Abstract
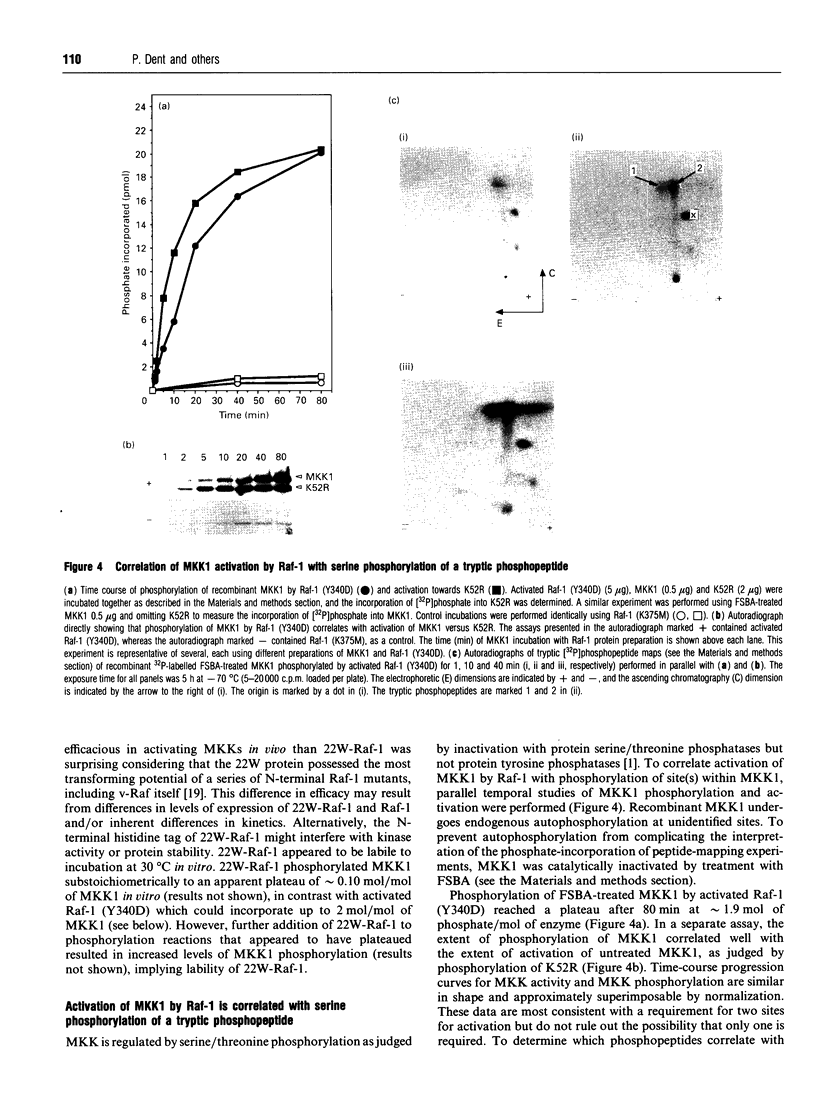
Mitogen-activated protein (MAP) kinase kinases (MKKs) are dual-specificity protein kinases which activate p42mapk and p44mapk by phosphorylation of regulatory tyrosine and threonine residues. cDNAs for two isotypes of MKK, MKK1 and MKK2, have been isolated from several species. Here we describe construction of recombinant baculoviruses for high-level expression of histidine-tagged rat MKK1 and MKK2, and procedures for production of nearly homogeneous MKK1 and MKK2 fusion proteins, in both inactive and active forms. Co-infection of Sf9 cells with either MKK1 or MKK2 virus together with recombinant viruses for Raf-1, pp60src (Y527F) and c-Ha-Ras resulted in activations of 250-fold and 150-fold for MKK1 and MKK2 respectively. Specific activities towards kinase-defective p42mapk were of the order of several hundred nanomoles of phosphate transferred/min per mg of MKK protein. The Michaelis constants for both enzymes were approx. 1 microM. Preparations of activated MKK were apparently free of Raf-1 as assessed by Western blotting. Raf-1 phosphorylated MKK1 on one major tryptic phosphopeptide, the phosphorylation of which increased with time. This phosphopeptide contained only phosphoserine and possessed neutral overall charge at pH 1.9 on two-dimensional peptide mapping. Phosphorylation of MKK1 by Raf-1 correlated with activation and reached a plateau of approximately 2 mol/mol.
Full text
PDF




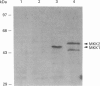
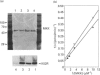
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M. H., Robbins D. J., Boulton T. G. ERKs, extracellular signal-regulated MAP-2 kinases. Curr Opin Cell Biol. 1991 Dec;3(6):1025–1032. doi: 10.1016/0955-0674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Fabian J. R., Daar I. O., Morrison D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993 Nov;13(11):7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Seth A., Raden D. L., Bowman D. S., Fay F. S., Davis R. J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993 Sep;122(5):1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Dent P., Wu J., Haystead C. M., Sturgill T. W. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 1992 Jul 13;306(1):17–22. doi: 10.1016/0014-5793(92)80828-5. [DOI] [PubMed] [Google Scholar]
- Huang W., Alessandrini A., Crews C. M., Erikson R. L. Raf-1 forms a stable complex with Mek1 and activates Mek1 by serine phosphorylation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10947–10951. doi: 10.1073/pnas.90.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma S. C., McGlynn E., Siegmann M., Reinhard C., Ferrari S., Thomas G. Active baculovirus recombinant p70s6k and p85s6k produced as a function of the infectious response. J Biol Chem. 1993 Apr 5;268(10):7134–7138. [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lenormand P., Sardet C., Pagès G., L'Allemain G., Brunet A., Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993 Sep;122(5):1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Marshall M. S., Gibbs J. B., Jove R. Reconstitution of interactions between the Src tyrosine kinases and Ras GTPase-activating protein using a baculovirus expression system. J Biol Chem. 1992 Jun 5;267(16):11612–11618. [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Dent P., Sturgill T. W., Marshak D. R. Mitogen-activated protein kinase kinase 1 (MKK1) is negatively regulated by threonine phosphorylation. Mol Cell Biol. 1994 Mar;14(3):1594–1602. doi: 10.1128/mcb.14.3.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Gonzalez F. A., Gupta S., Raden D. L., Davis R. J. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992 Dec 5;267(34):24796–24804. [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C., Campbell D. G., Cohen P. Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2. Identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur J Biochem. 1993 Mar 1;212(2):581–588. doi: 10.1111/j.1432-1033.1993.tb17696.x. [DOI] [PubMed] [Google Scholar]
- Teague M. A., Chaleff D. T., Errede B. Nucleotide sequence of the yeast regulatory gene STE7 predicts a protein homologous to protein kinases. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7371–7375. doi: 10.1073/pnas.83.19.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S., Gomez N., Paterson H., Marshall C., Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992 Dec 1;288(Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Dent P., Lynch K. R., Weber M. J., Sturgill T. W. Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol. 1993 Aug;13(8):4539–4548. doi: 10.1128/mcb.13.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Harrison J. K., Vincent L. A., Haystead C., Haystead T. A., Michel H., Hunt D. F., Lynch K. R., Sturgill T. W. Molecular structure of a protein-tyrosine/threonine kinase activating p42 mitogen-activated protein (MAP) kinase: MAP kinase kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):173–177. doi: 10.1073/pnas.90.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994 Mar 1;13(5):1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem. 1993 May 25;268(15):11435–11439. [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]