Abstract
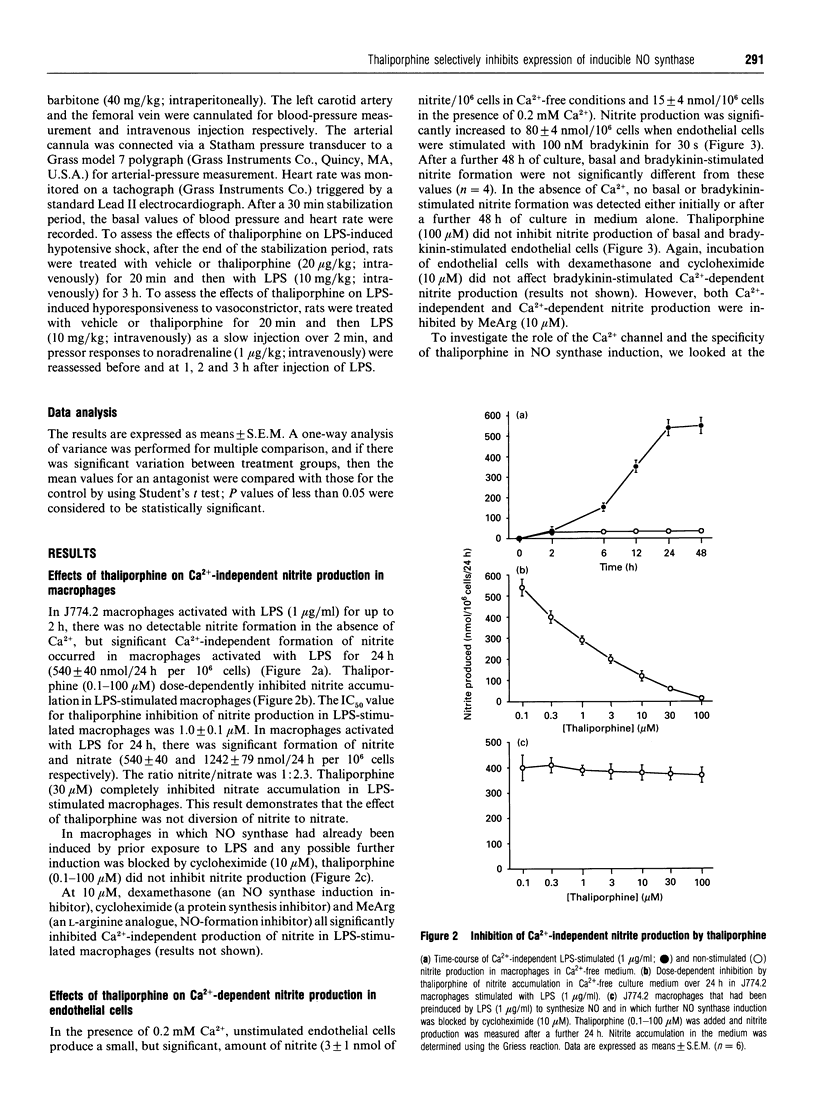
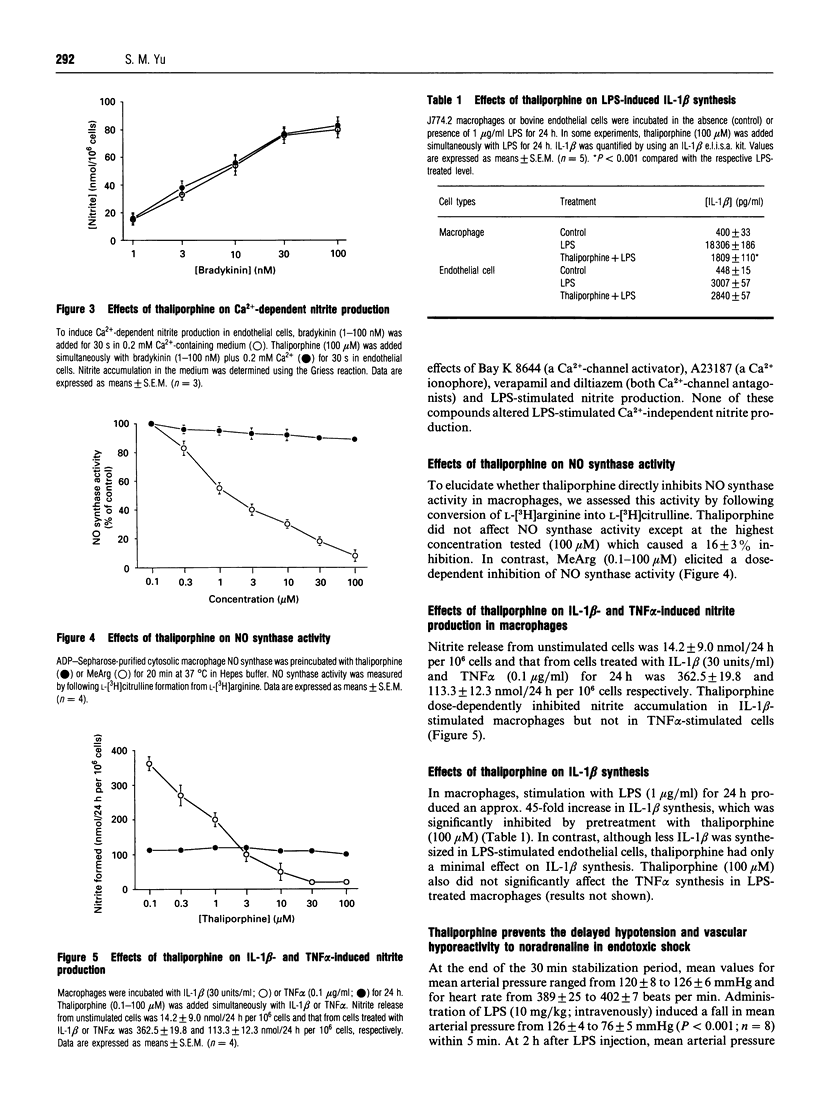
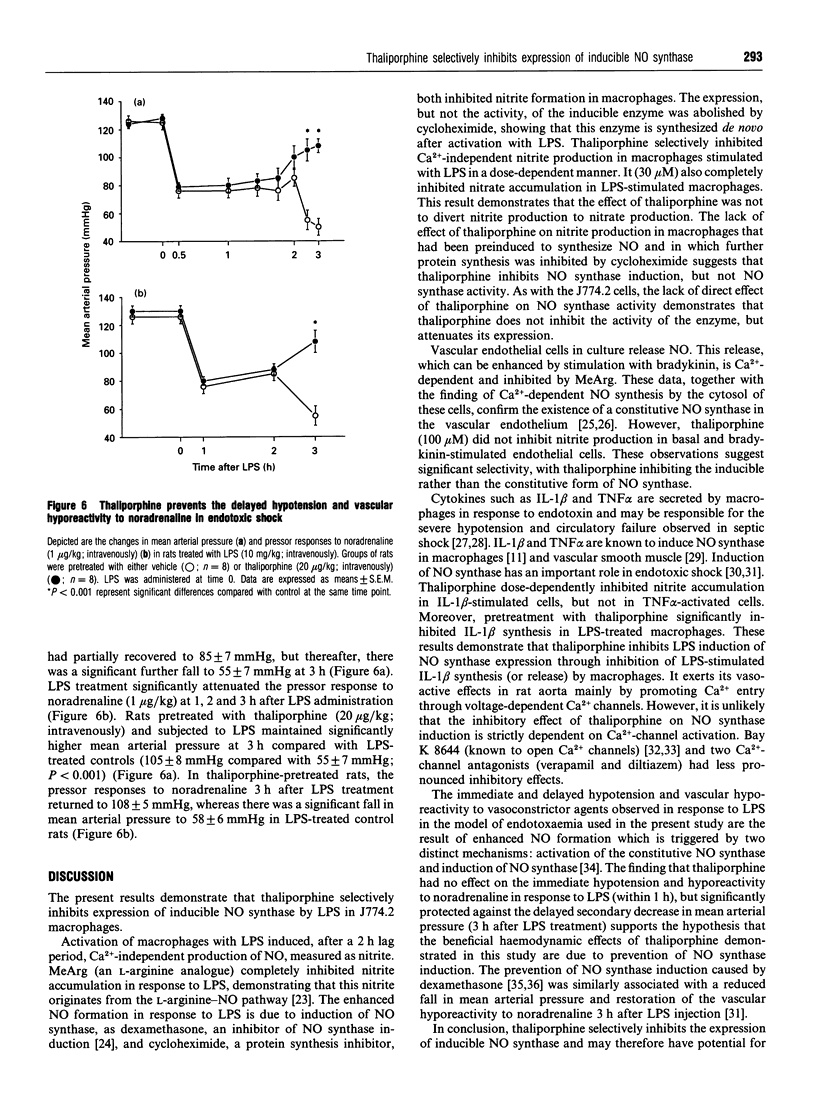
Formation of nitrites/nitrates caused by lipopolysaccharide (LPS) in J774.2 macrophages was inhibited by thaliporphine, an aporphine derivative isolated from the plant Neolitsea konishii K. This inhibition of nitrite synthesis in LPS-stimulated macrophages by thaliporphine was similar to that by cycloheximide, NG-methyl-L-arginine (MeArg) and dexamethasone. Thaliporphine, but not MeArg, inhibited expression of inducible NO synthase without directly affecting enzyme activity. However, thaliporphine did not inhibit nitrite production by NO synthase that had already been induced by prior exposure to LPS for which any possible further induction was inhibited by cycloheximide. In endothelial cells, nitrite formation induced by bradykinin (in the presence of 0.2 mM Ca2+) was inhibited by MeArg. However, incubation of endothelial cells with dexamethasone, cycloheximide and thaliporphine did not affect this Ca(2+)-dependent nitrite production. Thaliporphine (0.1-100 microM) dose-dependently inhibited nitrite accumulation in macrophages stimulated by interleukin-1 beta (IL-1 beta) whereas nitrite formation induced by tumour necrosis factor alpha was not inhibited. LPS-stimulated IL-1 beta synthesis in macrophages was significantly inhibited by thaliporphine, but thaliporphine had only minimal effect on LPS-stimulated IL-1 beta synthesis in endothelial cells. These results demonstrate that thaliporphine inhibits LPS induction of NO synthase expression, and that the mechanism of action of thaliporphine is via inhibition of LPS-stimulated IL-1 beta synthesis in macrophages. In anaesthetized rats subjected to LPS, pretreatment with thaliporphine partially restored the fall in mean arterial pressure and the vascular hyporeactivity to noradrenaline 3 h after LPS injection. In conclusion, thaliporphine selectively inhibited expression of inducible NO synthase, and may thus hold potential for the treatment of endotoxaemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad R., Saá J. M., Cava M. P. Regioselective O-demethylation in the aporphine alkaloid series. J Org Chem. 1977 Apr 1;42(7):1228–1230. doi: 10.1021/jo00427a028. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Invest. 1991 Dec;21(6):559–573. doi: 10.1111/j.1365-2362.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Bone R. C. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991 Feb 15;114(4):332–333. doi: 10.7326/0003-4819-114-4-332. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990 Jun 4;265(1-2):133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Franckowiak G., Bechem M., Schramm M., Thomas G. The optical isomers of the 1,4-dihydropyridine BAY K 8644 show opposite effects on Ca channels. Eur J Pharmacol. 1985 Aug 15;114(2):223–226. doi: 10.1016/0014-2999(85)90631-4. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Bridge L., Foxwell N. A., Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol. 1992 Jan;105(1):11–12. doi: 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1993 May;265(2):674–680. [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Teng C. M., Yu S. M., Ko F. N., Chen C. C., Huang Y. L., Huang T. F. Dicentrine, a natural vascular alpha 1-adrenoceptor antagonist, isolated from Lindera megaphylla. Br J Pharmacol. 1991 Nov;104(3):651–656. doi: 10.1111/j.1476-5381.1991.tb12484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. M., Yu S. M., Lee S. S., Ko F. N., Su M. J., Huang T. F. Vasoconstricting effect in rat aorta caused by thaliporphine isolated from the plant Neolitsea konishii K. Eur J Pharmacol. 1993 Mar 16;233(1):7–12. doi: 10.1016/0014-2999(93)90342-f. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yu S. M., Ko F. N., Su M. J., Wu T. S., Wang M. L., Huang T. F., Teng C. M. Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):349–355. doi: 10.1007/BF00168697. [DOI] [PubMed] [Google Scholar]
- Yu S. M., Ko F. N., Su M. J., Wu T. S., Wang M. L., Huang T. F., Teng C. M. Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):349–355. doi: 10.1007/BF00168697. [DOI] [PubMed] [Google Scholar]


