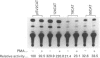Abstract
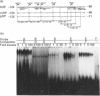
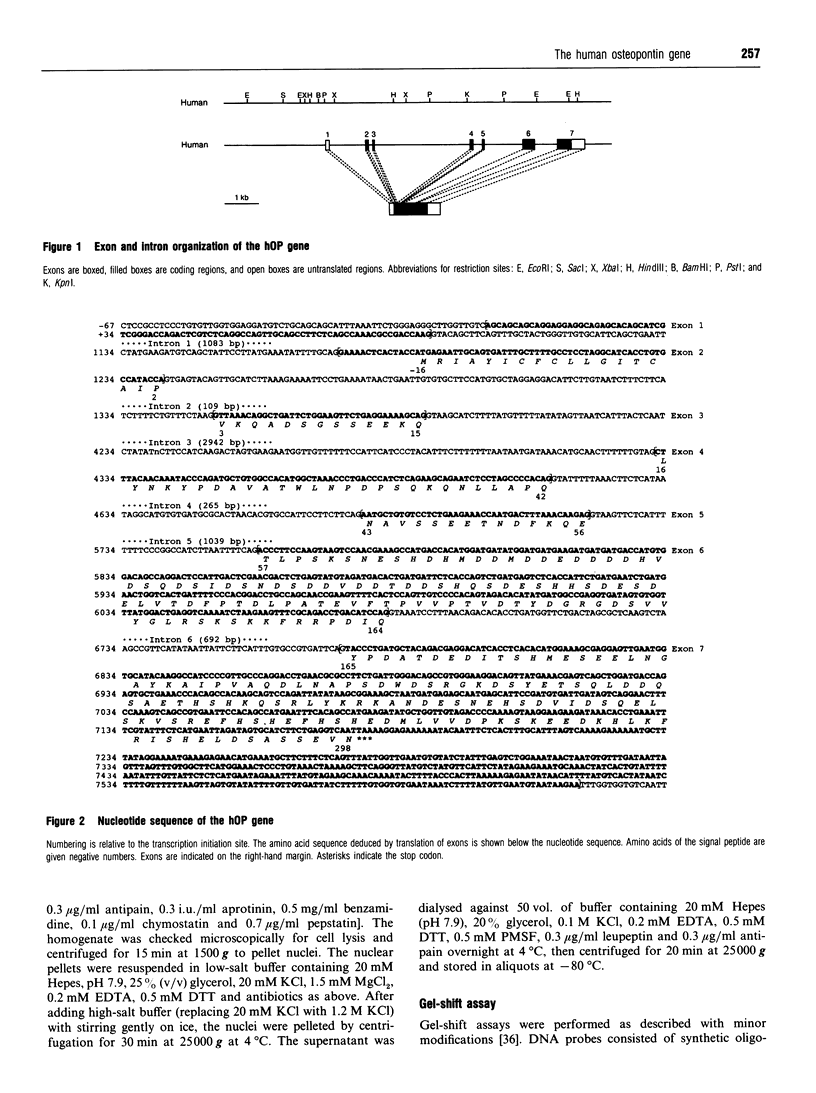
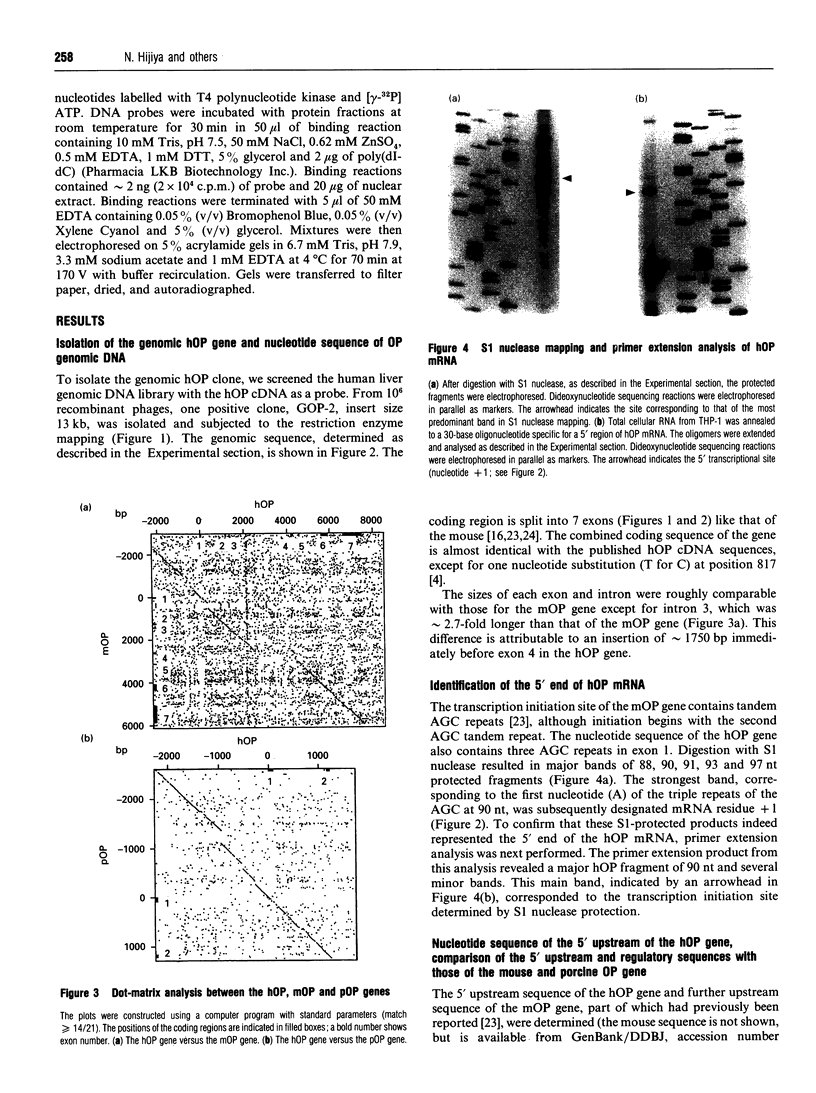
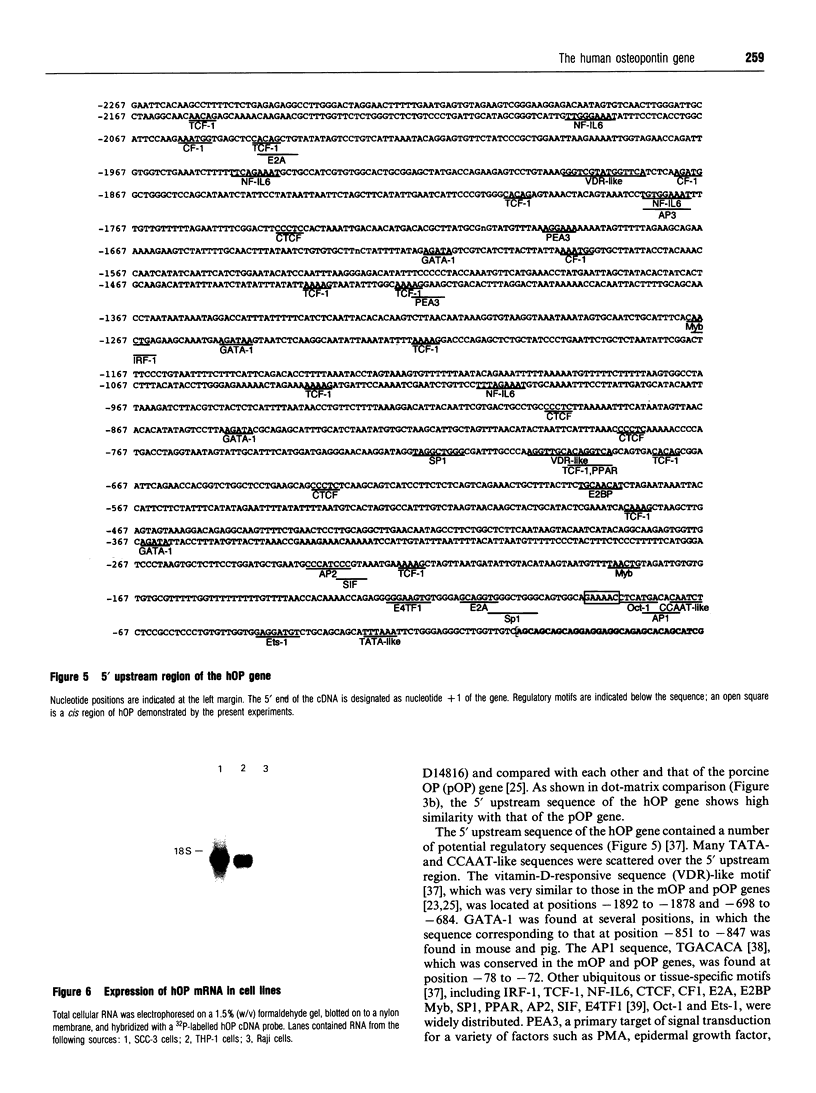
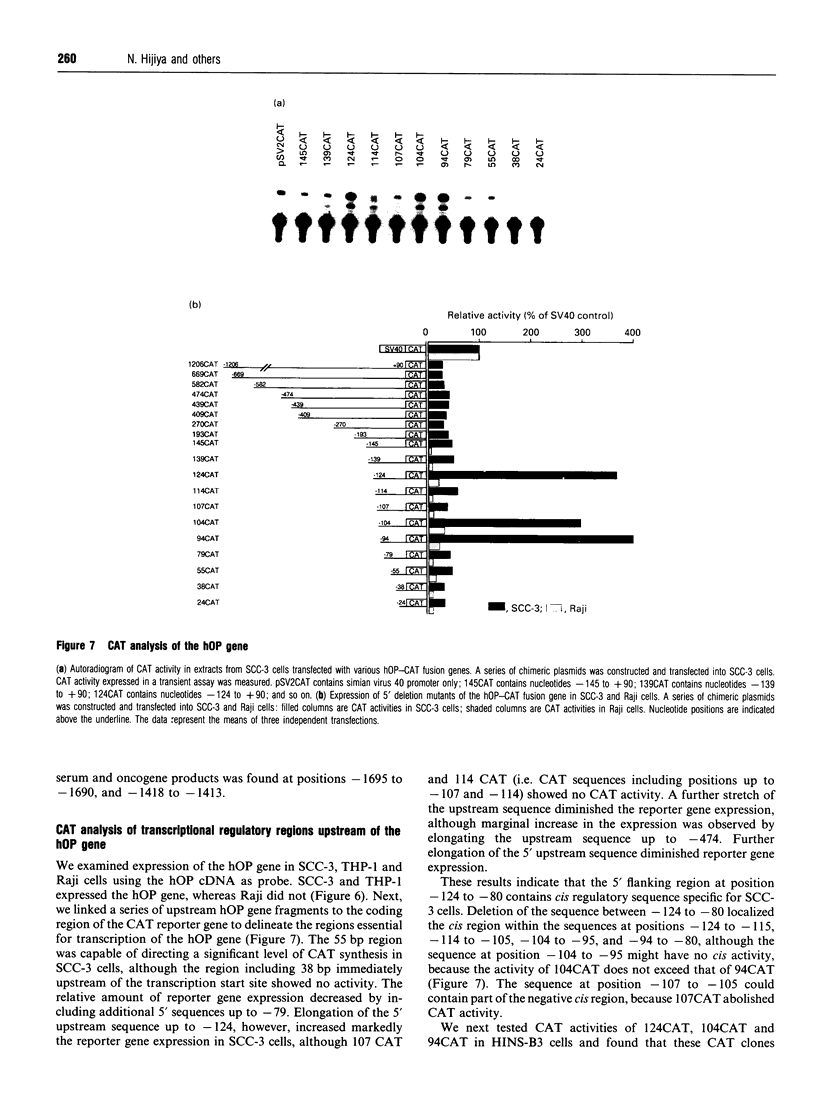
We isolated the human osteopontin (hOP) gene and the 5' upstream region, and analysed its exon-intron structure and potential regulatory sequences of the promoter region in comparison with those of the mouse and porcine gene. The coding sequence is split into 7 exons which are similar to those of the mouse gene, although the hOP gene is longer than the mouse gene. The difference in length is mainly due to variations in intron 3, which is approximately 2.7-fold longer than that of the mouse OP gene. The 5' upstream region of the hOP, which is highly conserved up to nucleotide -250, contains a number of potential cis regulatory consensus sequences. A series of sequentially 5'-deleted chimeric clones was tested for the ability to stimulate chloramphenicol acetyltransferase (CAT). Initial CAT analysis demonstrated that nucleotides at positions -474 to -270, -124 to -80, and -55 to -39 contained cis-acting enhancing sequences in a human monocyte cell line, SCC-3, although the -124 to -80 region was much more active than other regions. Deletion of the sequences between -474 and -270 localized this cis region to the sequence at positions -439 to -410, whereas the deletion between -124 to -80 localized the regions to -124 to -115, and -94 to -80. Gel-shift analysis using as probes synthesized double-stranded DNA corresponding to the 10 and 15 bp region at positions -124 to -115 and -94 to -80 respectively revealed that each probe formed a major band complexed with nuclear proteins prepared from SCC-3 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Behrend E. I., Chambers A. F., Wilson S. M., Denhardt D. T. Comparative analysis of two alternative first exons reported for the mouse osteopontin gene. J Biol Chem. 1993 May 25;268(15):11172–11175. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castagnola P., Bet P., Quarto R., Gennari M., Cancedda R. cDNA cloning and gene expression of chicken osteopontin. Expression of osteopontin mRNA in chondrocytes is enhanced by trypsin treatment of cells. J Biol Chem. 1991 May 25;266(15):9944–9949. [PubMed] [Google Scholar]
- Craig A. M., Denhardt D. T. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene. 1991 Apr;100:163–171. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss G. H., Lieber M. R. DEAE-dextran enhances electroporation of mammalian cells. Nucleic Acids Res. 1992 Dec 25;20(24):6739–6740. doi: 10.1093/nar/20.24.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y., Setoguchi M., Yoshida S., Akizuki S., Yamamoto S. Enhancement of c-fos expression is associated with activated macrophages. Oncogene. 1988 May;2(5):515–521. [PubMed] [Google Scholar]
- Kasugai S., Nagata T., Sodek J. Temporal studies on the tissue compartmentalization of bone sialoprotein (BSP), osteopontin (OPN), and SPARC protein during bone formation in vitro. J Cell Physiol. 1992 Sep;152(3):467–477. doi: 10.1002/jcp.1041520305. [DOI] [PubMed] [Google Scholar]
- Kasugai S., Todescan R., Jr, Nagata T., Yao K. L., Butler W. T., Sodek J. Expression of bone matrix proteins associated with mineralized tissue formation by adult rat bone marrow cells in vitro: inductive effects of dexamethasone on the osteoblastic phenotype. J Cell Physiol. 1991 Apr;147(1):111–120. doi: 10.1002/jcp.1041470115. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Bauer D. M., Barr P. J. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989 Apr 25;17(8):3306–3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Toki H., Okabe K., Suzuki K., Hayakawa T., Miyamoto K. Establishment and characterization of a monocytic cell line which expresses the interleukin-2 receptor. Jpn J Cancer Res. 1986 Sep;77(9):862–865. [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- Matsuura K., Ishida T., Setoguchi M., Higuchi Y., Akizuki S., Yamamoto S. Identification of a tissue-specific regulatory element within the murine CD14 gene. J Biol Chem. 1992 Oct 25;267(30):21787–21794. [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. Nucleotide sequence of cDNA for mouse osteopontin-like protein. Nucleic Acids Res. 1989 Apr 25;17(8):3298–3298. doi: 10.1093/nar/17.8.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone. J Cell Biol. 1989 Feb;108(2):713–718. doi: 10.1083/jcb.108.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Hasson D. M., Rodan G. A. Leukemia inhibitory factor suppresses proliferation, alkaline phosphatase activity, and type I collagen messenger ribonucleic acid level and enhances osteopontin mRNA level in murine osteoblast-like (MC3T3E1) cells. Endocrinology. 1990 Jul;127(1):185–190. doi: 10.1210/endo-127-1-185. [DOI] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., Regnier D. C., Kozak C. A., Mock B. A., Morse H. C., 3rd Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989 Jul 1;170(1):145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Oosawa T., Butler W. T., Tomana M., Bhown A. S., Bhown M., Schrohenloher R. E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987 Feb 25;262(6):2900–2907. [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Yoon K., Rodan G. A. Opposing effects of fibroblast growth factor and pertussis toxin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. J Biol Chem. 1989 Nov 25;264(33):19934–19941. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Smith J. H., Denhardt D. T. Molecular cloning of a tumor promoter-inducible mRNA found in JB6 mouse epidermal cells: induction is stable at high, but not at low, cell densities. J Cell Biochem. 1987 May;34(1):13–22. doi: 10.1002/jcb.240340103. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Sauk J. J., Foster R. A., Butler W. T. Mechanism of fibroblast attachment to bone extracellular matrix: role of a 44 kilodalton bone phosphoprotein. J Bone Miner Res. 1987 Jun;2(3):259–265. doi: 10.1002/jbmr.5650020313. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Wada T., Handa H. Transcription factor E4TF1 contains two subunits with different functions. EMBO J. 1990 Mar;9(3):841–847. doi: 10.1002/j.1460-2075.1990.tb08181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Zhang Q., Sodek J. Full length cDNA sequence of porcine secreted phosphoprotein-I (SPP-I, osteopontin). Nucleic Acids Res. 1989 Dec 11;17(23):10119–10119. doi: 10.1093/nar/17.23.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wrana J. L., Sodek J. Characterization of the promoter region of the porcine opn (osteopontin, secreted phosphoprotein 1) gene. Identification of positive and negative regulatory elements and a 'silent' second promoter. Eur J Biochem. 1992 Jul 15;207(2):649–659. doi: 10.1111/j.1432-1033.1992.tb17092.x. [DOI] [PubMed] [Google Scholar]