welding fume type) induced no pulmonary response, whereas doses above 5 mg/rat caused significant lung inflammation and prominent particle lung burden. The goal was to choose a dose between the 0.2 and 5.0 mg/rat dose range used in the earlier study (Antonini et al., 2014). Also, the 2.0 mg/rat dose was determined to approximate an exposure of 52.1 days or 10.4 weeks of exposure, based on 5-day work week (Antonini et al., 2014).
2.3. Isolation of PBMCs
At 30 d after exposure, animals were euthanized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg body weight, IP; Fatal-Plus Solution, Vortech Pharmaceutical, Inc., Dearborn, MI, USA) and then exsanguinated by severing the abdominal aorta. Whole blood was drawn using an 18-gauge needle from the abdominal vena cava and collected in BD Vacutainer tubes (Becton, Dickinson, and Co., Franklin Lakes, NJ). The PBMCs were isolated from heparinized blood collected from each animal using Accuspin 12-ml tubes (Sigma-Aldrich Co., St. Louis, MO, USA) containing Histopaque-1083 (Sigma-Aldrich Co., St. Louis, MO) by centrifuging at 1250×g for 30 min. PBMCs were collected in 15-ml falcon tubes and washed once with PBS at 450×g for 20 min. Finally, PBMCs were re-suspended in 1 ml PBS (Lonza, Walkersville, MD, USA) for genomic DNA (gDNA) isolation. All steps were performed at room temperature.
2.4. gDNA isolation and telomere length analysis
gDNA was extracted from the collected PBMCs (Shoeb et al., 2017a) and lung tissue isolated using DNeasy Blood & Tissue Kit (Qiagen Sciences Inc., Germantown, MD). The DNA concentration was measured using a Nano-Drop 2000 spectrophotometer. Samples were diluted to a final concentration of 25 ng/1.5 μl to measure telomere length. Quantitative PCR (qPCR) was performed using the SYBR Select Master Mix (Life Technologies, Carlsbad, CA) with a step one plus real time PCR system (Applied Biosystems, Foster City, CA, USA). The parameters used were as follows: 95 °C for 10 min (enzyme activation), 95 °C for 15 s (denaturing), and 60 °C for 60 s (annealing), 60 cycles. Primers used were as follows: Tel rat-F 5′-GGT TTT TGA GGG TGA GGG TGA GGG TGA GGG TGA GGG t-3′, and Tel rat-R 5′-TCC CGA CTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA- 3′; AT1 rat-F 5′-ACG TGT TCT CAG CAT CGA CCG CTA CC-3′ and AT1 rat-R 5′-AGA ATG ATA AGG AAA GGG AAC AAG AAG CCC-3′ (Invitrogen Corporation, Carlsbad, CA). The relative telomere length was measured by comparing the ratio of telomere repeat copy number (T as Tel1) and single gene copy number (S as AT1), expressed as telomere length (T/S) ratio. Each individual value obtained by qPCR were processed through formula T/S = 2−ΔCT, where ΔCT = CT telomere – CTAT1. This ratio was then compared with the ratio of the reference DNA. Each DNA sample collected was measured in duplicate.
2.5. RNA isolation from lung samples
Lung RNA was isolated, and reverse transcribed to cDNA following the conclusion of 30 d exposure to saline control or welding fume. RNeasy Fibrous Tissue Mini Kit (Qiagen Inc.; Valencia, CA, USA) was used according to kit instructions. Briefly, 25–30 mg of lung tissue was used to isolate total RNA from the right apical lobe of the control and welding-exposed lungs, homogenized in buffer RLT and two 2.4 mm Zirconia beads (BioSpec Products Inc.; Bartlesville, OK, USA) using a mini beadbeater-8 (BioSpec Products Inc.) for 20 s. The tissue homogenate was centrifuged at 10,000×g for 10 min at room temperature, and the RNA present in the supernatant was extracted and purified using RNeasy columns. Following RNA quantification, samples were reverse transcribed using random hexamers (Applied Biosystems, Foster City, CA, USA) and Superscript III (Invitrogen; Carlsbad, CA, USA). Gene expression was analyzed using transcribed cDNA, and hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as the endogenous control.
2.6. Shelterin components analysis
Relative mRNA levels of shelterin components were determined by quantitative PCR (qPCR). Gene expression was determined using the StepOne Plus (Applied Biosystems, Carlsbad, CA, USA) with pre-designed Assays-on-Demand TaqMan probes and primers including Pot1 (Rn01747967_m1), Tpp1 (Rn00580350_m1), Tin2 (Rn01481999_g1), Trf1 (Rn01749291_m1), Trf2 (Rn01432601_m1) and Rap1 (Rn01762131_m1), (Thermo Fisher Scientific, Waltham, MA, USA). Using 96-well plates, cDNA was used for gene expression. HPRT was used as the endogenous control.
2.7. Statistical analysis
Results are means ± standard error of measurement. The significance of difference between exposure groups was analyzed using a one-way analysis of variance (ANOVA) and the Tukey post-hoc test. The criterion of significance was set at p < 0.05.
3. Results
3.1. Welding fume-induced telomere alteration and TERT activation
Previously, we observed inflammatory response in the rat lungs tissue and oxidative stress in isolated PBMCs after ITI exposure to the welding fumes (Shoeb et al., 2017a). No significant epigenetic changes (DNA methylation) were observed comparing between the welding fume and control groups (Shoeb et al., 2017a). However, increased telomere length after 30 d of welding fume exposure was observed (Shoeb et al., 2017a). In this study 30 d welding fume exposure results reconfirmed significant telomere elongation in isolated PBMCs as well as in lung tissues (Figs. 2A and 4A) after a single exposure compared to the control groups as analyzed by qPCR analysis. Also, at this timepoint (), we did not observe any changes in PBMCs and lung tissue TERT mRNA expression in the welding fume-exposed animals compared to the control group (Figs. 2B and 4B).
Fig. 2.
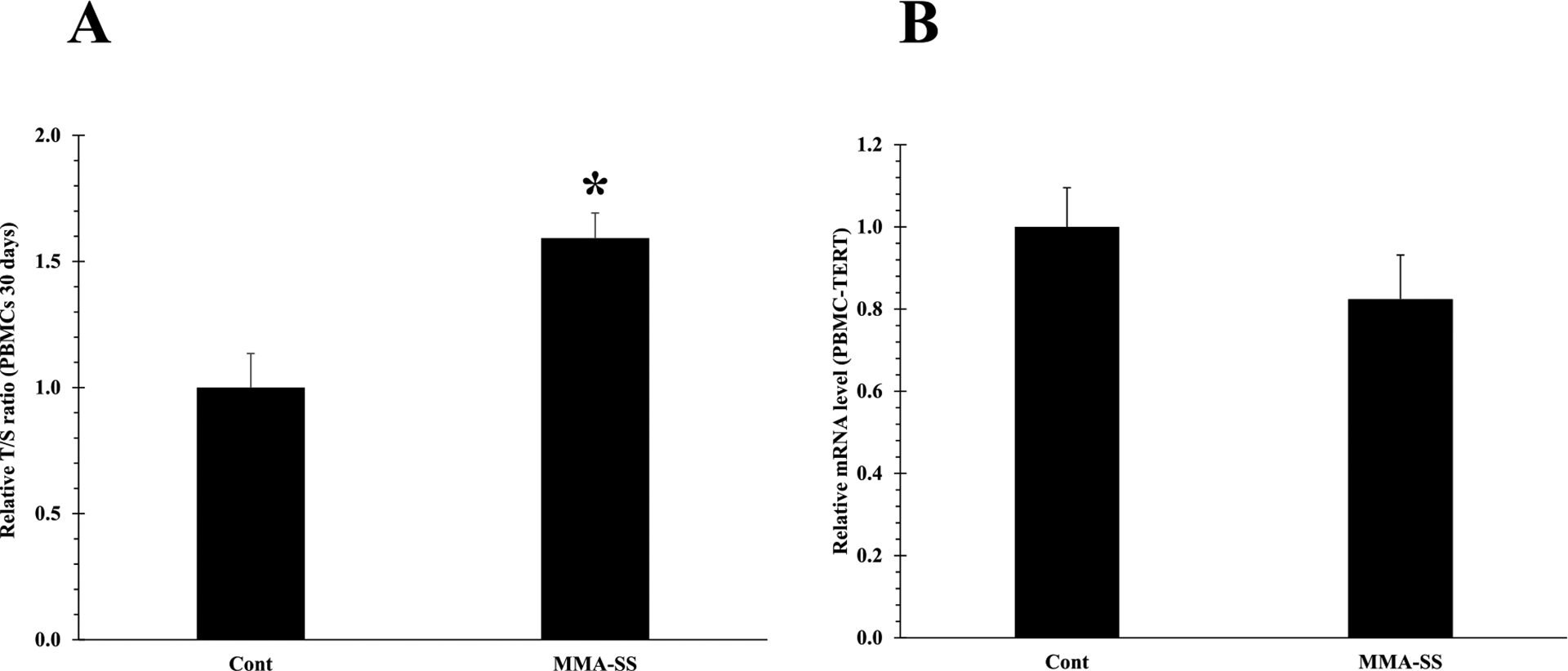
Telomere length of PBMCs isolated at 30 d after ITI exposure to 2.0 mg/rat of MMA-SS welding fume. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from MMA-SS groups). (A) The relative telomere length was measured by comparing the ratio of telomere repeat copy number (T) and single gene copy number (S), expressed as telomere length (T/S) ratio. (B) TERT mRNA expression.
Fig. 4.
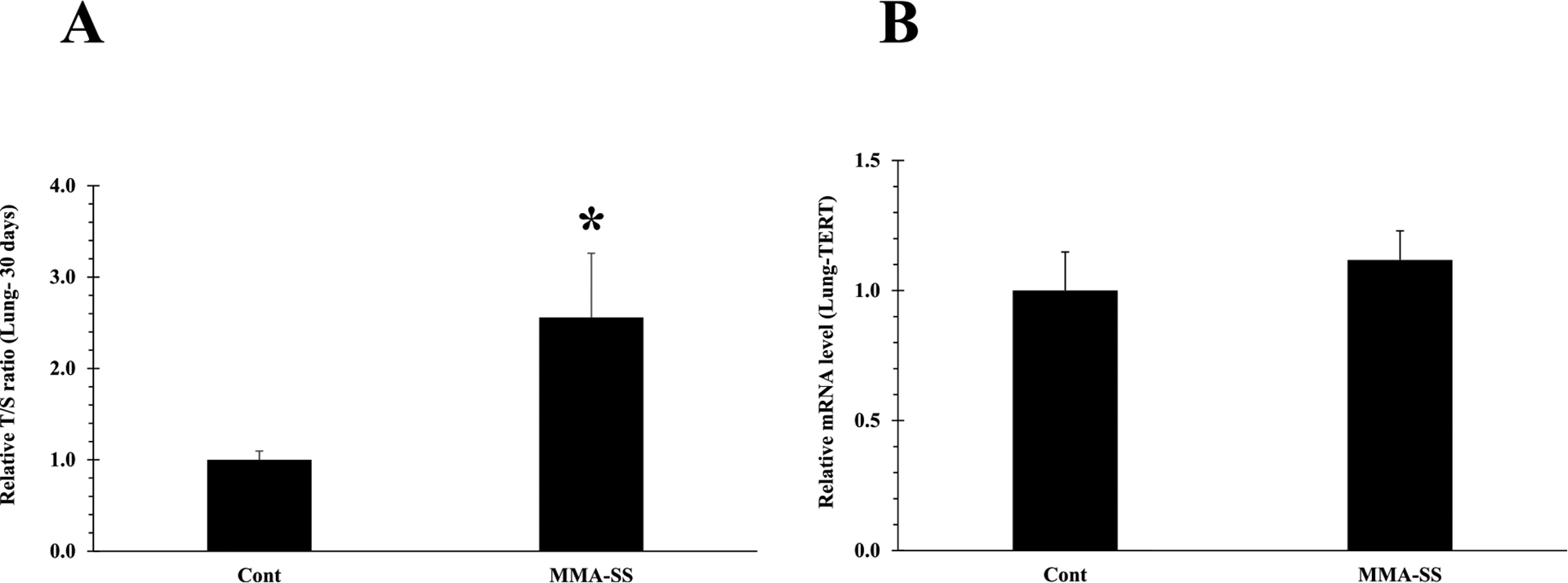
Telomere length of lung tissue isolated at 30 d after ITI exposure to 2.0 mg/rat of MMA-SS welding fume. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from MMA-SS groups). (A) The relative telomere length was measured by comparing the ratio of telomere repeat copy number (T) and single gene copy number (S), expressed as telomere length (T/S) ratio. (B) TERT mRNA expression.
3.2. Welding fume-induced dysregulation of shelterin complex
The shelterin complex protect telomeres from DDR (Shoeb et al., 2021). Pot1 forms a heterodimer with Tpp1 on ssDNA, required to maintain telomere length and stability by recruiting telomerase. Here, we investigated the impact of welding fumes exposure on the shelterin components, in rat PBMCs and lung tissue. Gene expression analysis indicated significant alteration of Tpp1 in PBMCs after welding fume exposure as compared to control (Fig. 3B), rest of the shelterin proteins in PBMCs remained unchanged. Next, we analyzed the shelterin protein expression in lung tissue. Gene expression analysis indicated significant alteration in all shelterin proteins except Tin2, Trf1 and Trf2 (Fig. 5C, D, and E). We observed significantly decreased expression of Pot1 (Fig. 5A) in the welding fume-exposed group as compared to control. Furthermore, Tpp1 and Rap1 was also significantly down-regulated (Fig. 5B and F) in welding fume-exposed group vs control. Pot1 alteration could result in activation of the ATR-dependent DDR (Shoeb et al., 2021). Since decreased expression of Pot1 was observed in welding fume exposed lung tissue (Fig. 5A), we selectively examined the expression of ATR kinases in isolated lung tissue. Significantly reduced expression of the ATR gene was observed in welding fume exposed-lung tissue (Fig. 6).
Fig. 3.
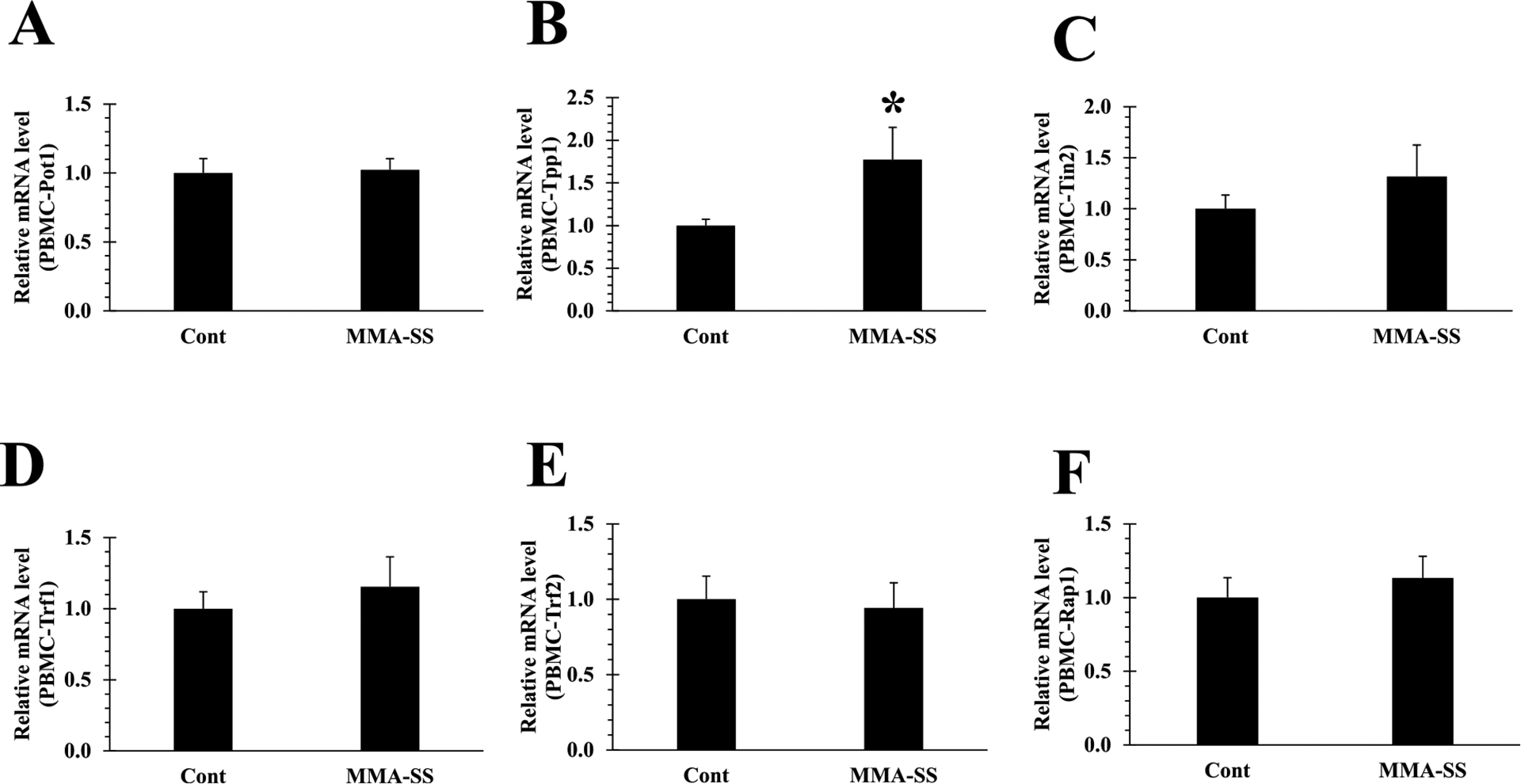
Initiation of shelterin core complex (B) Tpp1 disruption in isolated PBMCs after ITI exposure to 2.0 mg/rat of MMA-SS welding fume (n = 4–6; values are means ± standard error; *significantly different from MMA-SS groups). (A) Pot1, (C) Tin2 (D) Trf1 (E) Trf2 and (F) Rap1 expression remained unchanged in PBMCs cDNA.
Fig. 5.
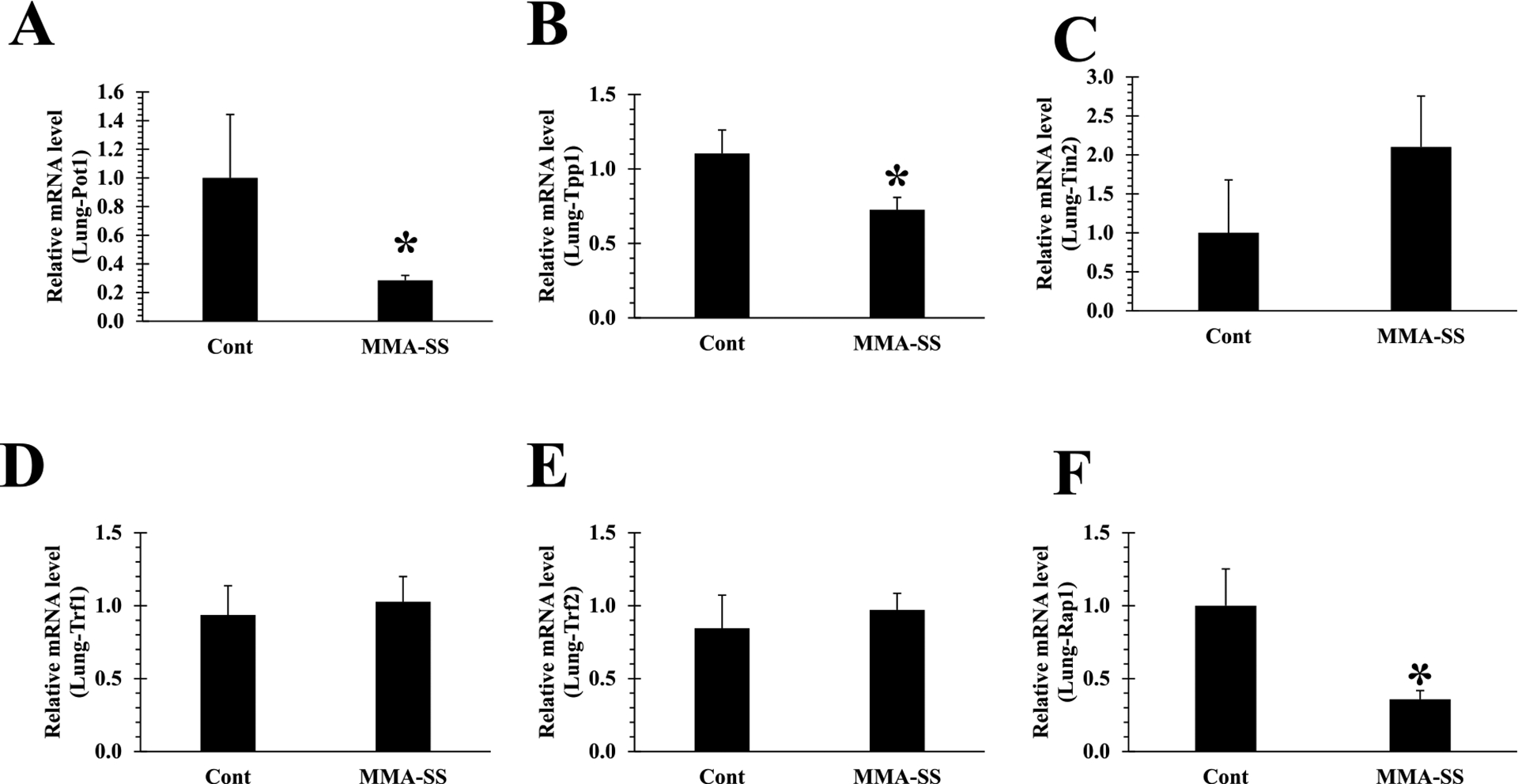
Initiation of shelterin core complex (B) Tpp1 disruption, inactivation of (A) Pot1 and (F) Rap1 expression in isolated lung tissue after ITI exposure to 2.0 mg/rat of MMA-SS welding fume (n = 4–6; values are means ± standard error; *significantly different from MMA-SS groups). (C) Tin2, (D) Trf1 and (E) Trf2 expression remained unchanged in lung cDNA.
Fig. 6.
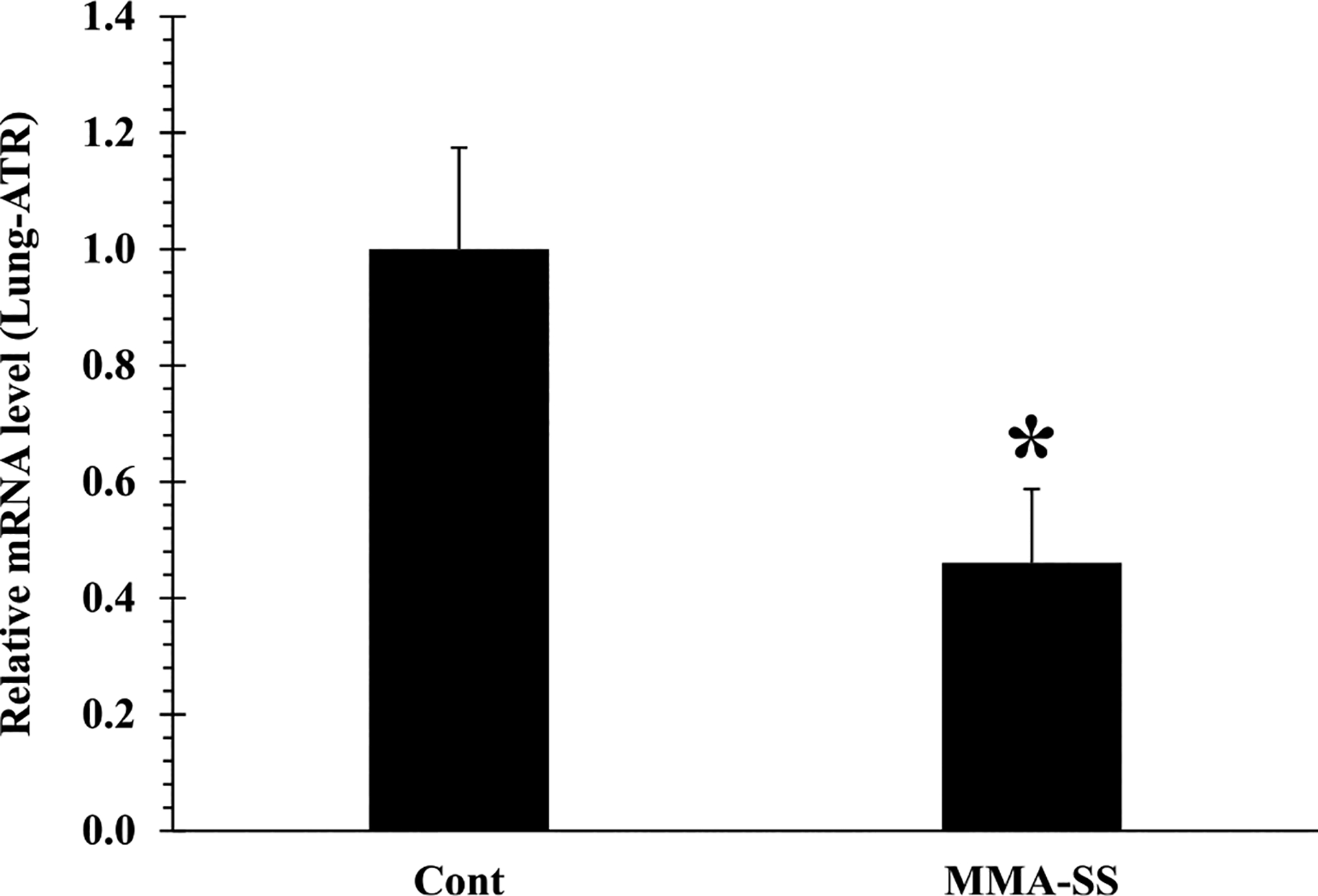
Expression of ATR gene in isolated lung tissue at 30 d after ITI exposure to 2.0 mg/rat of MMA-SS welding fume. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from MMA-SS groups).
4. Discussion
Exposures to cytotoxic metals, such as Ni and Cr, have been documented to cause genotoxic and carcinogenic effects (Agency for Toxic Substances and Disease Registry (ATSDR), 2005; Agency for Toxic Substances and Disease Registry (ATSDR), 2012). Genotoxic properties of welding fumes may vary depending on the metal composition, particle size, solubility, type of welding, and the electrodes/consumables (stainless steel or mild steel) being used during the welding process (Shoeb et al., 2017b). Welding exposure is known to cause pulmonary effects such as bronchitis, airway irritation, metal fume fever, chemical pneumonitis, lung function changes, and lung cancer in welders (Sferlazza and Beckett, 1991). However, pinpointing the mechanistic causes of these effects has been problematic due to multiple cellular targets. Importantly, an increase in the incidence of lung cancer has been reported in workers in the welding industry (Ambroise et al., 2006; Siew et al., 2008; Kjuus et al., 1986).
A growing body of evidence has suggested exposure-induced telomere alterations (Shoeb et al., 2021). However, associations between welding fume exposure and telomere length alterations are not extensively investigated. Telomeres are chromatin structures maintained by a group of six proteins called shelterin (Fig. 1A) and an enzyme, telomerase, which is essentially a ribonucleoprotein complex consisting of TERT, and a telomerase RNA component (TERC) (Gilson and Geli, 2007; Londono-Vallejo and Wellinger, 2012). Exposure to hazardous substances may result in the alteration of telomere length homeostasis and dysregulation of shelterin proteins (Shoeb et al., 2021). This study aims to investigate the effect of pulmonary exposure to welding fumes on shelterin proteins dysregulation and TERT expression in isolated PBMCs and lung tissue in an animal model.
Previously, we have observed increased telomere length after 30 d of welding fume exposure (Shoeb et al., 2017a), but the possible reason for this elongation has never been elucidated. Currently, 30 d welding fume exposure results reconfirmed telomere elongation in isolated PBMCs (Fig. 2A). Also, elongated telomeres were observed in lung tissues (Fig. 4A) as analyzed by qPCR analysis. Dysfunctional telomeres can lead to activation of DNA damage and may increase the risk of tumorigenesis by activating anti-tumorigenic mechanisms (Shoeb et al., 2021). Binary telomere length has been linked to lung and kidney cancer (Hou et al., 2012). Both short and long telomeres can lead to DNA damage and epigenetic modifications that may result in the development of various disease conditions. Activation of TERT has been reported to decrease mortality of cancer cells and therefore, extend the life span of various types of cancer (Blasco, 2005; Martinez and Blasco, 2011). It is possible that hazardous exposures may contribute to tumor formation due to a mutation in TERT (Martinez and Blasco, 2011; Barthel et al., 2017), whereby the senescent stage is possibly bypassed leading to unwanted activation of telomerase or telomeric fusion through formation of critically short telomeres (reduced telomerase activity). However, at this timepoint (30 d), we did not observe any changes in PBMCs and lung tissue TERT mRNA expression in the welding fume-exposed animals compared to the control group (Figs. 2B and 4B). Telomere elongation with no change in TERT expression was previously observed in animal brain tissue following welding fume inhalation exposure (Shoeb et al., 2020). These results suggest that telomere lengthening after welding exposures may not be due to telomerase activation.
Shelterin proteins and telomerase (TERT) activity are required to maintain telomere integrity by adding TTAGGG repeats at the ends of chromosomes. Shelterin complex proteins also prevent double- and single-stranded DNA from being recognized as sites of DNA damage during the DNA replication process thereby averting unwanted DNA repair (de Lange, 2005, 2018). Alteration or removal of any of these proteins would result in shelterin dysregulation, telomere length alteration, and genomic instability (Shoeb et al., 2019). The interaction of Tpp1 is the basis for shelterin complex integrity (Fig. 1A). We observed significant alteration of this foundational protein (Tpp1) in PBMCs after welding fume exposure as compared to control (Fig. 3B). However, the rest of the shelterin proteins in PBMCs remained unchanged. We then analyzed the shelterin protein expression in lung tissue to determine the effect of welding fume exposure at the primary site of exposure. Significant alteration in all shelterin proteins except Tin2, Trf1 and Trf2 were observed (Fig. 5C, D, and E). Shelterin Pot1 binds to single stranded DNA and is required to protect telomeres from being identified as a single strand DNA break (de Lange, 2010). We noticed significantly decreased expression of Pot1 (Fig. 5A) in the welding fume-exposed group as compared to control. In addition, Tpp1, which is required in the recruitment of telomerase (Wang et al., 2007; Sandin and Rhodes, 2014), was also significantly down-regulated (Fig. 5B). Dysregulation of Rap1 can cause telomeric fusion (Sfeir and de Lange, 2012) and telomere elongation was also significantly downregulated in welding fume-exposed group vs control (Fig. 5F).
In addition, ATM and ATR kinases are responsible for controlling DNA damage response in cells (Shoeb et al., 2021). Telomere alterations and dysfunctional shelterin complex proteins are reported to induce these kinases. Alteration in Trf1/Trf2 could result in the activation of DNA-double strand breaks which subsequently recruits ATM kinase for the repair and survival of the cell (de Lange, 2005; Sfeir and de Lange, 2012). However, we did not observe any changes in Trf1/Trf2 expression in isolated PBMCs and lung tissues after welding fume exposure. In contrast, Pot1 alteration could result in DNA-single strand breaks and activation of the ATR-dependent DNA damage response pathway (de Lange, 2010). Because decreased expression of Pot1 was observed in lung tissue (Fig. 5A), we selectively examined the expression of ATR kinases in isolated lung tissue. Significantly reduced expression of the ATR gene was observed in welding fume exposed-lung tissue (Fig. 6). Alteration of shelterin components has been reported to cause dysfunctional telomeres, leading to cancer initiation and progression (Shoeb et al., 2019). Interactions of Pot1-Tpp1 protect the single stranded telomere (3′ telomere) from being recognized as damaged DNA (Chen et al., 2017; Denchi and de Lange, 2007; Hockemeyer et al., 2007; Baumann and Cech, 2001; Hockemeyer et al., 2005). Therefore, alteration of Pot1 at ssDNA could result in the accumulation of increased DDR through the ATR pathway (Shoeb et al., 2021). On the other hand, Rap1 is a highly conserved shelterin component involved in protecting catastrophic telomere loss (Shoeb et al., 2019; Rai et al., 2016). Altered Pot1 and Rap1 observed in lung tissue after welding fume exposure could be because the lungs were the primary site of exposure.
5. Conclusions
Collectively, our results suggest that welding fume exposure result in telomere elongation and initiation of shelterin complex dysregulation in PBMCs and lung tissue. Furthermore, our data suggest that elongation of telomeres occurred without affecting telomerase (TERT activation). We also demonstrated that dysregulation of shelterin complex is initiated by disrupting Tpp1, an integral component responsible for the integrity of double- and single-stranded DNA. Taken together, DNA damage is a primary concern for cancer cell progression, and telomere alteration is one of the indicators of DNA damage manifestation. Therefore, identification of telomere length homeostasis, its regulatory proteins, and the factors influencing telomerase activation (TERT) could be beneficial in better understanding the molecular mechanisms involved in development of adverse health effects from exposure to various environmental and occupational exposures (as seen with welding fume exposure). This could potentially be utilized as predictive biomarkers to improve diagnosis and management of various exposure related disease conditions, and in the possible discovery of new therapeutic opportunities. Our work supports the need for future research in this area.
Acknowledgements
This study was supported by funding from NIOSH, United States and ATSDR, United States.
List of abbreviations
- PBMCs
Peripheral blood mononuclear cells
- ITI
Intratracheal instillation
- MMA-SS
Manual metal arc-stainless steel
- TERT
Telomerase reverse transcriptase
- IARC
International Agency for Research on Cancer
- DNA
Deoxyribonucleic acid
- DDR
DNA damage response
- ATM
Ataxia telangiectasia mutated
- (ATR) kinases
Ataxia telangiectasia Rad3-related
- Trf1 and 2
Telomere repeat factors 1 and 2
- Pot1
Protection of telomere 1
- Tpp1
Tripeptidyl peptidase 1
- Tin2
Trf1-interacting protein 2
- Rap1
Repressor/activator protein 1
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
- USP
United States Pharmacopeia
- gDNA
Genomic DNA
- qPCR
Quantitative PCR
- cDNA
complementary DNA
- RNA
Ribonucleic acid
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Agency for Toxic Substances and Disease Registry, the National Center for Environmental Health, and National Institute for Occupational Safety and Health The use of product names in this presentation does not constitute an endorsement of any manufacturer’s product.
CRediT authorship contribution statement
Mohammad Shoeb: Conceptualization. Terence Meighan: Investigation. Vamsi K. Kodali: Data curation, Writing – review & editing. Henry Abadin: Visualization. Obaid Faroon: Formal analysis. Gregory M. Zarus: Writing – review & editing. Aaron Erdely: Writing – review & editing. James M. Antonini: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Agency for Toxic Substances and Disease Registry (ATSDR), 2005. Toxicological Profile for Nickel. TOXICOLOGICAL PROFILE FOR NICKEL (cdc.gov), Atlanta, GA. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2012. Toxicological Profile for Chromium. Toxicological Profile for Chromium (cdc.gov), Atlanta, GA. [PubMed] [Google Scholar]
- Ambroise D, Wild P, Moulin JJ, 2006. Update of a meta-analysis on lung cancer and welding. Scand. J. Work. Environ. Health 32, 22–31. [DOI] [PubMed] [Google Scholar]
- Antonini JM, 2003. Health effects of welding. Crit. Rev. Toxicol 33, 61–203. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Krishna Murthy GG, Rogers RA, et al. , 1996. Pneumotoxicity and pulmonary clearance of different welding fume particles after intratracheal instillation in the rat. Toxicol. Appl. Pharmacol 40, 188–199. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Clarke RW, Krishna Murthy GG, et al. , 1998. Freshly generated stainless steel welding fume induces greater lung inflammation in rats as compared to aged fume. Toxicol. Lett 98 (1–2), 77–86. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Schwegler-Berry D, Mercer RR, 2013. Comparative microscopic study of human and rat lungs after overexposure to welding fume. Ann. Occup. Hyg 57 (9), 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini JM, Badding MA, Meighan TG, et al. , 2014. Evaluation of the pulmonary toxicity of a fume generated from a nickel-, copper-based electrode to be used as a substitute in stainless steel welding. Environ. Health Insights 8 (Suppl. 1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel FP, Wei W, Tang M, et al. , 2017. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet 49 (3), 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Cech TR, 2001. Pot1, the putative telomere end binding protein in fission yeast and humans. Science 292, 1171–1175. [DOI] [PubMed] [Google Scholar]
- Blasco MA, 2005. Telomeres and human disease: ageing, cancer and beyond. Nat. Rev. Genet 6, 611–622. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. , 2017. Structural insights into POT1-TPP1 interaction and POT1 C-terminal mutations in human cancer. Nat. Commun 10 (8), 14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T, 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Gene Dev. 19, 2100–2110. [DOI] [PubMed] [Google Scholar]
- de Lange T, 2010. How shelterin solves the telomere end-protection problem. Cold Spring Harbor Symp. Quant. Biol 75, 167–177. [DOI] [PubMed] [Google Scholar]
- de Lange T, 2018. Shelterin-mediated telomere protection. Annu. Rev. Genet 52, 223–247. [DOI] [PubMed] [Google Scholar]
- Denchi EL, de Lange T, 2007. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wang M, Tian T, et al. , 2019. The effect of hexavalent chromium on the incidence and mortality of human cancers: a meta-analysis based on published epidemiological cohort studies. Front. Oncol 9, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Errico A, Pasian S, Baratti A, et al. , 2009. A case-control study on occupational risk factors for sino-nasal cancer. Occup. Environ. Med 66, 448e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E, Geli V, 2007. How telomeres are replicated. Nat. Rev. Mol. Cell Biol 8, 825–838. [DOI] [PubMed] [Google Scholar]
- Guha N, Loomis D, Guyton KZ, et al. , 2017. Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol. 18 (5), 581–582. [DOI] [PubMed] [Google Scholar]
- Hernberg S, Westerholm P, Schultz-Larsen K, et al. , 1983. Nasal and sinonasal cancer. Connection with occupational exposures in Denmark, Finland and Sweden. Scand. J. Work. Environ. Health 9, 315e26. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Sfeir AJ, Shay JW, Wright WE, de Lange T, 2005. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24, 2667–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, et al. , 2007. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat. Struct. Mol. Biol 14, 754–761, 2007. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhang X, Gawron AJ, et al. , 2012. Surrogate tissue telomere length and cancer risk: shorter or longer. Cancer Lett. 319, 130–135. [DOI] [PubMed] [Google Scholar]
- Kjuus H, Skjaerven R, Langard S, Lien JT, Aamodt T, 1986. A case-referent study of lung cancer, occupational exposures and smoking. I. Comparison of title-based and exposure-based occupational information. Scand. J. Work. Environ. Health 12, 193e202. [DOI] [PubMed] [Google Scholar]
- Li JS, Miralles Fusté J, Simavorian T, Bartocci C, Tsai J, et al. , 2017. TZAP: a telomere-associated protein involved in telomere length control. Science 355 (6325), 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Wellinger RJ, 2012. Telomeres and telomerase dance to the rhythm of the cell cycle. Trends Biochem. Sci 37, 391–399. [DOI] [PubMed] [Google Scholar]
- Martinez P, Blasco MA, 2011. Telomeric and extratelomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 11, 161–176. [DOI] [PubMed] [Google Scholar]
- Rai R, Chen Y, Lei M, Chang S, 2016. TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination mediated deletions and fusions. Nat. Commun 4 (7), 10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Rhodes D, 2014. Telomerase structure. Curr. Opin. Struct. Biol 25, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, de Lange T, 2012. Removal of shelterin reveals the telomere end protection problem. Science 336, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferlazza SJ, Beckett WS, 1991. The respiratory health of welders. Am. Rev. Respir. Dis 143, 1134–1148. [DOI] [PubMed] [Google Scholar]
- Shoeb M, Kodali VK, Farris BY, et al. , 2017a. Oxidative Stress, DNA methylation, and telomere length changes in peripheral blood mononuclear cells after pulmonary exposure to metal-rich welding nanoparticles. NanoImpact 5, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Kodali VB, Farris LM, et al. , 2017b. Evaluation of the molecular mechanisms associated with cytotoxicity and inflammation after pulmonary exposure to different metal-rich welding particles. Nanotoxicology 11 (6), 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Joseph P, Kodali V, et al. , 2017c. Silica inhalation altered telomere length and gene expression of telomere regulatory proteins in lung tissue of rats. Sci. Rep 7 (1), 17284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Mustafa G, Joseph J, et al. , 2019. Initiation of pulmonary fibrosis after silica inhalation in rats is linked with dysfunctional shelterin complex and DNA damage response. Sci. Rep 9, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Mustafa GM, Kodali VK, et al. , 2020. A possible relationship between telomere length and markers of neurodegeneration in rat brain after welding fume inhalation exposure. Environ. Res 180, 108900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Meier HCS, Antonini JM, 2021. Telomeres in toxicology: occupational health. Pharmacol. Therapeut 220, 107742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Zarus G, Abadin H, 2023. Profiling metal-induced genotoxic endpoints. JEH 86 (3). Nov-Dec. [Google Scholar]
- Siew SS, Kauppinen T, Kyyronen P, et al. , 2008. Exposure to iron and welding fumes and the risk of lung cancer. Scand. J. Work. Environ. Health 34, 444e50. [DOI] [PubMed] [Google Scholar]
- Wang F, et al. , 2007. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC1, Erdely A, Antonini JM, 2012. Immunotoxicology of arc welding fume: worker and experimental animal studies. J. Immunot 9 (4), 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


