Abstract

In this study, an add-on preconcentration device powered by parallelized pretraps (PPTs) was utilized to measure the sub-pmol/mol levels of NF3 in N2. The add-on preconcentrator was coupled to the detachable trap preconcentrator (DTP) with a gas chromatograph–mass spectrometer [Anal. Chem.2019, 91, 3342–3349]. The breakthrough volume of the parallel configuration was found to be substantially higher than that of the serial configuration with the same amount of adsorbent (HayeSep D). Liquid oxygen (LO2) cooling (−183 °C) exhibited better preconcentration performance for NF3 in N2 compared to NF3 in air (N2 + O2) with liquid nitrogen cooling (−195 °C) and NF3 in air with LO2 cooling. The DTP unit was essential to discriminate residual species, such as N2, O2, CO2, and CF4, of which the preconcentrated portion in the PPT can be excessive, enabling the overwhelm filtering capability of the quadrupole mass spectrometer. The limit of detection of NF3 in N2 of the PPT/DTP/gas chromatograph–mass spectrometer was 0.01 ppt, which is significantly better than that determined without using the add-on preconcentration device (0.21 ppt).
1. Introduction
Nitrogen trifluoride (NF3) is a long-lived greenhouse gas (GHG) with an atmospheric lifetime of 500 years.1 Although its concentration in the atmosphere is only approximately 2 pmol/mol, its global warming potential value at the 100 year scale (GWP100) is 16,100-fold greater than that of carbon dioxide.1,2 The use of NF3 has been increasing in the manufacturing of semiconductors, photovoltaic cells, and display devices; consequently, the background concentration of NF3 in the atmosphere has been increasing by approximately 0.1 pmol/mol per year.2−4 As a result, NF3 is now subject to global and regional regulations, making the quantitative assessment of its emissions increasingly critical.3−5 However, to accurately validate GHG emissions, it is essential to reconcile the discrepancy between the “bottom-up” report of industrial measurements and the “top-down” report of atmospheric measurements.6,7 To improve the reliability of emission estimates, it is essential to harmonize the measurement values reported by various stakeholders. Achieving this requires the development of suitable and reliable reference materials, along with sensitive and repeatable measurement methods. However, given that the ambient concentration of NF3 is approximately 2 pmol/mol, preparing and verifying a reference gas mixture (RGM) with acceptable uncertainty present a significant challenge. A previous study of our group reported the limit of detection (LOD) of NF3 to be 0.2 pmol/mol and measurement precision to be 0.35% (1σ) against an ambient-level sample (2 pmol/mol) using a detachable trap preconcentrator (DTP) with a gas chromatograph–mass spectrometer.8 However, the DTP/gas chromatograph–mass spectrometer may not provide sufficient measurement capability for the verification of the preparation process of the atmospheric levels of NF3 in air RGM. One of the primary factors influencing the uncertainty of the RGM in a few pmol/mol is the measurement capability of the target substance presented in the raw gases of matrix components, namely, high-purity N2 and O2. For instance, the sensitivity coefficient of SF6 impurity in N2 raw gas was 7 × 1011 for the gravimetric preparation of RGM of SF6 in air.9 According to ISO 19229:2015, half of the LOD value accounts for the mean value of the nondetected impurity.10 Therefore, for the verification of an atmospheric-level NF3 RGM (∼2 pmol/mol), the LOD must be improved to achieve an unbiased reference value of the RGM and improved uncertainty. Long-term monitoring data of NF3 reported by the AGAGE, of which the calibration standard was maintained by the Scripps Institute of Oceanography (SIO-12, gravimetric, 2% uncertainty), is the only result publicly available up to date.11 By the same group, a clear pattern of monthly NF3 variation has been shown with a measurement precision of 2% (1σ).5 However, improved uncertainty (combined uncertainties of RGM, calibration, and long-term reproducibility) and harmonized measurement among various observation networks have the potential to help a emission tracking model in greater spatial resolution, allowing for region-specific emission estimates.12−15 The present study demonstrates an improved analytical capability for determining the LOD of atmospheric-level NF3 using an add-on preconcentration device, which was powered by parallelized pretraps (PPTs), coupled with a DTP–gas chromatograph–mass spectrometer. The analytical method was optimized by adjusting several factors, including the cooling temperature, number of traps, amount of adsorbent, and feeding flow rate of the sample. Because the LOD is well correlated to the measurement precision around the limit of quantification level,8 the LOD test can be a representative parameter of the measurement capability for the trace-level NF3 at sub-pmol/mol concentrations.
2. Experimental Section
To enhance the preconcentration volume for the atmospheric level of NF3, PPTs were added to the DTP/gas chromatograph–mass spectrometer (the gas chromatograph–mass spectrometer was Agilent 7890, 5957C), as published in the previous study of our group.8 In general, to enhance the detection sensitivity (= MS (mass spectrometric) response/concentration) when using a preconcentrator, the preconcentration volume of the analyte can be increased by decreasing the flow rate of the analyte stream or the preconcentration dwell time. Alternatively, the size of the preconcentration trap, i.e., the amount of adsorbent, can be increased to enlarge the preconcentration volume. In addition, the PPTs must be cooled to enhance the adsorption rate. However, these enhancements are often restricted by the capacity of a cooling instrument. For instance, the DTP/gas chromatograph–mass spectrometer showed that the trap temperature with the PCC Cryotiger PT-14 could not be reduced below −130 °C owing to the limitations of the thermal capacitance of the cold end. Therefore, an extra cooling source such as liquid nitrogen (LN2, boiling point −195 °C) or liquid oxygen (LO2, bp −183 °C) is required. In this study, PPTs were added on to the DTP/gas chromatograph–mass spectrometer. HayeSep D 100/120 was placed in a stainless-steel tube (1/8 in.) and blocked with silanized glass wool to hold the adsorbent granules during gas flow (Figure 1). The trap configurations tested in this study are listed in Table 1. The detection sensitivity depends on the loading amount of the adsorbent. The detection sensitivities of single tubes (100 and 1000 mm) and parallelized tubes (4 and 8 mm each) were compared. Before desorption, the PPT was backflushed with high-purity helium. The cooled DTP was removed from the cold dewar and placed in boiling water with a constant carrier gas (He, 99.999%) flowing into the DTP–gas chromatograph–mass spectrometer (Figure 2). The gas chromatograph–mass spectrometer was operated in the selective ion-monitoring mode at a target mass-to-charge ratio (m/z) of 52 and a qualifier m/z of 71. Gravimetrically prepared NF3 in N2 gas mixtures was analyzed to the performance of the add-on preconcentrator. NF3 in N2 or air gas mixtures was prepared by using high-purity N2, O2 and NF3 gases. Aluminum cylinders (47 L capacity) with electropolished inner surfaces were evacuated to approximately 1 × 10–6 Torr before being used as containers of the NF2/N2 and NF3/air gas mixtures. Further, 0.7 pmol/mol levels of NF3 in N2 or air were fed into the PPT by using a well-calibrated mass flow controller (Brooks Instruments). The amount of sample was quantified by multiplying the flow rate by the preconcentration time.
Figure 1.
Overview of the instrumental configuration of the add-on preconcentrator powered by eight PPTs and the DTP/gas chromatograph–mass spectrometer. Functionality of each component is given in the gray box.
Table 1. Types of Traps for the Add-on Preconcentrator Tested in This Studya.
| adsorbent | length | adsorbent weight | number of trap | |
|---|---|---|---|---|
| short pretrap | HayeSep D 100/120 | 100 mm | 100 mg | 1 |
| long pretrap | HayeSep D 100/120 | 1000 mm | 1000 mg | 1 |
| 4 parallelized pretrap (4-PPT) | HayeSep D 100/120 | 100 mm, each | 100 mg, each | 4 |
| 8 parallelized pretrap (8-PPT) | HayeSep D 100/120 | 100 mm, each | 100 mg, each | 8 |
The inner diameter of the tubes was 1/8 in.
Figure 2.
Schematics of the add-on preconcentrator equipped with a parallelized configuration of HayeSep D adsorbent pretraps cooled by LO2. Preconcentrated NF3 was directed through the detachable traps to effectively separate potent residuals of the matrix and impurities. The chromatogram (inset) was obtained using 0.7 pmol/mol of NF3 in N2. PT denotes the pretrap, DT represents the detachable trap, MFC indicates the mass flow controller, EPC stands for the electric pressure controller, and V denotes the valve.
3. Results and Discussion
3.1. Cooling Temperature Effect
The detection sensitivity for NF3 was investigated in various thermodynamical environments (Figure 3). A short pretrap (HayeSep D 100/120, 100 mg) was employed at a flow rate of 1600 mL/min. Adsorptivity of NF3 (bp −129 °C) with the LN2 cooling (−195 °C) was expected to surpass that with LO2 cooling (−183 °C). However, the NF3 detection sensitivity did not improve in either the N2 matrix with LN2 cooling or the O2 matrix with LO2 cooling, indicating that N2 and O2 predominated at their respective boiling points. Conversely, NF3 in N2 at the LO2 temperature exhibited a significant sensitivity enhancement by increasing sampling amount (= flow rate × sampling time), suggesting a release of free adsorption sites for NF3. Although fine adjustment of the cooling temperature using such a He cooler above the LO2 temperature may further optimize the detection sensitivity of NF3 in air, NF3 in N2 at the LO2 temperature serves as the preset condition for subsequent discussion due to the limitations of our resources.
Figure 3.

MS response as a function of the amount of NF3 injected into the add-on preconcentrator. The sensitivity for NF3 in N2 increases substantially with respect to the sample amount with LO2 cooling (blue triangle). Measurements of NF3 in the air with LO2 cooling (red circle) and that in N2 with LN2 cooling (black rectangular) are not sensitive. A short pretrap (single, HayeSep D 100/120, 100 mg) was used at a flow rate of 1600 mL/min. The amount of NF3 was estimated by multiplying the sampling flow rate, the preconcentration time, and the concentration of NF3 (0.7 pmol/mol). The lines are provided as visual guides without any statistical inference.
3.2. Parallelized Trap Configuration
To assess the preconcentration efficiencies of various pretrap configurations (Table 1), the detection sensitivities of NF3 were measured across different sample amounts (Figure 4). With all analytical parameters held constant except for the pretrap configuration, the slope of the tangential line represents the preconcentration efficiency. The 8-PPT demonstrated the most favorable performance in terms of preconcentration efficiency. Notably, the 8-PPT exhibited a linear response to the sampling amount, namely, the preconcentration time, below a sensitivity threshold of 5000. Although the total amount of adsorbent in 8-PPT (800 mg) was lower than that in the long pretrap (1000 mg), the preconcentration efficiency of the 8-PPT surpassed that of the long pretrap. Even the 4-PPT (400 mg) demonstrated superior preconcentration efficiency compared to that of the long pretrap (1000 mg). This suggests an increase in the number of active sites for NF3 in the PPT configurations. To elucidate this behavior, the Brunauer–Emmett–Teller (BET) isotherm, which is a common adsorption model at low temperatures, provided below can be considered.16,17
| 1 |
where q(p) is the absolute loading of the adsorbed phase as a function of pressure, qsat is the saturation loading, and bs and bl are the equilibrium constants of adsorption on the bare surface and on a layer of previously adsorbed adsorbates, respectively. The BET model extends the Langmuir model by allowing for multilayer formation, where the binding energy of sites in the second layer and beyond is equal to the heat of liquefaction. This model is effective for characterizing various sorbents at low temperatures. It has been reported that the BET isotherm describes the adsorption behaviors of N2 and CH4 into the HayeSep D well.18 HayeSep D demonstrated superior selectivity for CH4 over N2 in a stable cold bath at −192 °C. However, NF3 at 0.7 pmol/mol exhibited lower selectivity to HayeSep D than matrix N2 at −192 °C (as shown in black in Figure 3). Even at −182 °C, matrix O2 showed dominant adsorption selectivity to HayeSep D compared to 0.7 pmol/mol of NF3 (as shown in red in Figure 3). As discussed in the previous section, these phenomena suggest that the adsorption of O2 and N2 to HayeSep D significantly increases at their respective boiling points, implying that NF3 should be mixed with a matrix gas with a boiling point higher than that of the bath coolant. This scenario can be tested by the ideal adsorption solution theory, which can predict multicomponent adsorption isotherms from only the pure-component adsorption isotherms at the same temperature.17,19 However, the lack of isotherm parameters for NF3 in HayeSep D results in an incomplete quantitative interpretation of the above scenario. Generally, the component of the binary mixture with the highest saturation loading dominates adsorption at high pressures, potentially displacing the other component (as shown in Figure 3 in ref (17)). It should be noted that the optimal isotherm model and equilibrium constants for NF3 and HayeSep D need to be investigated using a single adsorption bay.17,19,20
Figure 4.

MS response as a function of the amount of NF3 injected into the add-on preconcentrator, compared across different configurations: 8-PPT (green diamond), 4-PPT (blue triangle), long single pretrap (red rectangle), and short single pretrap (black rectangle). Detailed configurations can be found in Table 1. All measurements were carried out at an identical sample flow rate of 1600 mL/min. The amount of NF3 was estimated by multiplying the sampling flow rate, the preconcentration time, and the concentration of NF3 (0.7 pmol/mol). The lines are provided as visual guides without any statistical inference.
Qualitatively speaking, trace-level NF3 mixed with the matrix components had a chance to be adsorbed by free sites in the initial part of the adsorption column. However, free sites in the later part of the pretrap were strongly preferred by the predominant matrix components. Once the adsorption sites are occupied by matrix gas O2 or N2 due to high selectivity, it appears that the second layer is filled with the same species, leaving no opportunity for additional adsorption of NF3. As indicated by the saturated plateau of the sensitivity plot (black in Figure 4), no further adsorption of NF3 occurred, suggesting the validity of this scenario. The fixed-bed adsorption simulation, a numerical method that takes into account molecular diffusion and mass transfer,17 can predict the gas uptake of each component of a mixture under flowing conditions. However, the absence of isotherm parameters limits the precise prediction of preconcentration parameters, such as flow rate and preconcentration temperature. Although the lack of isotherm parameters hinders the precise prediction of preconcentration parameters such as the flow rate, amount of absorbent, preconcentration time, and cooling temperature, this limitation was overcome by employing a parallelized configuration of multiple columns with reduced length. As demonstrated in Figure 4, the sensitivity of the 8-PPT (green, with a total of 800 mg of HayeSep D) is even higher than that of a single long trap (red, with a total of 1000 mg of HayeSep D). The number of parallelized traps can be further increased to enhance preconcentration efficiency while maintaining the flow rate at each individual trap.
3.3. Flow Rate Effect
The detection sensitivities of NF3 at various flow rates (1600, 2000, 2400, and 3200 mL/min) with the 8-PPT were tested to address the dynamic isotherm. For further analysis, the modified Wheeler model (MHM) can be considered (eq 2). The breakthrough volume was defined as an appropriate collection volume for a sample mixture in which the analyte does not pass through the adsorbent beds during preconcentration21
| 2 |
where We is the
kinetic adsorption capacity (adsorbate mass/adsorbent mass),  is the residence time (min), ρb is the packed adsorbent density (g/cm3), Q is the volumetric flow rate (cm3/min), Wb is the adsorbent mass (g), ka is the adsorption kinetic constant (min–1), C0 is the inlet concentration (g/cm3), and Cx is
the outlet concentration (g/cm3). The term
is the residence time (min), ρb is the packed adsorbent density (g/cm3), Q is the volumetric flow rate (cm3/min), Wb is the adsorbent mass (g), ka is the adsorption kinetic constant (min–1), C0 is the inlet concentration (g/cm3), and Cx is
the outlet concentration (g/cm3). The term 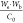 represents the total preconcentration volume
of the sample at C0 required to reach
thermodynamic equilibrium, Vt. The adsorption
efficiency corresponds to the term
represents the total preconcentration volume
of the sample at C0 required to reach
thermodynamic equilibrium, Vt. The adsorption
efficiency corresponds to the term 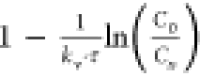 ; therefore,
; therefore,  is the fractional unused bed capacity (FUBC)
for the adsorbate. The breakthrough volume can be interpreted as the
preconcentration efficiency in the ideal mass transfer of adsorbed
analytes from the PPT to the subsequent stage. The slope of the sensitivity
curve remained constant until the flow rate reached 2000 mL/min, indicating
an increase in the chance of contact (adsorbate–adsorbent contact
rate) with the analyte for adsorption on the seed site (Figure 5). However, when operating
at flow rates of >2400 mL/min, a reduction in preconcentration
efficiency
was noted. This phenomenon could be attributed to the shorter residence
time of the analyte in the pretrap. According to the MHM, the breakthrough
volume drastically decreases at low residence times, i.e., high flow
rates, as demonstrated in our results. However, the residence time
only scaled the FUBC. Therefore, the decline in the preconcentration
volume can be explained by the conjecture that Cx is higher than C0, indicating that desorption of the adsorbed NF3 was activated
at high flow rates of the sweep gas N2.22 The decline in the preconcentration volume in the short
pretrap at 1600 mL/min (black of Figure 4) can be explained by the same origin. Under
the same input flow rate, the flow rate at the end point of the short
pretrap appeared to be higher in some extent compared to that of the
long pretrap due to a weak pressure drop.
is the fractional unused bed capacity (FUBC)
for the adsorbate. The breakthrough volume can be interpreted as the
preconcentration efficiency in the ideal mass transfer of adsorbed
analytes from the PPT to the subsequent stage. The slope of the sensitivity
curve remained constant until the flow rate reached 2000 mL/min, indicating
an increase in the chance of contact (adsorbate–adsorbent contact
rate) with the analyte for adsorption on the seed site (Figure 5). However, when operating
at flow rates of >2400 mL/min, a reduction in preconcentration
efficiency
was noted. This phenomenon could be attributed to the shorter residence
time of the analyte in the pretrap. According to the MHM, the breakthrough
volume drastically decreases at low residence times, i.e., high flow
rates, as demonstrated in our results. However, the residence time
only scaled the FUBC. Therefore, the decline in the preconcentration
volume can be explained by the conjecture that Cx is higher than C0, indicating that desorption of the adsorbed NF3 was activated
at high flow rates of the sweep gas N2.22 The decline in the preconcentration volume in the short
pretrap at 1600 mL/min (black of Figure 4) can be explained by the same origin. Under
the same input flow rate, the flow rate at the end point of the short
pretrap appeared to be higher in some extent compared to that of the
long pretrap due to a weak pressure drop.
Figure 5.

MS response as a function of the amount of NF3 injected into the add-on preconcentrator, compared across different flow rates: 1600 mL/min (green triangle), 2000 mL/min (blue triangle), 2400 mL/min (red circle), and 3200 mL/min (black rectangle) with 8-PPT. The amount of NF3 was estimated by multiplying the sampling flow rate, the preconcentration time, and the concentration of NF3 (0.7 pmol/mol). The lines are provided as visual guides without any statistical inference.
4. Conclusions
This study demonstrated that a parallelized adsorption trap configuration can enhance preconcentration efficiency when coupled with a DTP/gas chromatograph–mass spectrometer. In comparison to the serial configuration, which involves single and elongated columns with similar amounts of adsorbent per unit length, the parallelized configuration preserves active sites for ultratrace level of NF3 in a flowing condition. Due to the unavailability of adsorption isotherm parameters for NF3 and N2 with HayeSep D at −183 °C, precise modeling and trap design were challenging. Despite this constraint, we observed that the 8-PPT configuration (8 × 100 mg) delivered optimal performance at a flow rate of 2000 mL/min for preconcentrating sub-pmol/mol NF3 in N2 mixtures. At this temperature and flow rate, the physisorption of N2 was less effective than under this condition. Therefore, achieving further improvement in NF3 preconcentration in air using PPTs necessitates temperature adjustment above the LO2 temperature, which can be achieved with refrigerant-based cooling devices such as a He cooler with a temperature compensating unit. While fine temperature adjustment was not pursued in this study, it presents a complementary approach for NF3 preconcentration, which could enhance the measurement capability of advanced preconcentration technologies like Medusa-like preconcentrators.5,8,23−25 Experimental and theoretical determination of adsorption isotherm parameters and model for N2, O2, and NF3 with HayeSep D at temperatures other than LN2 is imperative to further enhance the preconcentration efficiency of atmospheric NF3 samples. Nevertheless, the incorporation of PPT add-on devices substantially improved the detection sensitivity of NF3 by a factor of 20 compared to that obtained using a bare DTP/gas chromatograph–mass spectrometer, with an LOD of NF3 at 0.2 ppt. This enhancement facilitates high-quality measurement of sub-pmol/mol levels of NF3 for gravimetric standard preparation and atmospheric NF3 monitoring. It is noteworthy that DTP- or Medusa-based preconcentrators are essential for discriminating interfering substances, such as CO2, N2, O2, and CF4, preconcentrated in the PPT. These substances can interfere with the chromatogram of NF3 due to excessive appearance by substantial ultracold physisorption at LO2 and LN2 temperatures.
Acknowledgments
The authors thank for fruitful discussion by Dr. Jeongsoon Lee and financial support by Dr. Dohyun Kwon. This work was funded by the Korea Meteorological Administration (KMA) under the project of development of continuous measurement of halogenated greenhouse gases at the background atmospheric concentration level (KMI2022-01410), the Ministry of Trade, Industry and Energy (MOTIE) under the project of development of monitoring and analysis technologies for greenhouse gases in the semiconductor manufacturing etching process (RS-2023-00265582), and the Korea Research Institute of Standards and Science (KRISS) under the basic R&D project of establishing measurement standards for climate monitoring based on molecular spectroscopy (grant no. 24011107).
Author Contributions
∥ D.Y. and T.K. equally contributed to this work.
The authors declare no competing financial interest.
References
- Chen D.; Rojas M.; Samset B. H.; Cobb K.; Diongue-Niang A.; Edwards P.; Emori S.; Faria S. H.; Hawkins E.; Hope P.; Huybrechts P.; Meinshausen M.; Mustafa S. K. E. A. R.; Plattner G.-K.; Treguier A. M.; Lai H.-W.; Villaseñor T.; Barimalala R.; Carmona R.; Cox P. M.; Cramer W.; Doblas-Reyes F. J.; Dolman H.; Dosio A.; Eyring V.; Flato G. M.; Forster P.; Frame D.; Frieler K.; Fuglestvedt J. S.; Fyfe J. C.; Garschagen M.; Gergis J.; Gillett N. P.; Grose M.; Guilyardi E.; Guivarch C.; Hassol S.; Hausfather Z.; Hersbach H.; Hewitt H. T.; Howden M.; Huggel C.; Hurlbert M.; Jones C.; Jones R. G.; Kaufman D. S.; Kopp R. E.; Leiserowitz A.; Lempert R. J.; Lewis J.; Liao H.; Lovenduski N.; Lund M. T.; Mach K.; Maraun D.; Marotzke J.; Minx J.; Nicholls Z. R. J.; O’Neill B. C.; Ogaz M. G.; Otto F.; Parker W.; Parmesan C.; Pearce W.; Pedace R.; Reisinger A.; Renwick J.; Riahi K.; Ritchie P.; Rogelj J.; Sapiains R.; Satoh Y.; Seneviratne S. I.; Shepherd T. G.; Sillmann J.; Silva L.; Slangen A. B. A.; Sörensson A. A.; Steinle P.; Stocker T. F.; Stockhause M.; Stone D.; Swann A.; Szopa S.; Takayabu I.; Tebaldi C.; Terray L.; Thorne P. W.; Trewin B.; Trigo I.; van Aalst M. K.; van den Hurk B.; van Vuuren D.; Vautard R.; Vera C.; Viner D.; von Engeln A.; von Schuckmann K.; Zhang X.; Chuersuwan N.; Hegerl G.; Yasunari T.; Lai H.-W.; Villaseñor T.. IPCC Sixth Assessment Report (AR6) Working Group 1: the Physical Science Basis; University Press: UK, 2021; Vol. 7.http://www.ipcc.ch/report/ar6/wg1/downloads.
- Trisna B. A.; Park S. N.; Park I.; Lee J.; Lim J. S. Measurement Report: Radiative Efficiencies of (CF3)2CFCN, CF3OCFCF2, and CF3OCF2CF3. Atmos. Chem. Phys. 2023, 23 (7), 4489–4500. 10.5194/acp-23-4489-2023. [DOI] [Google Scholar]
- Weiss R. F.; Mühle J.; Salameh P. K.; Harth C. M. Nitrogen Trifluoride in the Global Atmosphere. Geophys. Res. Lett. 2008, 35 (20), 1–3. 10.1029/2008gl035913. [DOI] [Google Scholar]
- Arnold T.; Harth C. M.; Mühle J.; Manning A. J.; Sala-meh P. K.; Kim J.; Ivy D. J.; Steele L. P.; Petrenko V. V.; Severinghaus J. P.; Baggenstos D.; Weiss R. F. Nitrogen Trifluoride Global Emissions Estimated from Updated Atmospheric Measurements. Proc. Natl. Acad. Sci. U.S.A. 2013, 110 (6), 2029–2034. 10.1073/pnas.1212346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T.; Mühle J.; Salameh P. K.; Harth C. M.; Ivy D. J.; Weiss R. F. Automated Measurement of Nitrogen Trifluoride in Ambient Air. Anal. Chem. 2012, 84 (11), 4798–4804. 10.1021/ac300373e. [DOI] [PubMed] [Google Scholar]
- Weiss R. F.; Prinn R. G. Quantifying Greenhouse-gas Emissions from Atmospheric Measurements: A Critical Reality Check for Climate Legislation. Philos. Trans. R. Soc., A 2011, 369 (1943), 1925–1942. 10.1098/rsta.2011.0006. [DOI] [PubMed] [Google Scholar]
- Arnold T.; Manning A. J.; Kim J.; Li S.; Webster H.; Thomson D.; Mühle J.; Weiss R. F.; Park S.; O’Doherty S. Inverse Modelling of CF4 and NF3 Emissions in East Asia. Atmos. Chem. Phys. 2018, 18 (18), 13305–13320. 10.5194/acp-18-13305-2018. [DOI] [Google Scholar]
- Yoon D.; Lee J.; Lim J. S. Detachable Trap Preconcentrator with a Gas Chromatograph–Mass Spectrometer for the Analysis of Trace Halogenated Greenhouse Gases. Anal. Chem. 2019, 91 (5), 3342–3349. 10.1021/acs.analchem.8b04551. [DOI] [PubMed] [Google Scholar]
- Lim J. S.; Lee J.; Moon D.; Kim J. S.; Lee J.; Hall B. D. Gravimetric Standard Gas Mixtures for Global Monitoring of Atmospheric SF6. Anal. Chem. 2017, 89 (22), 12068–12075. 10.1021/acs.analchem.7b02545. [DOI] [PubMed] [Google Scholar]
- ISO . Gas Analysis-Purity Analysis and the Treatment of Purity Data. ISO 19229:2019.
- Prinn R. G.; Weiss R. F.; Arduini J.; Arnold T.; Fraser P. J.; Ganesan A. L.; Gasore J.; Harth C. M.; Hermansen O.; Kim J.; Krummel P. B.; Li S.; Loh Z. M.; Lunder C. R.; Maione M.; Manning A. J.; Miller B. R.; Mitrevski B.; Mühle J.; O’Doherty S.; Park S.; Reimann S.; Rigby M.; Salameh P. K.; Schmidt R.; Simmonds P. G.; Steele L. P.; Vollmer M. K.; Wang R. H.; Young D.. The ALE/GAGE/AGAGE Data Base. http://agage.mit.edu/data.
- Kim J.; Li S.; Kim K. R.; Stohl A.; Mühle J.; Kim S. K.; Park M. K.; Kang D. J.; Lee G.; Harth C. M.; Salameh P. K.; Weiss R. F. Regional Atmospheric Emissions Determined from Measurements at Jeju Island, Korea: Halogenated Compounds from China. Geophys. Res. Lett. 2010, 37, L12801. 10.1029/2010gl043263. [DOI] [Google Scholar]
- Stohl A.; Kim J.; Li S.; O’Doherty S.; Mühle J.; Salameh P. K.; Saito T.; Vollmer M. K.; Wan D.; Weiss R. F.; Yao B.; Yokouchi Y.; Zhou L. X. Hydrochlorofluorocarbon and Hydrofluorocarbon Emissions in East Asia Determined by Inverse Modeling. Atmos. Chem. Phys. 2010, 10 (8), 3545–3560. 10.5194/acp-10-3545-2010. [DOI] [Google Scholar]
- Li S.; Kim J.; Kim K.-R.; Mühle J.; Kim S.-K.; Park M.-K.; Stohl A.; Kang D.-J.; Arnold T.; Harth C. M.; Salameh P. K.; Weiss R. F. Emissions of Halogenated Compounds in East Asia Determined from Measurements at Jeju Island, Korea. Environ. Sci. Technol. 2011, 45 (13), 5668–5675. 10.1021/es104124k. [DOI] [PubMed] [Google Scholar]
- Saito T.; Yokouchi Y.; Stohl A.; Taguchi S.; Mukai H. Large Emissions of Perfluorocarbons in East Asia Deduced from Continuous Atmospheric Measurements. Environ. Sci. Technol. 2010, 44 (11), 4089–4095. 10.1021/es1001488. [DOI] [PubMed] [Google Scholar]
- Camara M.; Breuil P.; Briand D.; Viricelle J.-P.; Pijolat C.; de Rooij N. F. Preconcentration Modeling for the Optimization of a Micro Gas Preconcentrator Applied to Environmental Monitoring. Anal. Chem. 2015, 87 (8), 4455–4463. 10.1021/acs.analchem.5b00400. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Balestra S. R. G.; Baur R.; Agarwal U.; Zuidema E.; Rigutto M. S.; Calero S.; Vlugt T. J. H.; Dubbeldam D. R. U. P. T. U. R. A. RUPTURA: simulation code for breakthrough, ideal adsorption solution theory computations, and fitting of isotherm models. Mol. Simul. 2023, 49 (9), 893–953. 10.1080/08927022.2023.2202757. [DOI] [Google Scholar]
- Eyer S.; Stadie N.; Borgschulte A.; Emmenegger L.; Mohn J. Methane Preconcentration by Adsorption: a Methodology for Materials and Conditions Selection. Adsorption 2014, 20, 657–666. 10.1007/s10450-014-9609-9. [DOI] [Google Scholar]
- Simon C. M.; Smit B.; Haranczyk M. pyI. A. S. T. pyIAST: Ideal adsorbed solution theory (IAST) Python package. Comput. Phys. Commun. 2016, 200, 364–380. 10.1016/j.cpc.2015.11.016. [DOI] [Google Scholar]
- Mason J. A.; McDonald T. M.; Bae T.-H.; Bachman J. E.; Sumida K.; Dutton J. J.; Kaye S. S.; Long J. R. Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. 10.1021/jacs.5b00838. [DOI] [PubMed] [Google Scholar]
- Lu C.-J.; Zellers E. T. A Dual-Adsorbent Preconcentrator for a Portable Indoor-VOC Microsensor System. Anal. Chem. 2001, 73 (14), 3449–3457. 10.1021/ac001524k. [DOI] [PubMed] [Google Scholar]
- Sukaew T.; Zellers E. T. Evaluating the Dynamic Retention Capacities of Microfabricated Vapor Preconcentrators as a Function of Flow Rate. Sens. Actuators, B 2013, 183 (5), 163–171. 10.1016/j.snb.2013.03.105. [DOI] [Google Scholar]
- Rennick C.; Arnold T.; Safi E.; Drinkwater A.; Dylag C.; Webber E. M.; Hill-Pearce R.; Worton D. R.; Bausi F.; Lowry D. Boreas: A Sample Preparation-Coupled Laser Spectrometer System for Simultaneous High-Precision In Situ Analysis of δ13C and δ2H from Ambient Air Methane. Anal. Chem. 2021, 93 (29), 10141–10151. 10.1021/acs.analchem.1c01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorov I.; Mohn J. CleanEx: A Versatile Automated Methane Preconcentration Device for High-Precision Analysis of 13CH4, 12CH3D, and 13CH3D. Anal. Chem. 2022, 94 (28), 9981–9986. 10.1021/acs.analchem.2c01949. [DOI] [PubMed] [Google Scholar]
- Obersteiner F.; Bönisch H.; Keber T.; O’Doherty S.; Engel A. A Versatile, Refrigerant- and Cryogen-free Cryofocusing–thermodesorption Unit for Preconcentration of Traces Gases in air. Atmos. Meas. Tech. 2016, 9, 5265–5279. 10.5194/amt-9-5265-2016. [DOI] [Google Scholar]




