Abstract
Objectives
Critically ill patients with severe pancreatitis exhibit substantial muscle wasting, which limits in-hospital and post-hospital outcomes. Survivors of critical illness undergo extensive recovery processes. Previous studies have explored pancreatic function, quality of life, and costs post-hospitalization for AP patients, but none have comprehensively quantified muscle loss and recovery post-discharge. By applying an AI-based automated segmentation tool, we aimed to quantify muscle mass recovery in ICU patients after discharge.
Materials
Muscle segmentation was performed on 22 patients, with a minimum of three measurements taken during hospitalization and one clinically indicated examination after hospital discharge. Changes in psoas muscle area (PMA) between admission, discharge and follow up were calculated. T-Test was performed to identify significant differences between patients able and not able to recover their muscle mass.
Results
Monitoring PMA shows muscle loss during and gain after hospitalization: The mean PMA at the first scan before or at ICU admission (TP1) was 17.08 cm², at the last scan before discharge (TP2), mean PMA was 9.61 cm². The percentage change in PMA between TP1 and TP2 ranged from − 85.42% to -2.89%, with a mean change of -40.18%. The maximum muscle decay observed during the stay was − 50.61%. After a mean follow-up period of 438.73 days most patients (81%) were able to increase their muscle mass. Compared to muscle status at TP1, only 27% of patients exhibited full recovery, with the majority still presenting a deficit of 31.96%.
Conclusion
Muscle recovery in ICU patients suffering from severe AP is highly variable, with only about one third of patients recovering to their initial physical status. Opportunistic screening of post-ICU patient recovery using clinically indicated imaging and AI-based segmentation tools enables precise quantification of patients’ muscle status and can be employed to identify individuals who fail to recover and would benefit from secondary rehabilitation. Understanding the dynamics of muscle atrophy may improve prognosis and support personalized patient care.
Keywords: Critical care, Acute pancreatitis, Muscle wasting, Artificial intelligence, Computed tomography
Introduction
Among the various gastrointestinal conditions leading to hospitalization, acute pancreatitis (AP) is one of the most prevalent. The severity of AP varies significantly, ranging from self-limiting cases to those with rapidly fatal outcomes [1, 2]. In severe cases, patients often require prolonged hospitalization and admission to the intensive care unit (ICU) for comprehensive management [3]. While a multidisciplinary approach remains essential, the treatment strategy for severe AP has evolved to prioritize aggressive intensive care over early surgical intervention [4].
A common complication among ICU patients is severe loss of muscle mass, which, combined with loss of muscle function, is referred to as ICU-acquired weakness (ICUAW) [5, 6]. Muscle wasting typically begins in the first days after admission to the ICU and progresses over time. The extent of muscle loss correlates with the severity of the underlying illness and the length of hospital stay and it is particularly high in septic patients [7]. In general, survivors of critical illness generally undergo an extended and resource-intensive recovery process. Previous studies have predominantly focused on pancreatic function, quality of life, and associated costs following hospitalization for AP. However, to our knowledge, no comprehensive study has addressed the quantification of muscle loss and the subsequent rebuilding of muscle mass post-discharge [8, 9].
The aim of this study was to quantify the recovery of muscle mass in intensive care patients admitted for acute pancreatitis following hospitalization.
Materials and methods
Study design and patient population
In this retrospective cohort study, we examined body composition metrics in critically ill patients admitted for severe pancreatitis. The study received approval from the Institutional Review Board (Internal registration number: EA4/152/20) and adhered to the principles outlined in the Declaration of Helsinki. The ethics committee waived the need to obtain patient consent. We conducted a retrospective search in our database for adult patients (aged > 18 years) who were admitted to the ICU of our university hospital between 2012 and 2022 with pancreatitis. Inclusion criteria required a clinically or morphologically confirmed diagnosis of pancreatitis, a minimum ICU stay of 10 days, and the availability of three serial computed tomography (CT) datasets of the abdomen during hospitalization. To ensure a comparable baseline assessment, only patients who underwent a CT scan prior to admission or within 6 days of ICU admission were included. The ethics committee waived the requirement for patient consent.
Segmentation of tissue compartments
The quantification of patient tissue compartments was conducted using an AI-based automated image segmentation tool integrated into the hospital’s Picture Archiving and Communication System (PACS) software (Visage version 7.1., Visage Imaging GmbH, Berlin, Germany), a method validated in prior studies [10–12]. Following the automated identification of the third lumbar vertebra (L3) level, the system performed segmentation to categorize tissues into subcutaneous fat (SAT), skeletal muscle area (SMA), visceral fat (VAT), and psoas muscle area (PMA). Subsequently, the software computed the areas in square centimeters (cm2) for each of these components. An experienced radiologist reviewed each automated segmentation and applied manual corrections if necessary.
Definition of obesity, Sarcopenia and muscular recovery
Obesity was defined as internationally recognised by a BMI threshold of > 30. Sarcopenia was determined using gender-specific cut-offs; SMA < 34.3 cm² for women, and < 45.4 cm² for men, based on established literature [13]. In our study, we defined muscular recovery as the restoration of muscle area (PMA) to the level observed at hospital admission.
Statistics
Statistical analysis utilized SPSS software version 25.0 (IBM; New York, USA). Descriptive statistics are presented as means and standard deviations. Changes in muscle mass between admission, discharge and follow up were calculated by dividing the occurred PMA change at discharge or follow-up by the respective baseline PMA at admission or discharge. The Mann-Whitney U Test (for non-normally distributed data) and the Chi-Squared Test were used to compare patient subgroups. A sample size was not calculated. All p-values less than 0.05 were considered statistically significant.
Results
Demographic data and hospitalization
Out of 330 patients admitted to the designated ICUs during the study period, 230 were excluded due to not meeting inclusion criteria, lacking clinical data, or having fewer than three relevant CT examinations for muscle segmentation. One hundred patients meeting the criteria were included, among whom 41 died. The data of the long-term monitoring have previously been published [14]. Among the survivors, 22 patients received CT imaging after discharge and were enrolled in the study. The cohort consisted of 22 patients, with a mean age of 56.14 ± 11.5 years. The average BMI for the cohort was 25.76 ± 5.14 kg/m², with individual values ranging. Notably, the majority of patients were overweight upon admission (77.27%), and 45.45% were classified as obese. Sarcopenia was present in 40.90% of individuals. Average time from hospital to ICU admission was 6.68 ± 6.51 days. Hospital stays averaged 177 ± 86.11 days while ICU stays averaged 109.68 ± 58.2 days). Initial Sepsis-related organ failure assessment score (SOFA) was 6.16 ± 2.34. The main findings are summarized in Table 1.
Table 1.
Overview of enrolled patients. TP1 = Time point 1: first CT within prior or within 6 days of ICU admission. TP2 = time point 2: last CT prior to discharge. TP3 = Time point 3: follow up CT
| ID | Age | Sex | BMI | Hospital stay in days | ICU stay in days | PMA TP1 | PMA TP2 | PMA TP3 | Δ PMA TP 1/2 | Peak PMA loss during hospital stay | Time to follow up | Δ PMA TP2/3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | M | 25.56 | 336 | 192 | 16.71 | 5.05 | 10.17 | -69.78 | -69.98% | 99 | 101.39% |
| 2 | 55 | M | 37.81 | 51 | 38 | 20.52 | 12.46 | 22.41 | -39.28% | -39.28% | 1075 | 79.86% |
| 3 | 63 | M | 16.67 | 139 | 111 | 11.28 | 10.55 | 12.42 | -6.47 | -39.80% | 685 | 17.73% |
| 4 | 58 | F | 23.98 | 133 | 100 | 7.68 | 6.49 | 7.45 | -15.49% | -15.49% | 587 | 14.79% |
| 5 | 39 | M | 29.41 | 88 | 75 | 18.55 | 12.95 | 11.96 | -30.19% | -30.19% | 808 | -7.64% |
| 6 | 57 | F | 23.67 | 288 | 126 | 10.88 | 4.42 | 7.72 | -59.38% | -59.38% | 554 | 74.66% |
| 7 | 39 | M | 27.13 | 76 | 58 | 18.97 | 7.06 | 11.84 | -62.78% | -62.78% | 803 | 67.71% |
| 8 | 79 | F | 25.39 | 235 | 119 | 16.51 | 13.68 | 11.26 | -17.14 | -44.46% | 104 | -17.69% |
| 9 | 59 | M | 23.84 | 98 | 83 | 22.40 | 14.73 | 14.81 | -34.24 | -36.47% | 27 | 0.54% |
| 10 | 53 | M | 28.40 | 308 | 208 | 26.65 | 11.25 | 11.65 | -57.79 | -71.33% | 49 | 3.56% |
| 11 | 50 | M | 19.37 | 142 | 56 | 16.7 | 5.74 | 17.8 | -65.63% | -65.63% | 984 | 210.10% |
| 12 | 59 | M | 24.97 | 300 | 260 | 23.06 | 16.80 | 23.5 | -27.15 | -31.31% | 450 | 39.88% |
| 13 | 57 | M | 21.91 | 158 | 149 | 13.06 | 10.68 | 18.26 | -18.22 | -50.92% | 145 | 70.97% |
| 14 | 35 | M | 27.10 | 192 | 105 | 14.9 | 12.62 | 12.95 | -15.30 | -60.67% | 242 | 2.61% |
| 15 | 67 | M | 23.57 | 60 | 10 | 13.43 | 9.28 | 8.13 | -30.90 | -35.44% | 104 | -12.39% |
| 16 | 46 | F | 20.98 | 102 | 49 | 16.59 | 10.11 | 16.31 | -39.06 | -45.27% | 445 | 61.33% |
| 17 | 61 | M | 24.22 | 137 | 130 | 13.84 | 5.73 | 6.33 | -58.60 | -71.39% | 124 | 10.47% |
| 18 | 63 | F | 37.38 | 320 | 135 | 9.09 | 2.87 | 4.94 | -68.43% | -68.43% | 615 | 72.13% |
| 19 | 52 | M | 25.93 | 170 | 77 | 29.61 | 13.84 | 20.29 | -53.26 | -55.45% | 114 | 46.60% |
| 20 | 78 | F | 24.39 | 174 | 148 | 9.01 | 8.75 | 10.95 | -2.89 | -27.08% | 634 | 25.14% |
| 21 | 49 | F | 20.76 | 152 | 119 | 16.24 | 11.93 | 9.62 | -26.54 | -47.17% | 107 | -19.36% |
| 22 | 44 | M | 34.19 | 226 | 50 | 30.04 | 4.38 | 24.38 | -85.42% | -85.42% | 897 | 456.62% |
| Ø | 56.14 | 25.76 | 177 | 109 | 17.08 | 9.61 | 13.42 | -40.18 | -50.61% | 439 | 59.05% |
Muscle loss during and gain after hospitalization
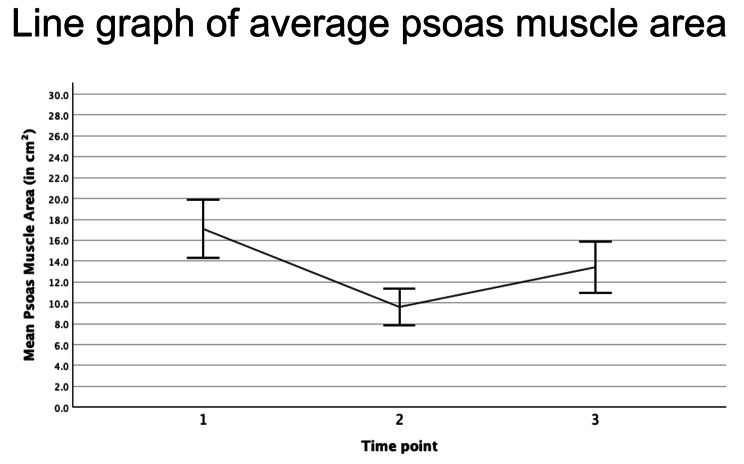
The mean PMA at the first scan prior to or at ICU admission (TP1), was 17.08 ± 6.15 cm², spanning from 7.68 to 30.04 cm². At the last scan prior to discharge (TP2), the mean PMA was 9.61 ± 3.81 cm², ranging from 2.87 to 22.41 cm². The percentage change in PMA between TP1 and TP2 among the patients was − 40.18 ± 22.78%. While the maximum muscle decay patients experienced during their stay, assessed over all available scans, was higher at -50.61 ± 13.02%. Follow-up periods post-discharge averaged at 478.14 ± 333 days. AT follow up (TP3), the mean PMA was 13.42 ± 5.5 cm², with a range from a minimum of 4.94 to 24.38 cm² (Fig. 1).
Fig. 1.
Line graph of average psoas muscle area of all 22 patients at ICU admission (1: 17.08 cm²), after a statistically significant decay (p < 0.001) prior to discharge (2: 9.61 cm².) and at follow up (3: 13.42 cm², p = 0.012)
Subgroup analysis
Statistical analysis unveiled noteworthy distinctions between patients demonstrating muscle recovery and those who did not. Notably, the recovering patients manifested a higher prevalence of obesity and consistently higher PMA across all three time points. Moreover, among individuals who attained full recovery to their initial physical state, a lesser proportion were female, as delineated in Table 2.
Table 2.
Results of t-test comparing individuals who showed recovery in muscle area (= 1) with those who did not (= 0), and comparison of those who recovered to their initial status at ICU admission (= 1) with those who did not (= 0)
| Recovery after discharge | N | Mean | SD | p | Recovery to TP1 | N | Mean | SD | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female Gender | 1 | 18 | 28% | 46% | 0.616 | Female Gender | 1 | 6 | 17% | 41% | 0.565 | ||
| 0 | 4 | 50% | 58% | 0 | 16 | 38% | 50% | ||||||
| Age | 1 | 18 | 55.61 | 10.62 | 0.831 | Age | 1 | 6 | 60.33 | 9.66 | 0.267 | ||
| 0 | 4 | 58.50 | 17.91 | 0 | 16 | 54.56 | 12.38 | ||||||
| Obesity | 1 | 18 | 17% | 51% | 1.00 | Obesity | 1 | 6 | 13% | 52% | 1.00 | ||
| 0 | 4 | 0% | 0% | 0 | 16 | 17% | 50% | ||||||
| Sarcopenia | 1 | 18 | 44% | 51% | 0.655 | Sarcopenia | 1 | 6 | 50% | 55% | 0.616 | ||
| 0 | 4 | 25% | 50% | 0 | 16 | 38% | 50% | ||||||
| PMA at TP1 | 1 | 18 | 17.27 | 6.93 | 0.837 | PMA at TP1 | 1 | 6 | 15.61 | 5.47 | 0.641 | ||
| 0 | 4 | 16.18 | 2.10 | 0 | 16 | 17.63 | 6.67 | ||||||
| PMA at TP2 | 1 | 18 | 9.09 | 4.06 | 0.195 | PMA at TP2 | 1 | 6 | 10.83 | 3.70 | 0.541 | ||
| 0 | 4 | 11.96 | 1.93 | 0 | 16 | 9.15 | 3.98 | ||||||
| PMA at TP3 | 1 | 18 | 14.12 | 5.98 | 0.262 | PMA at TP3 | 1 | 6 | 17.56 | 5.09 | 0.040 | ||
| 0 | 4 | 10.24 | 1.72 | 0 | 16 | 11.86 | 5.13 | ||||||
| Time btw. TP2 and TP3 | 1 | 18 | 473.83 | 339.05 | 0.342 | Time btw. TP2 and TP3 | 1 | 6 | 662.17 | 342.87 | 0.040 | ||
| 0 | 4 | 280.75 | 351.50 | 0 | 16 | 354.94 | 310.62 | ||||||
| Hospital.in days | 1 | 18 | 186.11 | 89.46 | 0.342 | Hospital.in days | 1 | 6 | 160.67 | 80.55 | 0.802 | ||
| 0 | 4 | 133.75 | 77.71 | 0 | 16 | 182.56 | 92.59 | ||||||
Discussion
The primary aim of this study was to rigorously quantify alterations in muscle mass among ICU patients diagnosed with acute pancreatitis post-discharge. To our knowledge, we present the first quantitative follow-up data on muscle decay by utilizing an AI-based tool for PMA segmentation in clinically indicated CT scans. Our data provides several important insights. Firstly, opportunistic screening for muscle area proves to be a sufficient tool for monitoring the recovery of ICU patients by quantifying their gains and losses. Secondly, the average muscle loss during hospitalization in the patient population suffering from severe pancreatitis was high, at 40.18% ± 23.31% and showed high patient individual variability. Thirdly, after a mean follow-up period of 438.73 days most patients (81%) were able to increase their muscle mass, with mean gains of 75.33% ± 107.47% compared to the last obtained status during hospitalization. Notably, two patients already exhibited muscle gain as signs of recovery during hospitalization, being discharged with an overall loss of less than 10% (ID 3 and ID 21). However, when comparing muscle status to ICU admission, only 27% of patients exhibited recovery, with the majority still presenting a deficit of 31.96% ± 15.92% PMA at follow-up. Additionally, among the very few individuals presenting with a full recovery (ID 2, 3, 11, 12, 13, and 20), it is noticeable that some had more muscle area at the follow-up time than at the initial imaging. This difference is primarily attributed to muscle loss that occurred before the initial imaging study. Finally, in some patients, muscle deterioration continues even after more than 100 days post-discharge (ID 15 and ID 21).
The long-term health outcomes of individuals who survive ICU stays have become a growing concern in recent years. This is especially true as the number of ICU survivors rises due to increased demand for critical care and reduced ICU mortality rates. Survivors of intensive care often encounter post-intensive care sequelae, which are commonly categorized under the term “post-intensive care syndrome” (PICS). This syndrome encompasses cognitive, psychological, and/or physical impairments [15, 16]. One component of PICS is intensive care acquired weakness (ICUAW), which is marked by severe loss of muscle mass and function [5, 17, 18]. The consequences of PICS and ICUAW on quality of life, health-related costs and hospital readmissions are real public health problems.
Although the pathophysiology of muscle atrophy and dysfunction during critical illness seems to be partially elucidated, the mechanisms involved in persistent muscle dysfunction resulting in impaired physical recovery after ICU discharge remain poorly understood [19].
Typically, women and older individuals exhibit a higher susceptibility to experiencing inadequate functional recuperation following critical illness. The variances between genders still lack clarity but could potentially be linked to the lesser pre-existing muscle mass. Generally, low muscle mass has been linked to less favorable pre-ICU health condition and post-ICU outcomes [20, 21]. In our study the recovery to the initial muscle status was significantly associated with the time to follow-up. While gender, initial muscularity, obesity and sarcopenia were not associated with the recovery, in this relatively small cohorts.
Assessing muscle mass in the ICU is crucial, as a recent study underscored, where researchers applied bio-impedance analysis (BIA) to evaluate muscle decay and found a significant correlation between inadequate nutritional intake and increased muscle wasting. This highlights the need for early and adequate nutritional support to mitigate muscle loss and enhance recovery [22].
Limitations
Given the retrospective nature of our study, a selection bias is inevitable. The retrospective design presents challenges in establishing causality between the degree and timing of muscle recovery, as does the absence of data on post-discharge rehabilitation programs. In addition, the comparable small study population restricts the generalizability of our results. Future prospective studies with a more robust design could provide a clearer understanding of the dynamics of muscle loss and recovery rates.
Conclusion
Individuals who have experienced acute pancreatitis requiring intensive care often endure significant muscle depletion. The recuperation from these ICU stays varies widely among patients, with a majority displaying persistent deficits in muscle mass. Opportunistic screening of post-ICU patient recovery through the segmentation of clinically indicated transactional imaging enables precise quantification of patients’ muscle status and may be employed to identify individuals who could benefit from secondary rehabilitation.
Acknowledgements
Not applicable.
Abbreviations
- AI
Artificial intelligence
- BMI
Body mass index
- CT
Computed tomography
- ICU
Intensive care unit
- ICUAW
ICU-acquired weakness
- PACS
Picture archiving and communication system
- PICS
Post-intensive care syndrome
- PMA
Psoas muscle area
- SAT
Subcutaneous adipose tissue
- SMA
Skeletal muscle area
- VAT
Visceral adipose tissue
Author contributions
CH analyzed and interpreted the patient data and did the writing. UF and DG have provided the body composition software. NB, AE, BG, CP and TA helped during the proof reading process. JK had the idea for this project and was significantly involved in the data analysis and corrections. All authors read and approved the final manuscript. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
The study received approval from the Institutional Review Board Ethics committee Charité – Universtitätsklinikum Berlin (Internal registration number: EA4/152/20) and adhered to the principles outlined in the Declaration of Helsinki. The need for consent to participate was waived by the Institutional Review Board of Charité- Universitätsklinikum Berlin.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bugiantella W, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg. 2016;28(Suppl 1):S163–71. 10.1016/j.ijsu.2015.12.038 [DOI] [PubMed] [Google Scholar]
- 2.Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: Rev JAMA. 2021;325(4):382–90. [DOI] [PubMed] [Google Scholar]
- 3.Hirota M, et al. Fundamental and intensive care of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17(1):45–52. 10.1007/s00534-009-0210-7 [DOI] [PubMed] [Google Scholar]
- 4.Werner J, et al. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54(3):426–36. 10.1136/gut.2003.035907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med, 2020. 46(4): pp. 637–653. [DOI] [PMC free article] [PubMed]
- 6.Herridge MS, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 7.Puthucheary ZA, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 8.Soran A, et al. Outcome and quality of life of patients with acute pancreatitis requiring intensive care. J Surg Res. 2000;91(1):89–94. 10.1006/jsre.2000.5925 [DOI] [PubMed] [Google Scholar]
- 9.Andersson B, Pendse M-L, Andersson R. Pancreatic function, quality of life and costs at long-term follow-up after acute pancreatitis. World J Gastroenterology: WJG. 2010;16(39):4944. 10.3748/wjg.v16.i39.4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beetz NL, et al. Artificial intelligence-based analysis of body composition in Marfan: skeletal muscle density and psoas muscle index predict aortic enlargement. J Cachexia Sarcopenia Muscle. 2021;12(4):993–9. 10.1002/jcsm.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, et al. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin Nutr. 2019;38(6):2747–55. 10.1016/j.clnu.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Kolck J, et al. Intermittent body composition analysis as monitoring tool for muscle wasting in critically ill COVID-19 patients. Ann Intensive Care. 2023;13(1):61. 10.1186/s13613-023-01162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derstine BA, et al. Skeletal muscle cutoff values for Sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8(1):11369. 10.1038/s41598-018-29825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolck J, et al. Opportunistic screening for long-term muscle wasting in critically ill patients: insights from an acute pancreatitis cohort. Eur J Med Res. 2024;29(1):294. 10.1186/s40001-024-01884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue S, et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6(3):233–46. 10.1002/ams2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adrion C, et al. Enhanced recovery after Intensive Care (ERIC): study protocol for a German stepped wedge cluster randomised controlled trial to evaluate the effectiveness of a critical care telehealth program on process quality and functional outcomes. BMJ Open. 2020;10(9):e036096. 10.1136/bmjopen-2019-036096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermans G. Van Den Berghe, Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. 10.1186/s13054-015-0993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batt J, Herridge MS, Dos Santos CC. From skeletal muscle weakness to functional outcomes following critical illness: a translational biology perspective. Thorax. 2019;74(11):1091–8. 10.1136/thoraxjnl-2016-208312 [DOI] [PubMed] [Google Scholar]
- 20.Geense WW, et al. New Physical, Mental, and cognitive problems 1 year after ICU admission: a prospective Multicenter Study. Am J Respir Crit Care Med. 2021;203(12):1512–21. 10.1164/rccm.202009-3381OC [DOI] [PubMed] [Google Scholar]
- 21.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;371(3):287–8. 10.1056/NEJMc1406274 [DOI] [PubMed] [Google Scholar]
- 22.Deana C, et al. Bioimpedance-assessed muscle wasting and its relation to nutritional intake during the first week of ICU: a pre-planned secondary analysis of Nutriti Study. Ann Intensive Care. 2024;14(1):29. 10.1186/s13613-024-01262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].