Abstract
We perform molecular dynamics (MD) simulations of a nanoscale
water
capillary bridge (WCB) surrounded by carbon dioxide over a wide range
of temperatures and pressures (T = 280–400
K and carbon dioxide pressures 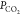 ≈ 0–80 MPa). The water–carbon
dioxide system is confined by two parallel silica-based surfaces (hydroxylated
β-cristobalite) separated by h = 5 nm. The
aim of this work is to study the WCB contact angle (θc) as a function of T and
≈ 0–80 MPa). The water–carbon
dioxide system is confined by two parallel silica-based surfaces (hydroxylated
β-cristobalite) separated by h = 5 nm. The
aim of this work is to study the WCB contact angle (θc) as a function of T and 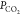 . Our simulations indicate that θc varies weakly with temperature and pressure: Δθc ≈ 10–20° for
. Our simulations indicate that θc varies weakly with temperature and pressure: Δθc ≈ 10–20° for 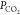 increasing from ≈0 to 80 MPa (T = 320 K); Δθc ≈ −10°
for T increasing from 320 to 360 K (with a fixed
amount of carbon dioxide). Interestingly, at all conditions studied,
a thin film of water (1–2 water layers-thick) forms under the
carbon dioxide volume. Our MD simulations suggest that this is due
to the enhanced ability of water, relative to carbon dioxide, to form
hydrogen-bonds with the walls. We also study the effects of adding
salt (NaCl) to the WCB and corresponding θc. It is
found that at the salt concentrations studied (mole fractions xNa = xCl = 3.50,
9.81%), the NaCl forms a large crystallite within the WCB with the
ions avoiding the water–carbon dioxide interface and the walls
surface. This results in θc being insensitive to
the presence of NaCl.
increasing from ≈0 to 80 MPa (T = 320 K); Δθc ≈ −10°
for T increasing from 320 to 360 K (with a fixed
amount of carbon dioxide). Interestingly, at all conditions studied,
a thin film of water (1–2 water layers-thick) forms under the
carbon dioxide volume. Our MD simulations suggest that this is due
to the enhanced ability of water, relative to carbon dioxide, to form
hydrogen-bonds with the walls. We also study the effects of adding
salt (NaCl) to the WCB and corresponding θc. It is
found that at the salt concentrations studied (mole fractions xNa = xCl = 3.50,
9.81%), the NaCl forms a large crystallite within the WCB with the
ions avoiding the water–carbon dioxide interface and the walls
surface. This results in θc being insensitive to
the presence of NaCl.
Introduction
To achieve net-zero carbon emissions, a broad suite of technologies should be deployed to transform the energy landscape. Carbon capture and storage (CCS) technologies play a pivotal role because they contribute both to reducing emissions in key sectors directly and to removing carbon dioxide (CO2) to balance emissions from hard-to-abate industries.1 After years of slow progress, CCS is gaining momentum behind new investment incentives and strengthened climate goals.
During commercial scale carbon capture and storage operations, CO2 is separated and captured from industrial sources and injected deep into and stored in a porous rock formation, such as a depleted hydrocarbon reservoir or saline aquifer. Researchers have proposed other geologic storage options, including in the form of gas hydrates,2 CO2 storage with enhanced gas recovery,3 and enhanced geothermal systems (EGS) using CO2 as working fluid.4 The target storage formations are greater than 800 m in depth to ensure the injected CO2 is in the supercritical state (dense phase), i.e., temperatures >304.25 K and pressure >7.4 MPa (1071 psia).5 Conditions for geologic CO2 storage typically range between 10–50 MPa in pressure and between 305–393 K in temperature6 so CO2 remains buoyant because its mass density is about 20% to 50% lower than that of brine. After injection, the buoyancy-driven vertically migrating CO2 plume will eventually reach the caprock and be physically held in place by low permeability caprock layers above the storage formation;7,8 see Figure 1.
Figure 1.

Schematic of CO2 structural trapping by a sealing caprock: a buoyant CO2 column is held by capillary forces at the caprock. The capillary pressure (eq 1) is a function of wetting properties: interfacial tension σ, contact angle θ, and the pore diameter ΦP.
There are four mechanisms that trap CO2 in sedimentary rocks.6 (i) Dissolution trapping occurs when injected CO2 dissolves within the formation brine. (ii) This dissolved CO2 can react with rock minerals over long time periods to form carbonate minerals resulting in mineral trapping. (iii) Residual trapping occurs when capillary forces trap “ganglia” of CO2 within pore spaces. However, (iv) structural trapping is the primary trapping mechanism for the first few decades after CO2 injection, where the caprock acts as a seal both in terms of its low permeability and its high capillary entry pressure.9,10
From a macroscopic perspective, the capillary breakthrough pressure Pc is the maximum pressure difference that exists across the CO2–brine interface before CO2 percolates across the porous medium8,11,12 (Figure 1). The capillary breakthrough pressure can be described using the well-known Young–Laplace equation:
| 1 |
where γ is the interfacial tension between CO2 and brine, θc is the contact angle of the interface with the mineral surface, and ΦP is the smallest equivalent pore throat diameter along the CO2 breakthrough path. CO2–brine interfacial tension ranges between 20–30 mN/m for typical CO2 storage conditions.11,13−16 The contact angle depends on the wetting properties of the caprock minerals in contact with brine and CO2 and is used as an indirect method to estimate the effectiveness of the caprock seal. If the caprock minerals are water wet, Pc is positive, the contact angle is less than 90°, and the pores will retain the buoyant CO2.17,18 If the caprock minerals are CO2 wet, Pc is negative, the contact angle is greater than 90°, and the CO2 is expected to be pulled into pores, potentially leading to leakage.8 Contact angle measurements have been reported extensively in the literature on a variety of rock samples including variations in pressure, temperature, and salinity. Most contact angle measurements of water/brine and CO2 on quartz and clay substrates report contact angle <50° at temperatures ranging from 296 to 323 K and pressures from 0.1 to 25 MPa.14,16,19−28 A few studies report contact angle increase with pressure with most of the change happening at lower pressures (below 10 MPa) and small changes in the supercritical CO2 regime.19,29−31
Molecular dynamics (MD) simulation studies have covered a broad range of temperatures (298–373 K), pressures (1–20 MPa), and salinities (0–6 M NaCl) and generally predict higher contact angles than experimental measurements. Most studies show the following trends: (i) slight decrease of contact angle (rock becoming more water-wet) with increasing temperature;32−34 (ii) increase in contact angle with pressure from 1 to 10 MPa and small changes in the supercritical CO2 regime but all mineral surfaces remain strongly water-wet;17,26,33−35 (iii) no evidence for a systematic increase or decrease of the contact angle and interfacial tension with salt concentration.17,33,36
In this study, we extend previous MD simulation work to temperatures (up to 400 K), pressures (up to 80 MPa), and salinities (up to 9.81 mol %) targeted for geologic CO2 storage to address the question as to whether CO2 will become the wetting phase instead of brine. This work is organized as follows. We first discuss the computer simulations details. We then present the results where we discuss the effects of temperature, carbon dioxide concentration, and salt (NaCl) on the hydration and contact angle of the water capillary bridge (WCB). We conclude with a brief summary where we discuss the implications of our findings to geological carbon storage.
Methods
We perform MD simulations of a WCB surrounded
by carbon dioxide
at T = 280–400 K and for (estimated) CO2 pressures in the range 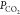 = 0–80 MPa. The WCB and carbon dioxide
volume are confined by two parallel silica-based (β-cristobalite)
walls. A snapshot of one of the systems studied is shown in Figure 2. The WCB is oriented
along the z-axis, from one wall to the other, and
is surrounded by carbon dioxide. The walls extend across the system
box along the x- and y-directions.
The system is periodic along the x-, y-, and z-directions with dimensions Lx = Ly = 140.000 Å and Lz = 25.987 Å; the separation between the walls is h = 50 Å and a large empty space is left behind the
walls in order to minimize any effect from the system periodicity
(along the z-direction). Given the geometry considered,
the WCB that form in our MD simulations are translationally symmetric
along the y-axis and hence, the WCB profiles depend
only on the z (WCB height) and x coordinates (WCB thickness).
= 0–80 MPa. The WCB and carbon dioxide
volume are confined by two parallel silica-based (β-cristobalite)
walls. A snapshot of one of the systems studied is shown in Figure 2. The WCB is oriented
along the z-axis, from one wall to the other, and
is surrounded by carbon dioxide. The walls extend across the system
box along the x- and y-directions.
The system is periodic along the x-, y-, and z-directions with dimensions Lx = Ly = 140.000 Å and Lz = 25.987 Å; the separation between the walls is h = 50 Å and a large empty space is left behind the
walls in order to minimize any effect from the system periodicity
(along the z-direction). Given the geometry considered,
the WCB that form in our MD simulations are translationally symmetric
along the y-axis and hence, the WCB profiles depend
only on the z (WCB height) and x coordinates (WCB thickness).
Figure 2.

(a) Snapshot of the system from an MD
simulation of water (N = 2756) and carbon dioxide
(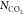 = 1114). Water molecules (center) form
a capillary bridge that is surrounded by carbon dioxide molecules
[the carbon dioxide molecules also form a capillary bridge (split
due to periodic boundary conditions)]. The z- and x-axis are shown; the origin o of the xz-reference frame is located at the midpoint between the
walls (z = 0). (b) Top and (c) side view of a section
of the silica walls employed (β-cristobalite). The top view
shows the silica tetrahedra forming an hexagonal structure with three
silanol groups per hexagon; the silanol groups arrange in a triangular
lattice. Only the wall surface in contact with the confined water/carbon
dioxide is hydroxylated. The planes containing the H atoms of the
walls are located at z = ±25 Å and hence,
the separation between the walls (defined as the distance between
the planes containing the H atoms of each wall) is h = 50 Å.
= 1114). Water molecules (center) form
a capillary bridge that is surrounded by carbon dioxide molecules
[the carbon dioxide molecules also form a capillary bridge (split
due to periodic boundary conditions)]. The z- and x-axis are shown; the origin o of the xz-reference frame is located at the midpoint between the
walls (z = 0). (b) Top and (c) side view of a section
of the silica walls employed (β-cristobalite). The top view
shows the silica tetrahedra forming an hexagonal structure with three
silanol groups per hexagon; the silanol groups arrange in a triangular
lattice. Only the wall surface in contact with the confined water/carbon
dioxide is hydroxylated. The planes containing the H atoms of the
walls are located at z = ±25 Å and hence,
the separation between the walls (defined as the distance between
the planes containing the H atoms of each wall) is h = 50 Å.
All systems considered are composed of N = 2756
water molecules while the number of carbon dioxide molecules varies
in the range 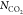 = 0–1502 (additional MD simulations
are performed using WCB composed of N = 1144 water
molecules; see Supporting Information).
We note that the WCB with N = 2756 have a minimum
thickness of ≈6 nm and hence, they are sufficiently large so
the different (wall–water and water–vapor/CO2) interfaces are separated by a volume of bulk-like water molecules
that is at least 2–3 nm-thick. As shown in ref.37, for capillarity theory/macroscopic
thermodynamics to correctly describe the profiles of nanoscale droplets
and WCB, the water–wall and water–vapor interfaces should
be separated by at least ≈1 nm.37 Water molecules are modeled using the SPC/E rigid water model38 while the EPM2 flexible model is used to represent
the CO2 molecules39 (see also
ref.40). The models
are validated in the Supporting Information where we compare a few properties obtained from MD simulations and
experiments. Each wall is composed of 2352 atoms and is modeled after
β-cristobalite. The structure of the walls is described in detail
in refs (41−43). Briefly, the walls are composed
of silica tetrahedra pointing perpendicularly to the walls surface.
The walls are hydroxylated on the confining surface and, hence, both
water and carbon dioxide molecules can form hydrogen-bonds (HB) with
the walls silanol groups. As in previous studies,42 the O and Si atoms of the walls are immobile during the
MD simulations. The H atoms of the surface silanol groups are able
to rotate in a plane parallel to the wall surface, and about the direction
defined by the O–Si covalent bond of the corresponding silanol
group.41,42
= 0–1502 (additional MD simulations
are performed using WCB composed of N = 1144 water
molecules; see Supporting Information).
We note that the WCB with N = 2756 have a minimum
thickness of ≈6 nm and hence, they are sufficiently large so
the different (wall–water and water–vapor/CO2) interfaces are separated by a volume of bulk-like water molecules
that is at least 2–3 nm-thick. As shown in ref.37, for capillarity theory/macroscopic
thermodynamics to correctly describe the profiles of nanoscale droplets
and WCB, the water–wall and water–vapor interfaces should
be separated by at least ≈1 nm.37 Water molecules are modeled using the SPC/E rigid water model38 while the EPM2 flexible model is used to represent
the CO2 molecules39 (see also
ref.40). The models
are validated in the Supporting Information where we compare a few properties obtained from MD simulations and
experiments. Each wall is composed of 2352 atoms and is modeled after
β-cristobalite. The structure of the walls is described in detail
in refs (41−43). Briefly, the walls are composed
of silica tetrahedra pointing perpendicularly to the walls surface.
The walls are hydroxylated on the confining surface and, hence, both
water and carbon dioxide molecules can form hydrogen-bonds (HB) with
the walls silanol groups. As in previous studies,42 the O and Si atoms of the walls are immobile during the
MD simulations. The H atoms of the surface silanol groups are able
to rotate in a plane parallel to the wall surface, and about the direction
defined by the O–Si covalent bond of the corresponding silanol
group.41,42
We also perform MD simulations of WCB containing salt. In these cases, the WCB contains equal numbers of Na+ and Cl– ions, NNa = NCl = N0 with N0 = 100, 300 (N = 2756). The Na+ and Cl– ions are modeled using the OPLS force field.44 As shown in Figure 3a, the surfaces considered are hydrophilic due to the ability of the wall silanol groups to form HB with the water molecules. As shown previously,42,45 the water contact angle for the studied surfaces is θc < 10–20°. Accordingly, the water molecules cover the whole wall surface, forming a thin film. The WCB that we study lie on the water films that remain adsorbed at the walls surface. For comparison, we include in Figure 3b the WCB that forms when the wall partial charges (located at the walls silanol groups) are removed, and the H atoms at the wall surfaces effectively vanish (see, e.g., ref.41). In this case, there is no water film covering the wall and the contact angle of water is >90°, i.e., the walls become hydrophobic; see also Figure S4.
Figure 3.

(a) A water capillary bridge formed between
two β-cristobalite
walls separated by h = 50 Å. The walls are hydrophilic
and are covered by a thin film of water above which lays the water
capillary bridge. (b) Same as (a) but after removing the walls partial
charges [when this is done, the surface H atoms (green spheres) have
no interactions with water and effectively vanish]. The surfaces are
hydrophobic with a water contact angle θc ≈
108°. (c), (d) Carbon dioxide confined between two β-cristobalite
walls separated by h = 50 Å (no water is present; 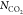 = 1670). Temperatures are (c) T = 320 K (supercritical carbon dioxide), and (d) T = 150 K (liquid carbon dioxide). The walls are solvophilic (i.e.,
they are appealing to the CO2 molecules) and are covered
by a thin film of carbon dioxide.
= 1670). Temperatures are (c) T = 320 K (supercritical carbon dioxide), and (d) T = 150 K (liquid carbon dioxide). The walls are solvophilic (i.e.,
they are appealing to the CO2 molecules) and are covered
by a thin film of carbon dioxide.
Our β-cristobalite surfaces are also solvophilic meaning that they are also appealing to CO2. As shown in Figure 3c,d, in the absence of water, the CO2 molecules cover the wall surface area. Moreover, at low temperatures for which carbon dioxide is stable in the liquid state, a carbon dioxide capillary bridge (CDCB) forms on top of carbon dioxide films adsorbed on the wall surfaces. Indeed, Figure 3d is reminiscent of Figure 3a for the case of water. The solvophilicity of the surface to CO2 can be explained by the ability of the surface silanol groups to form HB with the CO2 molecules. However, as shown in the Supporting Information, even when the partial charges of the walls (and the H atoms of the corresponding silanol groups) are removed, the walls remain solvophilic to CO2 (see Figure S3). It follows that, contrary to the case of water, the walls are appealing to CO2 not only due to the formation of wall–CO2 hydrogen-bonds but also due to the wall–CO2 Lennard-Jones interactions (see Supporting Information).
All MD simulations are performed using the LAMMPS software package.46 Simulations are performed for 20 ns; the first 10 ns are used for equilibration and the remaining 10 ns are used for data analysis. The simulation time step is dt = 0.001 ps. Our simulations seem to be long enough for the WCB to reach equilibrium; however, we cannot exclude the possibility that the WCB studied here (and in other computational studies) remain metastable during the simulated time – a limitation inherent to all MD simulations.47−50 MD simulations are performed at constant volume and temperature; the temperature is maintained using a Nosé–Hoover style thermostat with a coupling time constant of 0.1 ps. Electrostatic interactions are calculated using the particle–particle particle–mesh solver51 with a cut off distance of rcutoff = 10.0 Å. The same cut off distance is used to calculate the Lennard-Jones (LJ) interactions. Since we employ the same computational techniques used in our previous studies, we refer the reader to refs (42,50) for additional details.
Calculation of the Capillary Bridge Profiles
The WCB
profiles are calculated from 2000 snapshots taken every 5 ps during
the last 10 ns of the simulation. The procedure to calculate the WCB
is described in detail in ref.42. Briefly, for each snapshot, we first define a z-axis passing through the center of mass of the WCB, perpendicular
to the walls. The WCB is then covered with 20 overlapping slabs of
thickness 5 Å parallel to the walls and shifted vertically by
2.5 Å with respect to each other. For each slab, centered at
a distance z from the midpoint between the walls 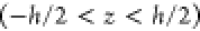 , we calculate the average density of water
ρslab-z(x) as a function of the distance x (WCB thickness)
from the z-axis [Figure 2a]. As expected, ρslab-z(x) is constant within the
WCB and it decays abruptly to practically zero in the vapor phase
or CO2 volume. Hence, we define the thickness of the WCB
at height z, xMD(z), as the distance x at which ρslab-z(x) = ρ0 = 0.2 g/cm3 (our results are not sensitive to
slight variations in ρ0). The function xMD(z) provides the WCB profile for the
given snapshot. By averaging xMD(z) over the 2000 snapshots considered, we obtain the average
WCB profile.
, we calculate the average density of water
ρslab-z(x) as a function of the distance x (WCB thickness)
from the z-axis [Figure 2a]. As expected, ρslab-z(x) is constant within the
WCB and it decays abruptly to practically zero in the vapor phase
or CO2 volume. Hence, we define the thickness of the WCB
at height z, xMD(z), as the distance x at which ρslab-z(x) = ρ0 = 0.2 g/cm3 (our results are not sensitive to
slight variations in ρ0). The function xMD(z) provides the WCB profile for the
given snapshot. By averaging xMD(z) over the 2000 snapshots considered, we obtain the average
WCB profile.
The procedure described so far to obtain xMD(z) applies to an isolated WCB and to a WCB surrounded by CO2 molecules – in the presence of carbon dioxide, only a small number of CO2 molecules are able to diffuse within the WCB (see below). However, the situation is different when salt is present since Na+ and Cl– ions remain within the WCB. Accordingly, when the WCB contains NaCl, we do not calculate the density profile ρslab-z(x) but, instead, we obtain the number density nslab-z(x) of the water O, as well as the Na+ and Cl- ions. Specifically, in the calculation of nslab-z(x), the water O, Na+, and Cl– ions all are treated identically as a “particle”. As expected, nslab-z(x) is constant within the WCB and it decays abruptly to zero in the vapor phase or CO2 volume. Hence, we define the thickness of the WCB (containing NaCl) at height z, xMD(z), as the distance x at which nslab-z(x) decays to n0 = 6.685 nm–3. This choice for the value of n0 is roughly equivalent to the condition ρslab-z(x) = ρ0 = 0.2 g/cm3 used to define xMD(z) in the case of WCB containing no salt.
Calculation of the Water Contact Angle from the Water Capillary Bridge Profile
Capillarity theory predicts that the profile of a translationally symmetric capillary bridge, such as the WCB formed in our MD simulations, should be a circle of radius R2 and centered at (xc,zc), where zc = 0 and xc = r0 – R2 (r0 is half the thickness of the capillary bridge at z = 0); see ref. (42). Accordingly, to calculate the contact angle of water from the WCB obtained in our simulations, we fit the corresponding average WCB profile xMD(z) with a circle:
| 2 |
The function x(z) that best fits the WCB profile xMD(z) using eq 2 can be used to calculate the contact angle of water. Specifically,
we get θc from the function x(z) using the expression 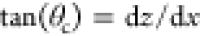 at z = z0. In principle, one would need to evaluate this expression
at the wall surface, i.e., z0 = h/2. However, as shown below, the walls studied are always
covered by a film of water which makes it unclear how to define the
height z0 at which the WCB ends and merges
with the water film adsorbed at the wall surface. In our simulations,
to avoid any artifact due to the water films on the walls, we only
fit the average WCB profile xMD(z) for |z| < 17.5 Å, and estimate
θc using z0 = 17 Å.
For comparison, we also estimate θc using the same
procedure described above but, instead of using eq 2, we fit the WCB profile with a second order
polynomial.
at z = z0. In principle, one would need to evaluate this expression
at the wall surface, i.e., z0 = h/2. However, as shown below, the walls studied are always
covered by a film of water which makes it unclear how to define the
height z0 at which the WCB ends and merges
with the water film adsorbed at the wall surface. In our simulations,
to avoid any artifact due to the water films on the walls, we only
fit the average WCB profile xMD(z) for |z| < 17.5 Å, and estimate
θc using z0 = 17 Å.
For comparison, we also estimate θc using the same
procedure described above but, instead of using eq 2, we fit the WCB profile with a second order
polynomial.
Results and Discussion
The results are organized as follows. We first discuss the walls hydration and the properties of the WCB in the presence of carbon dioxide at different temperatures and pressures. We then discuss the effects of adding salt (NaCl) on the wetting and WCB properties.
Water Capillary Bridges in the Presence of Carbon Dioxide
Wall Hydration
In order to characterize the hydration
of the walls, we first calculate the water and carbon dioxide density
profiles within the WCB and along the direction perpendicular
to the walls (z-direction), 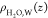 and
and 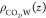 . To do so, we calculate the center of mass
of the WCB and consider only those H2O/CO2 molecules
within a distance Δx = 20 Å along the x-axis from the WCB center of mass. The value of Δx is small enough to exclude any artifact due to the water–carbon
dioxide interface. Figure 4a shows
. To do so, we calculate the center of mass
of the WCB and consider only those H2O/CO2 molecules
within a distance Δx = 20 Å along the x-axis from the WCB center of mass. The value of Δx is small enough to exclude any artifact due to the water–carbon
dioxide interface. Figure 4a shows 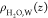 and
and 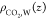 at T = 320 K and for different
amounts of carbon dioxide. At these conditions, carbon dioxide is
supercritical since the critical temperature of the flexible EPM2
carbon dioxide model is Tc ≈ 313
K.39 Nonetheless, similar results are obtained
at T = 280–400 K. Figure 4a shows that
at T = 320 K and for different
amounts of carbon dioxide. At these conditions, carbon dioxide is
supercritical since the critical temperature of the flexible EPM2
carbon dioxide model is Tc ≈ 313
K.39 Nonetheless, similar results are obtained
at T = 280–400 K. Figure 4a shows that 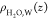 is practically independent of the presence
of CO2. In particular,
is practically independent of the presence
of CO2. In particular, 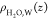 exhibits two maxima next to the walls,
at approximately |z| = 24.5 Å and |z| = 22.0 Å, indicating the formation of two well-defined hydration
layers next to the walls. At distances ≈8–10 Å
away from the wall surfaces, the density of water within the WCB is
constant and approximately equal to the density of bulk SPC/E water
at T = 320 K and low pressures.52 As shown in Figure 4a,
exhibits two maxima next to the walls,
at approximately |z| = 24.5 Å and |z| = 22.0 Å, indicating the formation of two well-defined hydration
layers next to the walls. At distances ≈8–10 Å
away from the wall surfaces, the density of water within the WCB is
constant and approximately equal to the density of bulk SPC/E water
at T = 320 K and low pressures.52 As shown in Figure 4a, 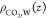 is rather small for all values of z indicating that only a few CO2 molecules are
able to diffuse into the WCB (the mass fraction of carbon dioxide
is <10%). The few CO2 molecules within the WCB tend
to locate preferentially at approximately |z| = 23
Å, in between water first and second hydration layers. Therefore,
under the WCB, the walls are preferentially solvated by water, as
expected.
is rather small for all values of z indicating that only a few CO2 molecules are
able to diffuse into the WCB (the mass fraction of carbon dioxide
is <10%). The few CO2 molecules within the WCB tend
to locate preferentially at approximately |z| = 23
Å, in between water first and second hydration layers. Therefore,
under the WCB, the walls are preferentially solvated by water, as
expected.
Figure 4.
(a) Density profile of water (upper solid lines; 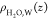 ) and carbon dioxide (lower dashed lines;
) and carbon dioxide (lower dashed lines;  ) within the water capillary bridge, and
along the direction perpendicular to the walls. Results are for T = 320 K and different number of carbon dioxide molecules
) within the water capillary bridge, and
along the direction perpendicular to the walls. Results are for T = 320 K and different number of carbon dioxide molecules  .
. 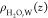 is practically independent of the amount
of carbon dioxide in the system; the two maxima next to the walls
(located at z = ± 25 Å) indicate that two
well-defined water layers form next to each wall.
is practically independent of the amount
of carbon dioxide in the system; the two maxima next to the walls
(located at z = ± 25 Å) indicate that two
well-defined water layers form next to each wall. 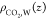 is small, i.e., the amount of CO2 within the WCB is minor at all conditions studied. The walls under
the WCB are solvated preferentially by water. (b) Density profile
of water (solid lines;
is small, i.e., the amount of CO2 within the WCB is minor at all conditions studied. The walls under
the WCB are solvated preferentially by water. (b) Density profile
of water (solid lines; 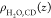 ) and carbon dioxide (dashed lines;
) and carbon dioxide (dashed lines; 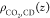 ) within the carbon-dioxide capillary bridge
(CDCB), and along the direction perpendicular to the walls; T = 320 K. The maxima of
) within the carbon-dioxide capillary bridge
(CDCB), and along the direction perpendicular to the walls; T = 320 K. The maxima of  indicate that water molecules preferentially
hydrate the walls (forming a thin film of water separating the carbon
dioxide volume and the walls). Not surprisingly,
indicate that water molecules preferentially
hydrate the walls (forming a thin film of water separating the carbon
dioxide volume and the walls). Not surprisingly, 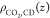 varies considerably with the amount of
carbon dioxide in the system, with densities from 0.25 g/cm3 (gas) to ≈1.1 g/cm3 (liquid) at the midpoint between
the walls (z = 0).
varies considerably with the amount of
carbon dioxide in the system, with densities from 0.25 g/cm3 (gas) to ≈1.1 g/cm3 (liquid) at the midpoint between
the walls (z = 0).
We also calculate the water and carbon dioxide
density profiles within the carbon dioxide capillary bridge
(CDCB) and along
the direction perpendicular to the walls (z-direction), 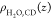 and
and  . To do so, we first calculate the center
of mass of the CDCB, and consider only those molecules within a distance
Δx = 20 Å from the CDCB center of mass.
Again, the value of Δx is small enough to exclude
any artifact due to the water–carbon dioxide interface. Figure 4b shows
. To do so, we first calculate the center
of mass of the CDCB, and consider only those molecules within a distance
Δx = 20 Å from the CDCB center of mass.
Again, the value of Δx is small enough to exclude
any artifact due to the water–carbon dioxide interface. Figure 4b shows 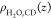 and
and 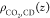 at T = 320 K for different
amounts of carbon dioxide (similar results are obtained at T = 280–400 K).
at T = 320 K for different
amounts of carbon dioxide (similar results are obtained at T = 280–400 K).  shows a single peak at |z| = 24.5 Å, practically independent of the presence of CO2, indicating the formation of a thin film of water adsorbed
at the wall surface. Interestingly,
shows a single peak at |z| = 24.5 Å, practically independent of the presence of CO2, indicating the formation of a thin film of water adsorbed
at the wall surface. Interestingly, 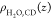 ≈ 0 for −19 < x < 19 Å meaning that water molecules do not diffuse
into the carbon dioxide volume. The CO2 molecules form
1–2 layers close to the walls. The first maximum of
≈ 0 for −19 < x < 19 Å meaning that water molecules do not diffuse
into the carbon dioxide volume. The CO2 molecules form
1–2 layers close to the walls. The first maximum of 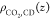 is located at |z| = 23
Å and, hence, the carbon dioxide volume is separated from the
walls by approximately one layer of water molecules.
is located at |z| = 23
Å and, hence, the carbon dioxide volume is separated from the
walls by approximately one layer of water molecules.
The results
discussed so far are for T = 320 K
but qualitatively similar results are found at T =
280, 300, 340, 360, and 400 K. To show this, we include in Figure 5a the 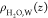 and
and 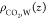 obtained for
obtained for 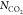 = 1114 and at all temperatures studied
(results for
= 1114 and at all temperatures studied
(results for 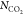 = 1502 are included in the Supporting Information). The main effect of increasing
the temperature, for a fixed
= 1502 are included in the Supporting Information). The main effect of increasing
the temperature, for a fixed  , is to decrease the height of the maxima
in
, is to decrease the height of the maxima
in 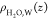 close to the walls and the density of the
“bulk” water away from the walls (i.e., at −19
< x < 19 Å). The decrease in the water
density within the WCB leads to an increase in
close to the walls and the density of the
“bulk” water away from the walls (i.e., at −19
< x < 19 Å). The decrease in the water
density within the WCB leads to an increase in 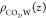 for all values of z. In
other words, more CO2 molecules are able to diffuse into
the WCB with increasing temperature, implying that the solubility
of CO2 in water increases upon heating. This may seem inconsistent
with experiments which show that the solubility of CO2 decreases
upon heating.53 However, experiments are
performed at constant pressure while our MD simulation are performed
at constant volume, and fixed amounts of water and carbon dioxide.
Nonetheless, the change of the carbon dioxide density within the WCB
is small,
for all values of z. In
other words, more CO2 molecules are able to diffuse into
the WCB with increasing temperature, implying that the solubility
of CO2 in water increases upon heating. This may seem inconsistent
with experiments which show that the solubility of CO2 decreases
upon heating.53 However, experiments are
performed at constant pressure while our MD simulation are performed
at constant volume, and fixed amounts of water and carbon dioxide.
Nonetheless, the change of the carbon dioxide density within the WCB
is small, 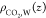 < 0.10–0.12 g/cm3 for
−20 < x < 20 Å, so the number of
CO2 molecules within the WCB remains low at all temperatures
studied. We also note that the locations of the water and carbon dioxide
layers within the WCB, in the proximity of the walls, do not change
with temperature.
< 0.10–0.12 g/cm3 for
−20 < x < 20 Å, so the number of
CO2 molecules within the WCB remains low at all temperatures
studied. We also note that the locations of the water and carbon dioxide
layers within the WCB, in the proximity of the walls, do not change
with temperature.
Figure 5.
(a) Temperature effects on the density profile of water
(upper
solid lines; 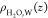 ) and carbon dioxide (lower dashed lines;
) and carbon dioxide (lower dashed lines; 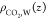 ) within the water capillary bridge, and
along the direction perpendicular to the walls. Results are for
) within the water capillary bridge, and
along the direction perpendicular to the walls. Results are for 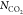 = 1114 and T = 280, 300,
320, 340, 360, 400 K (the critical temperature of carbon dioxide for
the model studied is Tc ≈ 313 K).
Varying the temperature causes slight changes in the local density
of water and carbon dioxide within the WCB but it does not affect
the location of the water/carbon dioxide layers (maxima in
= 1114 and T = 280, 300,
320, 340, 360, 400 K (the critical temperature of carbon dioxide for
the model studied is Tc ≈ 313 K).
Varying the temperature causes slight changes in the local density
of water and carbon dioxide within the WCB but it does not affect
the location of the water/carbon dioxide layers (maxima in 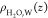 and
and  ) close to the walls. (b) Density profile
of water (solid lines;
) close to the walls. (b) Density profile
of water (solid lines; 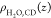 ) and carbon dioxide (dashed lines;
) and carbon dioxide (dashed lines; 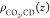 ) within the CDCB, and along the direction
perpendicular to the walls. Temperature variations affect slightly
the local density of water and carbon dioxide within the CDCB, increasing
the number of water molecules in the water films adsorbed at the walls
surfaces and pushing the CO2 molecules away from the walls
[toward the central region of the CDCB (z = 0)].
) within the CDCB, and along the direction
perpendicular to the walls. Temperature variations affect slightly
the local density of water and carbon dioxide within the CDCB, increasing
the number of water molecules in the water films adsorbed at the walls
surfaces and pushing the CO2 molecules away from the walls
[toward the central region of the CDCB (z = 0)].
The temperature effects on the density profiles
of water and carbon
dioxide within the CDCB are shown in Figure 5b. Included are the 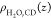 and
and 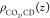 obtained for
obtained for 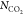 = 1114 at all temperatures studied (results
for
= 1114 at all temperatures studied (results
for 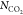 = 1502 are included in the Supporting Information). The main effect of increasing
the temperature, for a fixed value of
= 1502 are included in the Supporting Information). The main effect of increasing
the temperature, for a fixed value of 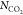 , is to thicken the water layer separating
the carbon dioxide volume and the walls. At the highest temperature
studied, T = 400 K, the water film on the walls seems
to be composed of two water layers while there is practically a single
water layer at T < 400 K. The thickening of the
water films next to the walls, with increasing temperature, leads
to a reduction of the volume available to the carbon dioxide. Accordingly,
as shown in Figure 5b, (i)
, is to thicken the water layer separating
the carbon dioxide volume and the walls. At the highest temperature
studied, T = 400 K, the water film on the walls seems
to be composed of two water layers while there is practically a single
water layer at T < 400 K. The thickening of the
water films next to the walls, with increasing temperature, leads
to a reduction of the volume available to the carbon dioxide. Accordingly,
as shown in Figure 5b, (i) 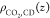 decreases in the proximity of the walls
as the temperature increases while (ii) it increases upon heating
at
decreases in the proximity of the walls
as the temperature increases while (ii) it increases upon heating
at 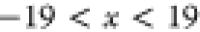 Å (corresponding to the “bulk”
carbon dioxide volume within the CDCB). Briefly, the CO2 molecules are pushed away from the wall as the temperature increases.
Å (corresponding to the “bulk”
carbon dioxide volume within the CDCB). Briefly, the CO2 molecules are pushed away from the wall as the temperature increases.
The results presented so far are given in terms of  . However, it is not practically feasible
to measure
. However, it is not practically feasible
to measure 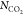 ; experiments usually have access to the
pressure of carbon dioxide,
; experiments usually have access to the
pressure of carbon dioxide, 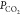 . We can estimate
. We can estimate 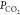 in our MD simulations [at a given (T,
in our MD simulations [at a given (T, 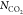 )] from the value of
)] from the value of 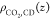 at z ≈ 0. Specifically,
the carbon dioxide within the CDCB at z ≈
0 is far from the walls and hence, it may be considered to have carbon
dioxide bulk-like properties. Hence, the pressure within the carbon
dioxide in our simulations can be estimated by the pressure of bulk
CO2 at a density equal to the value of
at z ≈ 0. Specifically,
the carbon dioxide within the CDCB at z ≈
0 is far from the walls and hence, it may be considered to have carbon
dioxide bulk-like properties. Hence, the pressure within the carbon
dioxide in our simulations can be estimated by the pressure of bulk
CO2 at a density equal to the value of 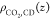 at z ≈ 0. We stress
that the pressure–density equation-of-state for bulk CO2 obtained from experiments and MD simulations (flexible EPM2
model) are in very good agreement; see Figure S1. Therefore, we estimate the pressure of carbon dioxide within
the CDCB as the experimental pressure of bulk carbon
dioxide at the density
at z ≈ 0. We stress
that the pressure–density equation-of-state for bulk CO2 obtained from experiments and MD simulations (flexible EPM2
model) are in very good agreement; see Figure S1. Therefore, we estimate the pressure of carbon dioxide within
the CDCB as the experimental pressure of bulk carbon
dioxide at the density 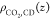 at z ≈ 0 (at the
temperature considered). Figure 6a shows
at z ≈ 0 (at the
temperature considered). Figure 6a shows 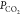 as a function of
as a function of  obtained by following the procedure described
above. The values of
obtained by following the procedure described
above. The values of 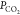 as a function of the CO2 density,
as defined by
as a function of the CO2 density,
as defined by  at z ≈ 0, is shown
in Figure 6b. It follows
from Figure 6 that
carbon dioxide is at supercritical conditions at T ≥ 320 K, with carbon dioxide being in a liquid-like state
for
at z ≈ 0, is shown
in Figure 6b. It follows
from Figure 6 that
carbon dioxide is at supercritical conditions at T ≥ 320 K, with carbon dioxide being in a liquid-like state
for 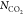 = 1382 and 1502 (the highest two density
values along an isotherm), and gas-like state for
= 1382 and 1502 (the highest two density
values along an isotherm), and gas-like state for  = 664 and 872 (the lowest two density values
along an isotherm).
= 664 and 872 (the lowest two density values
along an isotherm).
Figure 6.
(a) Density of carbon dioxide within the CDCB (defined
by the value
of 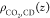 at z ≈ 0) for
at z ≈ 0) for 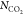 = 664, 872, 966, 1114, 1246, 1382, 1502
and all temperatures studied. (b) Estimated carbon dioxide pressure,
= 664, 872, 966, 1114, 1246, 1382, 1502
and all temperatures studied. (b) Estimated carbon dioxide pressure, 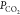 , as a function of density within the CDCB
(T = 280, 300, 320, 340 K, bottom to top). Lines
are the
, as a function of density within the CDCB
(T = 280, 300, 320, 340 K, bottom to top). Lines
are the 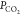 (ρ)-equation of state from experiments
of bulk carbon dioxide; circles are the pressures corresponding to
the densities included in (a) along a given isotherm.
(ρ)-equation of state from experiments
of bulk carbon dioxide; circles are the pressures corresponding to
the densities included in (a) along a given isotherm.
Water Capillary Bridge Profile and Contact Angle
In
order to explore the effects of carbon dioxide on the wetting behavior
of the WCB, we focus on the case of T = 320 K (at
which carbon dioxide is supercritical). Similar results are obtained
at the other temperatures studied. Figure 7 shows the average WCB profile in the presence
of  = 0, 664, 872, 966, 1114, 1246, 1382, and
1502 carbon dioxide molecules. As shown in Figure 6b (red line, T = 320 K),
the systems studied correspond to CO2 pressures in the
range
= 0, 664, 872, 966, 1114, 1246, 1382, and
1502 carbon dioxide molecules. As shown in Figure 6b (red line, T = 320 K),
the systems studied correspond to CO2 pressures in the
range 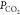 ≈ 0–80 MPa. It follows from Figure 7 that the WCB profiles
are weakly affected by variations in the amount of carbon dioxide.
≈ 0–80 MPa. It follows from Figure 7 that the WCB profiles
are weakly affected by variations in the amount of carbon dioxide.
Figure 7.
Profile of the WCB at T = 320 K and for different amounts of carbon dioxide. Similar results are obtained at the other temperatures studied. The dashed lines at z = ±25 Å indicate the location of the walls surface (defined by the planes containing the H atoms of the walls). The data points at heights z = ±22.5 Å are off due to the water film adsorbed on the wall surfaces and hence, these data points do not represent the WCB profile.
To estimate the contact angle of water, we fit
the WCB profiles
shown in Figure 7 with
either a circle, or a second order polynomial. The corresponding fits
to the WCB profiles are shown in Figure 8. Both fits work reasonably well at heights
|z|< 17 Å, corresponding to distances of
at least 8 Å away from the nearest wall. The contact angles of
water obtained from both fitting procedures are shown in Figure 9. Our MD simulations
indicate that θc ≈ 40–60° depending
on the conditions and the method considered. These values are not
inconsistent with experiments (where θ ≈ 0–60°
depending on the surface and experimental details54) and are slightly larger than the contact angles of WCB
reported from computer simulations using different model surfaces
and methods (e.g., θc ≈ 0–45°
in refs (40,54)). The main
point of Figure 9 is
that, independently of the method used to calculate the WCB contact
angle, our MD simulations indicate that θc increases
weakly with increasing the carbon dioxide density/pressure, Δθc ≈ 10–20° for 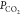 = 0–80 MPa (and T = 320 K).
= 0–80 MPa (and T = 320 K).
Figure 8.
(a) Upper half of the WCB shown in Figure 7 at T = 320 K and for different amounts of carbon dioxide (symbols). Lines are obtained by using a circle to fit the WCB profile (eq 2). (b) Same as (a) with the lines representing a second order polynomial fit of the WCB profiles. Only data points at z < 17.5 Å are used in the fitting procedure in order to exclude any contribution from the water films adsorbed on the walls. The wall surface is located at height z = 25 Å (dashed line); the dotted line corresponds to z = 17 Å at which the contact angle is evaluated (Figure 9).
Figure 9.
Water contact angle θc at T =
320 K obtained from the WCB shown in Figure 8. (a) θc as a function of
the carbon dioxide pressure, 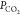 (see Figure 6). (b) θc as a function of the carbon
dioxide density,
(see Figure 6). (b) θc as a function of the carbon
dioxide density,  . Circles are the θc(T) resulting from the fits of the WCB in Figure 8a using a circle [eq 2]. Squares are the θc(T) resulting from the fits of the WCB in Figure 8b using a second
order polynomial. In both cases, θc(T) increases weakly (Δθc ≈ 10–20°)
as the amount of carbon dioxide increases. Estimated error bars are
approximately 2–4° (smaller than the symbols size).
. Circles are the θc(T) resulting from the fits of the WCB in Figure 8a using a circle [eq 2]. Squares are the θc(T) resulting from the fits of the WCB in Figure 8b using a second
order polynomial. In both cases, θc(T) increases weakly (Δθc ≈ 10–20°)
as the amount of carbon dioxide increases. Estimated error bars are
approximately 2–4° (smaller than the symbols size).
Our choice of (A) fitting the WCB profiles up to
|z| < zc = 17.5 Å,
and (B) evaluating
θc from the slope of the so-obtained WCB profile
at z0 = 17.0 Å is based on physical
grounds. (i) Macroscopic thermodynamics (capillarity theory) assumes
that the WCB is composed of a bulk water volume bounded by the water–wall
and water–vapor (or water–CO2) interfaces.
However, as shown in Figures 4a and 5a, two layers of water molecules
form next to the walls and 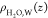 becomes constant only at a distance approximately d > 8–10 Å from the walls. Accordingly, from
a capillarity theory point of view, the “bulk” water
within the WCB extends up to, at most, the distance d from the walls and hence, evaluation of the WCB profile should exclude
those molecules located at |z| > (25 Å – d) (wall–water interfaces). (ii) We also note that
in a previous study,37 we tested the ability
of capillarity theory to predict the profile of WCB confined by the
same silica walls employed here but at smaller wall separations, h < 50 Å. It was found that capillarity theory correctly
predicted the WCB profile down to approximately h ≈ 25–30 Å. At smaller values of h, capillarity theory failed; at these separations there is not bulk-like
water between the walls (the two water-wall interfaces practically
touched each other). Briefly, the results of ref. (37) also indicate that, macroscopically,
it is not physically consistent to include the water molecules (films)
next to the wall (within a distance ≈d) when
applying capillarity theory (e.g., to fit the WCB profile and measure
the associated contact angle). To do so would require modifying capillarity
theory, see ref. (37). (iii) In addition, the film of water on the wall and under the
CO2 volume extends up to ≈7–8 Å from
the wall [see the density profile
becomes constant only at a distance approximately d > 8–10 Å from the walls. Accordingly, from
a capillarity theory point of view, the “bulk” water
within the WCB extends up to, at most, the distance d from the walls and hence, evaluation of the WCB profile should exclude
those molecules located at |z| > (25 Å – d) (wall–water interfaces). (ii) We also note that
in a previous study,37 we tested the ability
of capillarity theory to predict the profile of WCB confined by the
same silica walls employed here but at smaller wall separations, h < 50 Å. It was found that capillarity theory correctly
predicted the WCB profile down to approximately h ≈ 25–30 Å. At smaller values of h, capillarity theory failed; at these separations there is not bulk-like
water between the walls (the two water-wall interfaces practically
touched each other). Briefly, the results of ref. (37) also indicate that, macroscopically,
it is not physically consistent to include the water molecules (films)
next to the wall (within a distance ≈d) when
applying capillarity theory (e.g., to fit the WCB profile and measure
the associated contact angle). To do so would require modifying capillarity
theory, see ref. (37). (iii) In addition, the film of water on the wall and under the
CO2 volume extends up to ≈7–8 Å from
the wall [see the density profile 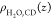 in Figures 4b and 5b]. This means that a
reliable WCB profile can only be calculated for approximately |z| < 17–18 Å. Otherwise,
when one calculates the radius of the WCB at a given position z, molecules from the water film may be (erroneously) included
in the calculations; see the Methods section.
in Figures 4b and 5b]. This means that a
reliable WCB profile can only be calculated for approximately |z| < 17–18 Å. Otherwise,
when one calculates the radius of the WCB at a given position z, molecules from the water film may be (erroneously) included
in the calculations; see the Methods section.
The Role of Temperature
Next, we focus on the role
of temperature on the WCB profile and water contact angle for a fixed
amount of carbon dioxide. The WCB profiles surrounded by  = 1114 carbon dioxide molecules at T = 280–400 K are shown in Figure 10a. Figure 10b shows the values of θc obtained
from Figure 10a when
the WCB profiles are fitted by a circle or a quadratic polynomial
(green and blue lines). Despite the noise in the data, our results
suggest that θc decreases slightly with increasing
temperature. For example, θc decreases by <10°
when the temperature increases from T = 320 to 360
K.
= 1114 carbon dioxide molecules at T = 280–400 K are shown in Figure 10a. Figure 10b shows the values of θc obtained
from Figure 10a when
the WCB profiles are fitted by a circle or a quadratic polynomial
(green and blue lines). Despite the noise in the data, our results
suggest that θc decreases slightly with increasing
temperature. For example, θc decreases by <10°
when the temperature increases from T = 320 to 360
K.
Figure 10.
(a) Profile of the WCB surrounded by carbon dioxide, 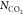 , at different temperatures. Similar results
are obtained for other values of
, at different temperatures. Similar results
are obtained for other values of 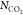 . The dashed lines indicate the location
of the walls surface (as defined by the plane containing the H atoms
of the wall). The WCB profiles for heights z = ±22.5
Å are off due to the presence of water films adsorbed on the
walls; these data points do not represent the WCB profile. (b) Water
contact angle θc obtained from (a) for
. The dashed lines indicate the location
of the walls surface (as defined by the plane containing the H atoms
of the wall). The WCB profiles for heights z = ±22.5
Å are off due to the presence of water films adsorbed on the
walls; these data points do not represent the WCB profile. (b) Water
contact angle θc obtained from (a) for 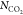 (green and blue lines). Also included,
are the results for
(green and blue lines). Also included,
are the results for 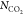 = 1502 (black and red lines). Estimated
error bars are approximately 2–4° (smaller than the symbols
size).
= 1502 (black and red lines). Estimated
error bars are approximately 2–4° (smaller than the symbols
size).
Why Does Water Form a Film between the Walls and Carbon Dioxide?
To answer this question, we calculate the number of HB that the walls silanol groups form with H2O/CO2 molecules. The main panel of Figure 11 shows the probability distribution for the number of HB, P(nHB), that a silanol group forms with nearby (i) H2O and (ii) CO2 molecules. In the case of CO2, the distribution P(nHB) > 0 only for nHB = 0, 1 meaning that the silanol groups can form at most 1 HB with CO2 molecules. Indeed, we find that most silanol groups do not form HB with CO2 molecules and only ≈5% of the surface OH groups form one HB with the CO2 molecules. Instead, for the case of water, P(nHB) > 0 for nHB = 1, 2, 3, i.e., the surface OH groups can form up to 3 HB with water molecules. About 50% and 35% of the OH groups form 2 and 3 HB with H2O molecules, respectively.
Figure 11.
Probability distribution
for the number of HB that a silanol group
of the walls forms with H2O and CO2 molecules.
Only silanol groups located under the CDCB are considered. Most of
these silanol groups form no HB with the CO2 molecules
and only ≈5% are able to form one HB with the CO2 molecules. Instead, these silanol groups can form up to 3 HB with
H2O molecules. Inset: probability distribution for the
number of HB that a H2O molecule forms with the wall silanol
groups. In this case, only molecules that form at least one HB are
included in the statistics. Results are based on a system composed
of N = 2756 and 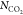 =1114 molecules at T =
320 K.
=1114 molecules at T =
320 K.
We also find that a given water molecule can form multiple HB with the walls silanol groups. To show this, we consider only those water molecules that form at least one HB with the walls and evaluate the total number of HB that they form with the surface silanol groups. As shown in the inset of Figure 11, such water molecules can form 1, 2, and even 3 HB with the silanol groups. To confirm these results, we include in Figure 12 a typical snapshot of the system showing only those H2O and CO2 molecules that form at least one HB with the walls OH groups. Water molecules are able to occupy the spaces between three silanol groups (at the center of the hexagons shown in Figure 2b), while forming up to three HB with the surface OH groups. Instead, the CO2 molecules tend to stick away from the walls and form only one HB with the surface OH groups. Clearly, in the case of β-cristobalite, the topography of the surface, and the distribution of silanol groups, are important factors that favor the formation of HB between water and the walls, relative to carbon dioxide. Similar results are expected for the case of other surfaces composed of silica tetrahedra, such as α-quartz. For example, in ref.55 it is found that water molecules can make 2–3 HB with silanol groups on the surface of silicalite-1, a widely studied zeolite. However, from a chemistry point of view, one may expect that, in general, water will preferentially wet the surface if the surface has the ability to form HB with H2O/CO2. This is because a CO2 molecule can only “accept” HB with its O atoms while, instead, a H2O molecule can “accept” two HB, with its O atom, and “donate” two HB, with its two H atoms.
Figure 12.

(a) Top and (b) side view of a section of one of the walls in contact with the carbon dioxide capillary bridge. Only the wall surface O and H atoms are shown (blue and white spheres, respectively). The wall surface OH groups are located in a triangular lattice [see also Figure 2b]. Also included are the (approximately 20) water and (three) carbon dioxide molecules that form HB with the surface OH groups. These CO2 molecules form one HB with the wall OH groups and they tend to stick away from the wall. The H2O molecules form mostly 2–3 HB with the OH groups by sitting flat and parallel to the walls, in between three of the surface OH groups [H2O molecules sit at the center of the triangular lattice formed by the OH groups or, equivalently, they sit at the center of the hexagonal lattice formed by the silica tetrahedra in Figure 2b].
Summarizing, the walls are preferentially solvated by water because of the ability of the H2O molecules to form more HB (per molecule) than the CO2 molecules. This allows the system to lower the enthalpy and the free energy (assuming the entropic contribution plays a secondary role).
The Role of Salt (NaCl) on the Wall Hydration, Water Capillary Bridges, and Water Contact Angle
Walls Hydration
Figure 13a shows a snapshot from our MD simulations of a WCB containing N0 = 100 pairs of Na+ and Cl– at T = 320 K, with no carbon dioxide. Our simulations show that the ions aggregate and form a crystallite that remains within the WCB; see Figure 13b. Hence, the Cl– and Na+ ions remain preferentially away from the water–vapor interface and do not diffuse into the empty space. Interestingly, the Cl– and Na+ ions also remain away from the water–wall interface. Similar results are found for the case of N0 = 300 pairs of Na+ and Cl–. It is not clear whether the tendency of NaCl to form crystallites within the WCB is due to (i) confinement (nm-scale WCB and nm-scale wall–wall separation), or to (ii) the Na/Cl/water interactions, or both. In the Supporting Information, we present results from MD simulations of NaCl in bulk water (T = 300 K, p = 0.1 MPa) and mole fractions xCl = xNa = 0.91, 1.80, 4.93, 9.40%. Crystallites are found for xCl = xNa = 4.93, 9.40% but not for xCl = xNa = 0.91, 1.80%. The effective concentration of ions within our WCB for N0 = 100 should be, at least, xCl = xNa = 100/(100 + 2756) = 3.50% since many water molecules belong to the films adsorbed on the walls. Accordingly, it seems plausible that the ions form a crystallite within the WCB mostly due to the Na–Cl–water interactions, with confinement effects playing a secondary role (note that at xCl = xNa = 4.93, 9.40%, we would need N0 175, 350 pairs of Na+ and Cl– ions in the WCB). We note that the force field used in our computational study (and other studies) has its own limitations. For example, the concentrations at which we find the formation of crystallites is smaller than the experimental solubility of NaCl in water, xCl,xNa ≈ 11%. Indeed, the properties of salts in water are very sensitive to the force field considered (see, e.g., refs (56,57)).
Figure 13.

(a) Water
capillary bridge containing N0 = 100 pairs
of Na+ and Cl−
ions at T = 320 K (N = 2756 water
molecules). (b) Na+ and Cl– ions within the WCB shown in (a).
The ions remain within the WCB at all times and stay away from the
walls and water–carbon dioxide interface. At the present concentration
(xCl = xNa = 100/(100 + 2756) = 3.50% mole fraction), the ions form a crystallite
inside the WCB. The rod-like crystallite extends through the WCB bridge
length (y-direction), and is effectively infinite
due to periodic boundary conditions. (c), (d) Same as (a), (b) for
a WCB in the presence of carbon dioxide (T = 320
K, N = 2756, 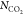 = 1502, N0 =
100).
= 1502, N0 =
100).
Figure 13c,d shows
a snapshot of a WCB containing N0 = 100
pairs of Na+ and Cl– and at T = 320 K, in
the presence of carbon dioxide (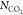 = 1502). As discussed above, the NaCl also
forms a crystallite within the WCB, avoiding the water–carbon
dioxide interface and the wall interface. While the ions are not able
to diffuse into the carbon-dioxide volume, some CO2 molecules
diffuse into the WCB.
= 1502). As discussed above, the NaCl also
forms a crystallite within the WCB, avoiding the water–carbon
dioxide interface and the wall interface. While the ions are not able
to diffuse into the carbon-dioxide volume, some CO2 molecules
diffuse into the WCB.
To confirm the qualitative picture resulting
from Figure 13, we
also calculate 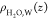 and
and 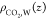 . As shown in Figure 14a, the water density profiles are practically
independent of the presence of NaCl in the WCB, particularly, at the
interfaces with the walls. Interestingly,
. As shown in Figure 14a, the water density profiles are practically
independent of the presence of NaCl in the WCB, particularly, at the
interfaces with the walls. Interestingly, 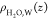 develops a pronounced minimum at −20
< z < 20 Å when the ions are present.
This minimum is due to the NaCl crystallite formed within the WCB.
The NaCl crystallite oscillates over time but remains away from the
water–wall interface. It is for this reason that
develops a pronounced minimum at −20
< z < 20 Å when the ions are present.
This minimum is due to the NaCl crystallite formed within the WCB.
The NaCl crystallite oscillates over time but remains away from the
water–wall interface. It is for this reason that 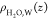 is indifferent to the presence of NaCl
close to the walls (|z| > 20 Å). Similar
conclusions
apply to the
is indifferent to the presence of NaCl
close to the walls (|z| > 20 Å). Similar
conclusions
apply to the 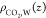 . The
. The 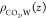 is weakly affected by the addition of salt;
see Figure 14b. In
all cases,
is weakly affected by the addition of salt;
see Figure 14b. In
all cases, 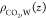 is small (<10–20% mass fraction
for −20 < z < 20 Å). As for the
case of water, a weak minimum develops in
is small (<10–20% mass fraction
for −20 < z < 20 Å). As for the
case of water, a weak minimum develops in 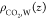 at −20 < z <
20 Å due to the excluded volume occupied by the NaCl crystallite.
at −20 < z <
20 Å due to the excluded volume occupied by the NaCl crystallite.
Figure 14.
Density profiles of water (a) and carbon dioxide (b) within the WCB containing N0 = 100 pairs of Na+ and Cl– ions, and along the direction perpendicular to the walls (dashed lines). Results are for T = 320 K and different number of CO2 molecules. For comparison, we also include the density profiles when the Na+ and Cl– ions are removed from Figure 4a (solid lines).
One may wonder if the presence of NaCl can affect
the distribution
of H2O and CO2 molecules within the carbon dioxide
volume. To address this question, we include in Figure 15 the density profiles of H2O and CO2 along the CDCB. A comparison of the corresponding
density profiles with (dashed lines) and without NaCl (solid lines)
shows that the  and
and 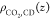 exhibit minor changes with the addition
of NaCl. It follows that, even in the presence of NaCl, a thin water
film is adsorbed on the walls surface. Again, this is because the
NaCl ions remain within the WCB and away from the walls and the CDCB.
exhibit minor changes with the addition
of NaCl. It follows that, even in the presence of NaCl, a thin water
film is adsorbed on the walls surface. Again, this is because the
NaCl ions remain within the WCB and away from the walls and the CDCB.
Figure 15.
Density
profiles of water (a) and carbon dioxide (b) within the
CDCB for the case where there are N0 =
100 pairs of Na+ and Cl– ions in the system (dashed lines).
Results are for T = 320 K and different numbed of
CO2 molecules. For comparison, we also include the density
profiles when the Na+ and Cl– ions are removed from Figure 4b (solid lines).
Both 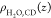 and
and 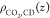 are weakly affected by the presence of
NaCl.
are weakly affected by the presence of
NaCl.
Interestingly, as shown in Figure 15b, the values of 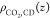 increase slightly when NaCl is added to
the WCB. For example, for the case
increase slightly when NaCl is added to
the WCB. For example, for the case 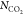 = 1502,
= 1502, 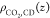 increases by 0.04–0.05 g/cm3 (≈3–4%) after adding N0 = 100 pairs of Na+ and Cl– ions. This indicates that
the presence of ions decreases slightly the solubility of CO2 molecules into the WCB. We note that, as found previously in the
absence of NaCl, the surface under the CDCB remains preferentially
hydrated by water (as opposed to CO2). Qualitative similar
results hold when N0 = 300 pairs of Na+
and Cl– are added to the WCB.
increases by 0.04–0.05 g/cm3 (≈3–4%) after adding N0 = 100 pairs of Na+ and Cl– ions. This indicates that
the presence of ions decreases slightly the solubility of CO2 molecules into the WCB. We note that, as found previously in the
absence of NaCl, the surface under the CDCB remains preferentially
hydrated by water (as opposed to CO2). Qualitative similar
results hold when N0 = 300 pairs of Na+
and Cl– are added to the WCB.
Water-and-Salt Capillary Bridges and Contact Angle
In order to explore the effects of adding salt to the wetting behavior
of the WCB, we focus on the case T = 320 K (supercritical
CO2) and N0 = 100 pairs of
Cl– and Na+ ions (similar results are obtained for N0 = 300). Figure 16a shows the average WCB profile (with NaCl)
in the presence of 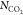 = 0, 664, 872, 966, 1114, 1246, 1382, and
1502 molecules. The effect of increasing the amount of carbon dioxide
on the WCB profile is rather mild. The corresponding water contact
angles are shown in Figure 16b. The values of θc fluctuate considerably.
Nonetheless, it is apparent that adding NaCl to the WCB does not affect
θc. We find an increase of Δθc = 1–20° with carbon dioxide in the range
= 0, 664, 872, 966, 1114, 1246, 1382, and
1502 molecules. The effect of increasing the amount of carbon dioxide
on the WCB profile is rather mild. The corresponding water contact
angles are shown in Figure 16b. The values of θc fluctuate considerably.
Nonetheless, it is apparent that adding NaCl to the WCB does not affect
θc. We find an increase of Δθc = 1–20° with carbon dioxide in the range 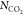 = 1502 (supercritical liquid-like carbon
dioxide). The changes in θc are consistent with the
observations above that Na+ and Cl– ions are located within
the WCB and away from the water–carbon dioxide and water–wall
interfaces. We also note that our results, within the fluctuations
in the data, seem to be rather independent of whether one uses a circle
or a second order polynomial to fit the (salty) WCB profiles.
= 1502 (supercritical liquid-like carbon
dioxide). The changes in θc are consistent with the
observations above that Na+ and Cl– ions are located within
the WCB and away from the water–carbon dioxide and water–wall
interfaces. We also note that our results, within the fluctuations
in the data, seem to be rather independent of whether one uses a circle
or a second order polynomial to fit the (salty) WCB profiles.
Figure 16.
(a) Profile
of the WCB containing N0 = 100 pairs of
Na+ and Cl– ions, and surrounded by carbon
dioxide; T = 320 K (similar results are obtained
for N0 = 300). The dashed lines indicate
the location of the walls surface (at z = ±25
Å). The density profiles at heights |z| = 20–22.5
Å are off due to the presence of water films adsorbed on the
walls; these data points do not represent the WCB profile. The effect
of increasing the amount of carbon dioxide (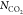 ) on the WCB profile is mild. (b) Water
contact angles θc obtained from (a) by fitting the
WCB using a circle and a second order polynomial (black and red lines,
respectively). For comparison, also included are the values of θc in the absence of ions (from Figure 7, orange and dark-green lines) and for the
case N0 = 300 (green and blue lines).
θc increases slightly with increasing amounts of
carbon dioxide (Δθc = 10–20° for
) on the WCB profile is mild. (b) Water
contact angles θc obtained from (a) by fitting the
WCB using a circle and a second order polynomial (black and red lines,
respectively). For comparison, also included are the values of θc in the absence of ions (from Figure 7, orange and dark-green lines) and for the
case N0 = 300 (green and blue lines).
θc increases slightly with increasing amounts of
carbon dioxide (Δθc = 10–20° for 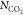 = 0–1502). Within the fluctuations
in the data, there is no effect on θc due to the addition of NaCl to the WCB. Estimated error bars are
approximately 2–4° (smaller than the symbols size).
= 0–1502). Within the fluctuations
in the data, there is no effect on θc due to the addition of NaCl to the WCB. Estimated error bars are
approximately 2–4° (smaller than the symbols size).
Conclusions
In this work, we study the behavior of
nanoscale water capillary
bridges surrounded by carbon dioxide over a wide range of temperatures
and pressures. The water and carbon dioxide system is confined by
hydroxylated silica surfaces (β-cristobalite) which can form
HB with both H2O and CO2 molecules. Our simulations
show that, consistent with studies based on α-quartz,40 our silica walls are preferentially hydrated
by water. Accordingly, the carbon dioxide fluid phase in the system
is separated from the walls by a thin film of water (1–2 water
layers thick). This conclusion holds at all temperatures (T = 280–400 K) and pressures studied (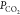 = 0–80 MPa). While the water film
adsorbed on the walls is practically insensitive to variations in
the CO2 content of the system, the water film becomes thicker
with increasing temperature. This implies that increasing the temperature
favors the release of CO2 away from the confining walls.
In order to understand why the walls are preferentially hydrated by
water, we also perform a molecular-level characterization of the walls
hydration. It is found that, relative to the CO2 molecules,
H2O molecules have an enhanced ability to form HB with
the surface silanol groups. Specifically, a given water molecule next
to the walls is able to form up to three HB with the silanol groups
while, instead, most CO2 molecules form zero or one HB
with the surface. Our MD simulations also show that the WCB contact
angle θc varies weakly with temperature and pressure.
For example, Δθc ≈ + 10–20°
for
= 0–80 MPa). While the water film
adsorbed on the walls is practically insensitive to variations in
the CO2 content of the system, the water film becomes thicker
with increasing temperature. This implies that increasing the temperature
favors the release of CO2 away from the confining walls.
In order to understand why the walls are preferentially hydrated by
water, we also perform a molecular-level characterization of the walls
hydration. It is found that, relative to the CO2 molecules,
H2O molecules have an enhanced ability to form HB with
the surface silanol groups. Specifically, a given water molecule next
to the walls is able to form up to three HB with the silanol groups
while, instead, most CO2 molecules form zero or one HB
with the surface. Our MD simulations also show that the WCB contact
angle θc varies weakly with temperature and pressure.
For example, Δθc ≈ + 10–20°
for 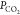 increasing from ≈0 to 80 MPa (T = 320 K), and Δθc ≈ −10°
for T increasing from 320 to 360 K (with a fixed
amount of carbon dioxide).
increasing from ≈0 to 80 MPa (T = 320 K), and Δθc ≈ −10°
for T increasing from 320 to 360 K (with a fixed
amount of carbon dioxide).
The effect of adding salt (NaCl)
to the water–carbon dioxide
system was also explored at T = 320 K (supercritical
CO2). The MD simulations show that at the salt concentrations
studied (mole fractions xNa = xCl = 3.50, 9.81%), the NaCl forms a large crystallite
within the WCB with the ions avoiding the water–carbon dioxide
interface and the walls surface. This results in θc being insensitive to the presence of NaCl, for all the concentrations
of CO2 studied (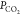 = 0–80 MPa). Our results on the
WCB containing salt are based on the OPLS model for NaCl, and the
SPC/E model for water. At the (realistic) concentrations studied,
these models predict the aggregation of the ions within the WCB. It
would be important in the future to systematically compare the effects
of using different models for NaCl, as well as water, on the WCB contact
angle. This is important because the solubility of NaCl in water is
sensitive to the water–NaCl model considered.56
= 0–80 MPa). Our results on the
WCB containing salt are based on the OPLS model for NaCl, and the
SPC/E model for water. At the (realistic) concentrations studied,
these models predict the aggregation of the ions within the WCB. It
would be important in the future to systematically compare the effects
of using different models for NaCl, as well as water, on the WCB contact
angle. This is important because the solubility of NaCl in water is
sensitive to the water–NaCl model considered.56
Our results are important for CO2 capture and storage technologies. Our MD simulations suggest that the contact angle of water on a hydroxylated silica-based surface, surrounded by carbon dioxide, remains <90° over a wide range of temperature, CO2 pressure, and irrespective of salt presence. Hence, caprocks comprised of hydrophilic materials, such as β-cristobalite, should remain water wet, entailing a positive capillary pressure. Using γ = 30 mN/m, θc = 60° and ΦP = 5 nm in eq 1, we estimate that at least 12 MPa is needed in order for CO2 to permeate across the entire caprock layer. Real rocks are obviously mineralogically more complex and heterogeneous than the system modeled in this study so the suitability of caprocks for geological storage should be individually assessed.
Acknowledgments
This work was supported by the ExxonMobil Technology and Engineering Company. APC is thankful for support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) through the CAPES PrInt program. N.G. is thankful for support from the NSF (grant number CHE-2223461) and to the NSF-CREST ‘Center for Interface Design and Engineered Assembly of Low Dimensional Systems (IDEALS)’ (grant number HRD-1547830). This work used computational resources at San Diego Supercomputer Center (SDSC) through allocation CHE230026 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296.58
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.4c01185.
(i) A comparison of the carbon-dioxide equation-of-state predicted by the (flexible) EPM2 model and experiments; (ii) a comparison of the carbon dioxide–water surface tension predicted by the SPC/E water and (flexible) EPM2 carbon dioxide models with experiments; (iii) effects of the surface hydrophobicity/hydrophilicitiy on the (a) carbon dioxide capillary bridges, and (b) water capillary bridges in contact with carbon dioxide; (iv) effects of temperature on the walls hydration; (v) size effects on the water capillary bridge profiles; (vi) additional MD simulations of bulk NaCl–water solutions at different concentrations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- International Energy Agency. Special Report On Carbon Capture Utilisation And Storage – CCUS In Clean Energy Transitions, International Energy Agency, 2020. www.iea.org.
- Zheng J.; Chong Z. R.; Qureshi M. F.; Linga P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–11059. 10.1021/acs.energyfuels.0c02309. [DOI] [Google Scholar]
- Liu S.-Y.; Ren B.; Li H.-Y.; Yang Y.-Y.; Wang Z.-Q.; Wang B.; Xu J.-C.; Agarwal R. CO2 storage with enhanced gas recovery (CSEGR): A review of experimental and numerical studies. Pet. Sci. 2022, 19, 594–607. 10.1016/j.petsci.2021.12.009. [DOI] [Google Scholar]
- Pruess K. Enhanced geothermal systems (EGS) using CO2 as working fluid – A novel approach for generating renewable energy with simultaneous sequestration. Geothermics 2006, 35, 351–367. 10.1016/j.geothermics.2006.08.002. [DOI] [Google Scholar]
- Span R.; Wagner W. A new equation of state for carbon dioxide covering the fluid region from the triplepoint temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. 10.1063/1.555991. [DOI] [Google Scholar]
- Ringrose P.How to store CO2 underground: Insights from early-mover CCS projects; Springer Nature: Switzerland, 2020. [Google Scholar]
- Tonnet N.; Broseta D.; Mouronval G.. Evaluation of the petrophysical properties of a carbonate-rich caprock for CO2 geological storage purposes. In SPE Europec featured at EAGE Conference and Exhibition; SPE, 2010. [Google Scholar]
- Guiltinan E. J.; Cardenas M. B.; Bennett P. C.; Zhang T.; Espinoza D. N. The effect of organic matter and thermal maturity on the wettability of supercritical CO2 on organic shales. Int. J. Greenhouse Gas Control 2017, 65, 15–22. 10.1016/j.ijggc.2017.08.006. [DOI] [Google Scholar]
- Schowalter T. T. Prediction of cap rock seal capacity. Bull. Am. Assoc. Pet. Geol. 1981, 65, 987. [Google Scholar]
- Schlomer S.; Kroos B. M. Experimental characterisation of the hydrocarbon sealing efficiency of cap rocks. Mar. Pet. Geol. 1997, 14, 565–580. 10.1016/S0264-8172(97)00022-6. [DOI] [Google Scholar]
- Hildenbrand A.; Schlömer S.; Krooss B. M.; Littke R. Gas breakthrough experiments on pelitic rocks: Comparative study with N2, CO2 and CH4. Geofluids 2004, 4, 61–80. 10.1111/j.1468-8123.2004.00073.x. [DOI] [Google Scholar]
- Amann-Hildenbrand A.; Bertier P.; Busch A.; Krooss B. M. Experimental investigation of the sealing capacity of generic clay-rich caprocks. Int. J. Greenhouse Gas Control 2013, 19, 620–641. 10.1016/j.ijggc.2013.01.040. [DOI] [Google Scholar]
- Chiquet P.; Daridon J.-L.; Broseta D.; Thibeau S. CO2/water interfacial tensions under pressure and temperature conditions of CO2 geological storage. Energy Convers. Manage. 2007, 48, 736–744. 10.1016/j.enconman.2006.09.011. [DOI] [Google Scholar]
- Espinoza D. N.; Santamarina J. C.. Water–CO2–mineral systems: Interfacial tension, contact angle, and diffusion–Implications to CO2 geological storage. Water Resour. Res., 2010, 46, 10.1029/2009WR008634. [DOI] [Google Scholar]
- Georgiadis A.; Maitland G.; Trusler J. P. M.; Bismarck A. Interfacial tension measurements of the (H2O+ CO2) system at elevated pressures and temperatures. J. Chem. Eng. Data 2010, 55, 4168–4175. 10.1021/je100198g. [DOI] [Google Scholar]
- Mutailipu M.; Liu Y.; Jiang L.; Zhang Y. Measurement and estimation of CO2–brine interfacial tension and rock wettability under CO2 sub-and super-critical conditions. J. Colloid Interface Sci. 2019, 534, 605–617. 10.1016/j.jcis.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Lv P.; Liu Y.; Wang Z.; Liu S.; Jiang L.; Chen J.; Song Y. In Situ Local Contact Angle Measurement in a CO2–Brine–Sand System Using Microfocused X-ray CT. Langmuir 2017, 33, 3358–3366. 10.1021/acs.langmuir.6b04533. [DOI] [PubMed] [Google Scholar]
- AlRatrout A.; Blunt M. J.; Bijeljic B. Spatial correlation of contact angle and curvature in pore–space images. Water Resour. Res. 2018, 54, 6133–6152. 10.1029/2017WR022124. [DOI] [Google Scholar]
- Chiquet P.; Broseta D.; Thibeau S. Wettability alteration of caprock minerals by carbon dioxide. Geofluids 2007, 7, 112–122. 10.1111/j.1468-8123.2007.00168.x. [DOI] [Google Scholar]
- Bikkina P. K. Contact angle measurements of CO2–water–quartz/calcite systems in the perspective of carbon sequestration. Int. J. Greenhouse Gas Control 2011, 5, 1259–1271. 10.1016/j.ijggc.2011.07.001. [DOI] [Google Scholar]
- Mills J.; Riazi M.; Sohrabi M.. Wettability of common rock-forming minerals in a CO2-brine system at reservoir conditions. In International symposium of the society of core analysts, Society of Core Analysts Fredericton, 2011, pp. 19–21. [Google Scholar]
- Farokhpoor R.; Bjo̷rkvik B. J. A.; Lindeberg E.; Torsæter O. CO2 wettability behavior during CO2 sequestration in saline aquifer - An experimental study on minerals representing sandstone and carbonate. Energy Procedia 2013, 37, 5339–5351. 10.1016/j.egypro.2013.06.452. [DOI] [Google Scholar]
- Wang S.; Edwards I. M.; Clarens A. F. Wettability phenomena at the CO2–brine–mineral interface: Implications for geologic carbon sequestration. Environ. Sci. Technol. 2013, 47, 234–241. 10.1021/es301297z. [DOI] [PubMed] [Google Scholar]
- Kaveh N. S.; Rudolph E. S. J.; Van Hemert P.; Rossen W. R.; Wolf K. H. Wettability evaluation of a CO2/water/bentheimer sandstone system: Contact angle, dissolution, and bubble size. Energy Fuels 2014, 28, 4002–4020. 10.1021/ef500034j. [DOI] [Google Scholar]
- Iglauer S.; Pentland C. H.; Busch A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo sequestration. Water Resour. Res. 2015, 51, 729–774. 10.1002/2014WR015553. [DOI] [Google Scholar]
- Liu Y.; Mutailipu M.; Jiang L.; Zhao J.; Song Y.; Chen L. Interfacial tension and contact angle measurements for the evaluation of CO2–brine two–phase flow characteristics in porous media. Environ. Prog. Sustainable Energy 2015, 34, 1756–1762. 10.1002/ep.12160. [DOI] [Google Scholar]
- Al-Yaseri A. Z.; Lebedev M.; Barifcani A.; Iglauer S. Receding and advancing (CO2+ brine+ quartz) contact angles as a function of pressure, temperature, surface roughness, salt type and salinity. J. Chem. Thermodyn. 2016, 93, 416–423. 10.1016/j.jct.2015.07.031. [DOI] [Google Scholar]
- Botto J.; Fuchs S. J.; Fouke B. W.; Clarens A. F.; Freiburg J. T.; Berger P. M.; Werth C. J. Effects of mineral surface properties on supercritical CO2 wettability in a siliciclastic reservoir. Energy Fuels 2017, 31, 5275–5285. 10.1021/acs.energyfuels.6b03336. [DOI] [Google Scholar]
- Yang D.; Tontiwachwuthikul P.; Gu Y. Interfacial interactions between reservoir brine and CO2 at high pressures and elevated temperatures. Energy Fuels 2005, 19, 216–223. 10.1021/ef049792z. [DOI] [Google Scholar]
- Dickson J. L.; Gupta G.; Horozov T. S.; Binks B. P.; Johnston K. P. Wetting phenomena at the CO2/water/glass interface. Langmuir 2006, 22, 2161–2170. 10.1021/la0527238. [DOI] [PubMed] [Google Scholar]
- Saraji S.; Goual L.; Piri M.; Plancher H. Wettability of supercritical carbon dioxide/water/quartz systems: Simultaneous measurement of contact angle and interfacial tension at reservoir conditions. Langmuir 2013, 29, 6856–6866. 10.1021/la3050863. [DOI] [PubMed] [Google Scholar]
- Javanbakht G.; Sedghi M.; Welch W.; Goual L. Molecular dynamics simulations of CO2/water/quartz interfacial properties: Impact of CO2 dissolution in water. Langmuir 2015, 31, 5812–5819. 10.1021/acs.langmuir.5b00445. [DOI] [PubMed] [Google Scholar]
- Chen C.; Dong B.; Zhang N.; Li W.; Song Y. Pressure and temperature dependence of contact angles for CO2/water/silica systems predicted by molecular dynamics simulations. Energy Fuels 2016, 30, 5027–5034. 10.1021/acs.energyfuels.6b00171. [DOI] [Google Scholar]
- Arif M.; Abu-Khamsin S. A.; Iglauer S. Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems: Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019, 268, 91–113. 10.1016/j.cis.2019.03.009. [DOI] [PubMed] [Google Scholar]
- McCaughan J.; Iglauer S.; Bresme F. Molecular dynamics simulation of water/CO2–quartz interfacial properties: Application to subsurface gas injection. Energy Procedia 2013, 37, 5387–5402. 10.1016/j.egypro.2013.06.457. [DOI] [Google Scholar]
- Iglauer S.; Mathew M. S.; Bresme F. Molecular dynamics computations of brine–CO2 interfacial tensions and brine–CO2–quartz contact angles and their effects on structural and residual trapping mechanisms in carbon geo-sequestration. J. Colloid Interface Sci. 2012, 386, 405–414. 10.1016/j.jcis.2012.06.052. [DOI] [PubMed] [Google Scholar]
- Almeida A. B.; Alencar A. M.; Buldyrev S. V.; Giovambattista N. How Small Is Too Small for the Capillarity Theory?. J. Phys. Chem. C 2021, 125, 5335–5348. 10.1021/acs.jpcc.0c11140. [DOI] [Google Scholar]
- Berendsen H. J.; Grigera J. R.; Straatsma T. P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. 10.1021/j100308a038. [DOI] [Google Scholar]
- Harris J. G.; Yung K. H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve And Critical Properties as Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. 10.1021/j100031a034. [DOI] [Google Scholar]
- Sun E. W.-H.; Bourg I. C. Molecular dynamics simulations of mineral surface wettability by water versus CO2: Thin films, contact angles, and capillary pressure in a silica nanopore. J. Phys. Chem. C 2020, 124, 25382–25395. 10.1021/acs.jpcc.0c07948. [DOI] [Google Scholar]
- Giovambattista N.; Rossky P. J.; Debenedetti P. G. Effect of pressure on the phase behavior and structure of water confined between nanoscale hydrophobic and hydrophilic plates. Phys. Rev. E 2006, 73, 041604. 10.1103/PhysRevE.73.041604. [DOI] [PubMed] [Google Scholar]
- Giovambattista N.; Almeida A. B.; Alencar A. M.; Buldyrev S. V. Validation of capillarity theory at the nanometer scale by atomistic computer simulations of water droplets and bridges in contact with hydrophobic and hydrophilic surfaces. J. Phys. Chem. C 2016, 120, 1597–1608. 10.1021/acs.jpcc.5b10377. [DOI] [Google Scholar]
- Tang B.; Buldyrev S. V.; Xu L.; Giovambattista N. Energy Stored in Nanoscale Water Capillary Bridges between Patchy Surfaces. Langmuir 2020, 36, 7246–7251. 10.1021/acs.langmuir.0c00549. [DOI] [PubMed] [Google Scholar]
- Patra M.; Karttunen M. Systematic comparison of force fields for microscopic simulations of NaCl in aqueous solutions: Diffusion, free energy of hydration, and structural properties. J. Comput. Chem. 2004, 25, 678–689. 10.1002/jcc.10417. [DOI] [PubMed] [Google Scholar]
- Giovambattista N.; Debenedetti P. G.; Rossky P. J. Effect of surface polarity on water contact angle and interfacial hydration structure. J. Phys. Chem. B 2007, 111, 9581–9587. 10.1021/jp071957s. [DOI] [PubMed] [Google Scholar]
- Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. 10.1006/jcph.1995.1039. [DOI] [Google Scholar]
- Vishnyakov A.; Neimark A. V. Nucleation of liquid bridges and bubbles in nanoscale capillaries. J. Chem. Phys. 2003, 119, 9755–9764. 10.1063/1.1615760. [DOI] [Google Scholar]
- Desgranges C.; Delhommelle J. Free energy calculations along entropic pathways. III. Nucleation of capillary bridges and bubbles. J. Chem. Phys. 2017, 146, 184104. 10.1063/1.4982943. [DOI] [Google Scholar]
- Giacomello A.; Roth R. Bubble formation in nanopores: A matter of hydrophobicity, geometry, and size. Adv. Phys.: X 2020, 5, 1817780. 10.1080/23746149.2020.1817780. [DOI] [Google Scholar]
- Almeida A. B.; Giovambattista N.; Buldyrev S. V.; Alencar A. M. Validation of capillarity theory at the nanometer scale. II: Stability and rupture of water capillary bridges in contact with hydrophobic and hydrophilic surfaces. J. Phys. Chem. C 2018, 122, 1556–1569. 10.1021/acs.jpcc.7b08577. [DOI] [Google Scholar]
- Hockney R. W.; Eastwood J. W.. Computer Simulation Using Particles; Adam Hilger: New York, 1989. [Google Scholar]
- Starr F. W.; Sciortino F.; Stanley H. E. Dynamics of simulated water under pressure. Phys. Rev. E 1999, 60, 6757–6768. 10.1103/PhysRevE.60.6757. [DOI] [PubMed] [Google Scholar]
- Rochelle C. A.; Moore Y. A.. The solubility of CO2 into pure water and synthetic Utsira porewater; British Geological Survey Comissioned Report, NERC, 2002. CR/02/052. [Google Scholar]
- Huang P.; Shen L.; Gan Y.; Maggi F.; El-Zein A.; Pan Z. Atomistic Study of Dynamic Contact Angles in CO2-Water-Silica System. Langmuir 2019, 35, 5324–5332. 10.1021/acs.langmuir.9b00076. [DOI] [PubMed] [Google Scholar]
- Agarwal A.; Rimer J. D.; Palmer J. C. Water Structure and Dynamics Near the Surfaces of Silicalite-1. J. Phys. Chem. C 2023, 127, 23887–23896. 10.1021/acs.jpcc.3c05723. [DOI] [Google Scholar]
- Benavides A. L.; Aragones J. L.; Vega C. Consensus on the solubility of NaCl in water from computer simulations using the chemical potential route. J. Chem. Phys. 2016, 144, 124504. 10.1063/1.4943780. [DOI] [PubMed] [Google Scholar]
- Maerzke K. A.; Yoon T. J.; Currier R. P. Consensus on the solubility of NaCl in water from computer simulations using the chemical potential route. J. Chem. Eng. Data 2022, 67, 3661–3671. 10.1021/acs.jced.2c00528. [DOI] [Google Scholar]
- Boerner T. J.; Deems S.; Furlani T. R.; Knuth S. L.; Towns J.. Practice and Experience in Advanced Research Computing (PEARC 23); ACM: Portland, OR, USA New York, NY, USA, 2023; p 4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.














