Abstract
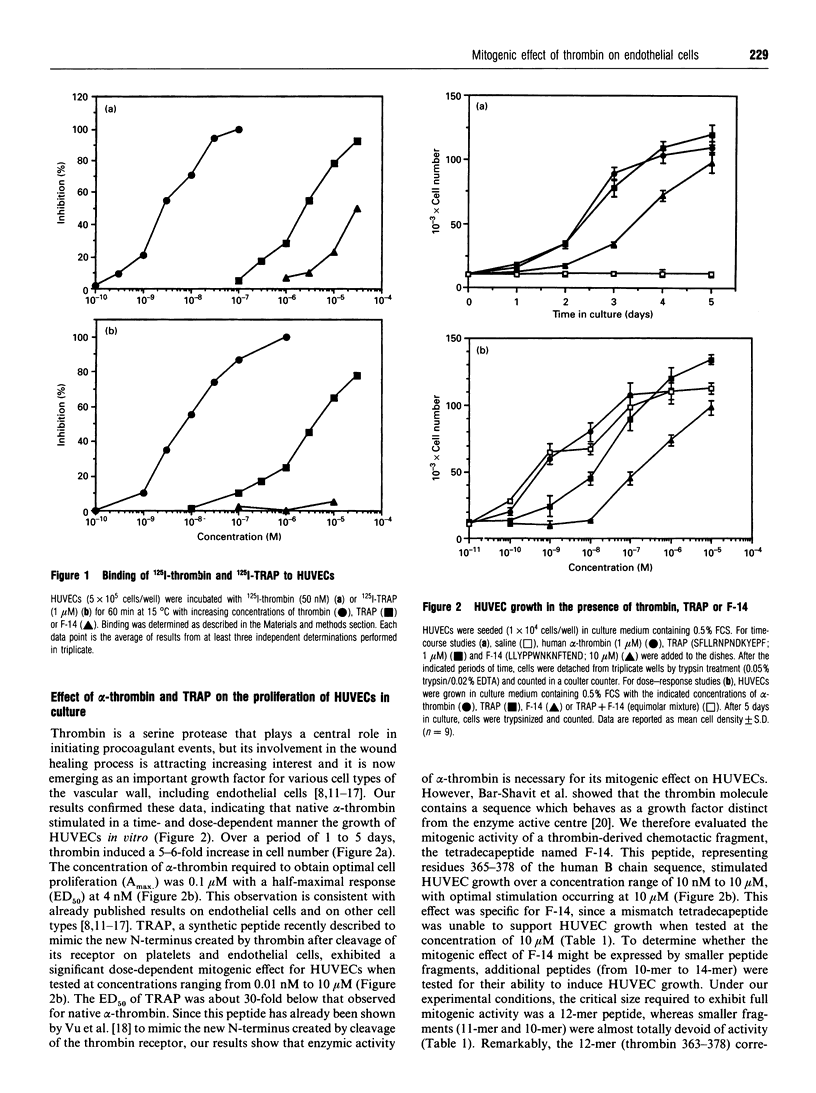
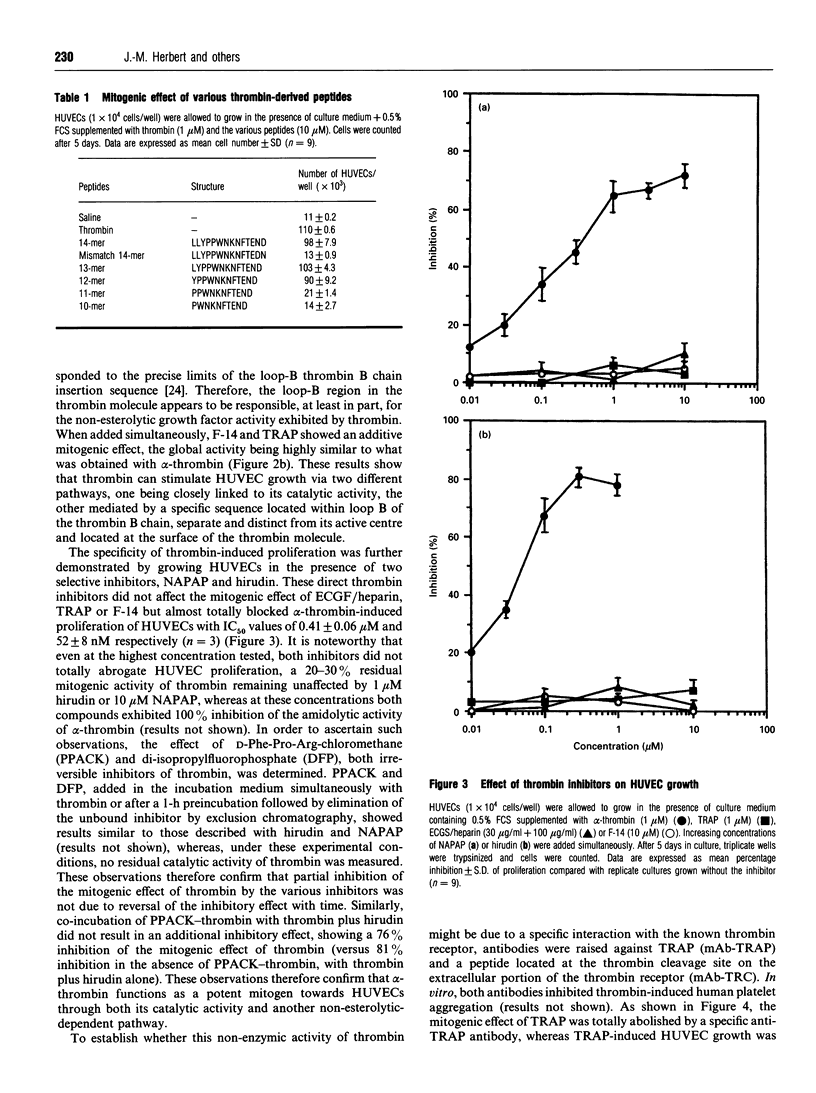
Binding of 125I-thrombin to human umbilical vein endothelial cells (HUVECs) was specifically displaced by the synthetic tetradecapeptide SFLLRNPNDKYEPF, named thrombin receptor agonist peptide (TRAP), which has recently been described as a peptide mimicking the new N-terminus created by cleavage of the thrombin receptor, and F-14, a tetradecapeptide representing residues 365-378 of the human alpha-thrombin B chain. Binding of 125I-TRAP to HUVECs was time-dependent, reversible and saturable, showing high affinity (KD = 1.5 +/- 0.4 microM) and high binding capacity (Bmax. = 7.1 +/- 0.6 x 10(6) sites/cell) (n = 3). Unlabelled thrombin and TRAP competitively and selectively inhibited the specific binding of 125I-TRAP with IC50 values of 5.8 +/- 0.7 nM and 2.8 +/- 0.4 microM respectively, whereas F-14 remained ineffective at displacing 125I-TRAP from its binding sites, suggesting the presence of at least two different types of thrombin-binding sites on HUVECs. TRAP was a potent mitogen for HUVECs in culture. Both TRAP and alpha-thrombin stimulated the proliferation of HUVECs with half-maximum mitogenic responses between 1 and 10 nM. F-14 also promoted HUVEC growth. The mitogenic effects of F-14 and TRAP were additive. N alpha-(2-Naphthylsulphonylglycyl)-DL-p-amidinophenylalanylpiper idine (NAPAP) and hirudin (two specific inhibitors of the enzyme activity of thrombin) specifically inhibited thrombin-induced HUVEC growth (IC50 values 400 +/- 60 and 52 +/- 8 nM respectively) but remained without effect on the mitogenic effect of TRAP or F-14. This demonstrated that the mitogenic effect of alpha-thrombin for HUVECs was intimately linked to its esterolytic activity but also showed that thrombin can stimulate HUVEC growth via another non-enzymic pathway. This hypothesis was further reinforced by the fact that F-14-induced proliferation of HUVECs remained unaltered by two antibodies directed against TRAP or the cleavage site on the extracellular portion of the thrombin receptor, which both strongly reduced thrombin-induced proliferation of HUVECs. Thrombin-, TRAP- or F-14-induced HUVEC proliferation was strongly inhibited by a neutralizing monoclonal antibody directed against basic fibroblast growth factor (bFGF), suggesting that thrombin regulates the autocrine release of bFGF in HUVECs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Benezra M., Eldor A., Hy-Am E., Fenton J. W., 2nd, Wilner G. D., Vlodavsky I. Thrombin immobilized to extracellular matrix is a potent mitogen for vascular smooth muscle cells: nonenzymatic mode of action. Cell Regul. 1990 May;1(6):453–463. doi: 10.1091/mbc.1.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A. J., Mann K. G., Wilner G. D. Identification of a thrombin sequence with growth factor activity on macrophages. Proc Natl Acad Sci U S A. 1986 Feb;83(4):976–980. doi: 10.1073/pnas.83.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Cell surface action of thrombin is sufficient to initiate division of chick cells. Cell. 1978 Aug;14(4):811–823. doi: 10.1016/0092-8674(78)90337-9. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Teng N. N., Buchanan J. M. Mitogenicity of thrombin and surface alterations on mouse splenocytes. Exp Cell Res. 1976 Aug;101(1):41–46. doi: 10.1016/0014-4827(76)90409-2. [DOI] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Dupuy E., Bikfalvi A., Rendu F., Toledano S. L., Tobelem G. Thrombin mitogenic responses and protein phosphorylation are different in cultured human endothelial cells derived from large and microvessels. Exp Cell Res. 1989 Dec;185(2):363–372. doi: 10.1016/0014-4827(89)90306-6. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):165–169. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J. M., Lamarche I., Dol F. Induction of vascular smooth muscle cell growth by selective activation of the thrombin receptor. Effect of heparin. FEBS Lett. 1992 Apr 20;301(2):155–158. doi: 10.1016/0014-5793(92)81237-g. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Ives H. E. Growth inhibition by protein kinase C late in mitogenesis. 1987 Oct 29-Nov 4Nature. 329(6142):849–850. doi: 10.1038/329849a0. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani M. G., Colotta F., Polentarutti N., Pedenovi M., Mantovani A., Dejana E. Thrombin induces c-fos expression in cultured human endothelial cells by a Ca2(+)-dependent mechanism. Blood. 1990 Sep 15;76(6):1173–1180. [PubMed] [Google Scholar]
- Lum H., Andersen T. T., Siflinger-Birnboim A., Tiruppathi C., Goligorsky M. S., Fenton J. W., 2nd, Malik A. B. Thrombin receptor peptide inhibits thrombin-induced increase in endothelial permeability by receptor desensitization. J Cell Biol. 1993 Mar;120(6):1491–1499. doi: 10.1083/jcb.120.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Moreno F., García-Barreno P. Mitogenic activity and inositide metabolism in thrombin-stimulated pig aorta endothelial cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1302–1309. doi: 10.1016/0006-291x(87)91579-8. [DOI] [PubMed] [Google Scholar]
- Nelken N. A., Soifer S. J., O'Keefe J., Vu T. K., Charo I. F., Coughlin S. R. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992 Oct;90(4):1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. J., Marks V. Methods for the preparation of enzyme-antibody conjugates for use in enzyme immunoassay. Methods Enzymol. 1981;73(Pt B):147–166. doi: 10.1016/0076-6879(81)73062-3. [DOI] [PubMed] [Google Scholar]
- Shuman M. A. Thrombin-cellular interactions. Ann N Y Acad Sci. 1986;485:228–239. doi: 10.1111/j.1749-6632.1986.tb34585.x. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weiss R. H., Maduri M. The mitogenic effect of thrombin in vascular smooth muscle cells is largely due to basic fibroblast growth factor. J Biol Chem. 1993 Mar 15;268(8):5724–5727. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]


