Abstract
Background
The number of frail older people is increasing worldwide, and all countries will be confronted with their growing needs for healthcare and social support. The aim of this umbrella review was to summarize the evidence on the factors associated with frailty in older people, using a socioecological approach.
Methods
PubMed (MEDLINE), Scopus, Web of Science, ScienceDirect, Hinari (research4life), and the Trip database were systematically searched up to April 2023. Systematic reviews of observational studies that explored factors associated with frailty in older adults aged 60 years and over were considered for inclusion. No language, geographical or setting restrictions were applied. However, we excluded systematic reviews that investigated frailty factors in the context of specific diseases. The Joanna Briggs Institute Critical Appraisal Checklist for Systematic Reviews and Research Syntheses and the ROBIS tool were used to assess the quality and risk of bias in the included studies.
Results
Forty-four systematic reviews were included, covering 1,150 primary studies with approximately 2,687,911 participants overall. Several risk factors, protective factors and biomarkers were found to be associated with frailty, especially in community-dwelling older people, including 67 significant associations from meta-analyses. The certainty of the evidence was rated as moderate or reached moderate levels for seven factors relevant to older people. These factors include depression (OR 4.66, 95% CI 4.07 to 5.34), loneliness (OR 3.51, 95% CI 2.70 to 4.56), limitations in activities of daily living (OR 2.59, 95% CI 1.71 to 3.48), risk of malnutrition (OR 3.52, 95% CI 2.96 to 4.17), Dietary Inflammatory Index score (OR 1.24, 95% CI 1.16 to 1.33), maximal walking speed (Standardized Mean Difference (SMD) -0.97, 95% CI -1.25 to -0.68), and self-reported masticatory dysfunction (OR 1.83, 95% CI 1.55 to 2.18). Additionally, only greater adherence to a Mediterranean diet showed a high level of evidence (OR 0.44, 95% CI 0.31 to 0.64).
Conclusions
This umbrella review will provide guidance for prevention strategies and clinical practice by promoting healthy lifestyles and addressing all modifiable risk factors associated with frailty. Future systematic reviews should consider heterogeneity and publication bias, as these were the main reasons for downgrading the level of evidence in our review.
Registration
PROSPERO 2022, CRD42022328902.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-024-05288-4.
Keywords: Frailty, Older people, Factors, Socioecological approach, Umbrella review
Background
With the gradual ageing of the population [1], the number of older people becoming frail is increasing worldwide [2–6]. The World Health Organization defines frailty as "a clinically recognizable state in which the ability of older people to cope with everyday or acute stressors is compromised by an increased vulnerability brought by age-associated declines in physiological reserve and function across multiple organ systems" [7].
The identification of frailty, pre-frailty and robustness status has often been done by considering the biological model of frailty known as Fried's physical phenotype or physical frailty [8, 9]. It is generally recognized by the presence of at least three of the following five criteria: unintentional weight loss, muscle weakness, self-reported exhaustion, slowness and low physical activity [8].
A recent systematic review found that 13.6% of non-frail (robust or pre-frail) people became frail, with a global incidence rate of frailty of 43.4 cases per 1000 person-years [2]. The prevalence of frailty varies considerably between countries and regions, and also according to the assessment tool used [4, 5]. The authors of a systematic review conducted in the context of low- and middle-income countries, showed that the prevalence of frailty and pre-frailty ranged from 3.9% to 59.4% and from 13.4% to 71.6%, respectively [4]. In a more recent systematic review of 62 countries around the world, the pooled prevalence of frailty was 12% using physical frailty and 24% using the deficit accumulation model [5].
As conceptual models of frailty have evolved, several authors have been interested in the multidimensional approach to frailty, taking into account all its domains: biological, psychological, and social [10, 11].
Given the diversity of theoretical definitions of frailty in older persons [12, 13], and despite the lack of consensus on an operational definition, it is crucial to identify frailty earlier in order to limit its negative consequences. Numerous studies have shown that frail older people have an increased risk of falls, fractures, disabilities, hospitalizations, institutionalization, and even death [14–18]. It is also important to note that frailty is considered a dynamic syndrome that can be reversed by addressing modifiable factors [19–21].
Frailty in the older population is influenced by multiple factors, and it is essential to approach it holistically by considering all the determinants related to the individuals, their relationships, and their living environment. The use of a socioecological approach will help to uncover the different levels of influence involved in the development of frailty and guide preventive interventions. According to commonly used models of application of this approach [22, 23], it is possible to examine frailty factors at various levels: the individual or intrapersonal level, including demographic characteristics, lifestyle factors, and health-related factors of the older person; the relationship or interpersonal level, involving the older individual's social network, such as family relationships and friendships; the community level, encompassing the physical and social environment; and the societal or policy level, which includes local cultures, laws, and policies.
Over the past decade, there has been a gradual increase in the number of systematic reviews addressing factors related to frailty in older people. However, most of these studies have focused on only one or a few determinants of frailty in older individuals [24–28]. In addition, other systematic reviews have been limited to frailty associated with specific diseases, countries, regions or contexts, and very few studies have examined a wide range of determinants of frailty [29, 30]. Therefore, the aim of this umbrella review was to summarize the best available evidence on the determinants and factors associated with frailty in older people from published systematic reviews, with or without meta-analysis, to guide clinical practice and health policies.
Methods
This umbrella review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Update guidelines [31] and the recommendations of the Joanna Briggs Institute (JBI) for conducting umbrella reviews [32].
Registration and protocol
The review protocol was registered on the international prospective register of systematic reviews (PROSPERO) on May 10, 2022, under the number CRD42022328902 and is available from the following link: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=328902.
Data sources and search strategy
A comprehensive search strategy was developed and a systematic search was conducted on September 29, 2022, in the following databases: PubMed (MEDLINE), Scopus, Web of Science, ScienceDirect, Hinari (research4life), and the Trip database.
The keywords and MeSH terms used were guided by our preliminary research and included older people as our population of interest, the factors associated with different levels of influence of the socioecological approach as the exposure, and the concept of frailty as the outcome. There were no predefined interventions or comparisons in this review, and the research was conducted without limitation of geographical area or setting. The keywords were linked using the Boolean operators "AND", "OR" and "NOT" and our search equations were adjusted for each database. Details of these equations and the filters used can be found in the supplementary material (Additional file 1).
We have completed by hand searching reference lists and citations of the articles included in this review. Subsequently, an additional search in Google Scholar was performed on December 30, 2022, with a continuous check of the alerts generated in three main databases (Scopus, Web of Science, and ScienceDirect). As planned in our protocol, a re-run of the search strategy was performed on April 16, 2023, before completing our final analysis to retrieve any eligible articles. For those articles that were not available in full text, a request was made to the authors.
Eligibility criteria
Systematic reviews of observational studies, with or without meta-analysis, published from 2011 to 2023 that explored factors associated with frailty in older adults aged 60 years and over were considered for inclusion in this umbrella review.
The chosen age limit remains a reference for talking about older people [1] and allows us to cover a wider population in our study than if we had considered only people aged 65 years and over by convention.
For exposure, we considered all the determinants or factors associated with frailty that belong to different levels of influence as defined by the socioecological approach. These factors include those at the individual or intrapersonal level (e.g., age, gender, education, income, lifestyle factors or behaviors, biological and health-related factors), the relationship or interpersonal level (e.g., marital status, social support, family income), the community level (e.g., physical environment, neighborhood, social interactions), and the societal or policy level (e.g., public policies, cultural norms, legislation) [22, 23]. Regarding the main outcomes, the studies to be included should address frailty as measured by any valid frailty assessment tool, and the nature of its association with the factor(s) studied.
In addition, systematic reviews that examined multiple factors associated with frailty with available and sufficient data in their qualitative synthesis, separated by age or study design, were also considered for inclusion.
We excluded systematic reviews that studied frailty factors in the context of specific diseases, included a population group under 60 years of age in their meta-analyses, or had no full text available. However, we did not apply any language, geographical or setting restrictions and no reviews were excluded after quality assessment.
Study selection process
The PRISMA 2020 flowchart was used to report our review process and the selection of studies. Records retrieved from all databases were transferred to a library created on the reference management software Zotero. This allowed two reviewers (MB and AS) to manually identify and remove duplicates. Subsequently, these reviewers independently screened all titles and abstracts of the remaining articles, making initial exclusions based on our eligibility criteria. The full texts of the articles were then assessed for inclusion.
Any disagreements between the two reviewers were resolved through discussion and, if necessary, the opinion of a third author (MK) was sought.
The combination of these results with those from the additional search helped determine which articles were retained and which were excluded at the end of the process.
Data collection process and data items
Data collection from eligible articles was conducted independently by two reviewers (MB and NEH) and with reference to the JBI Data Extraction Form for Review for Systematic Reviews and Research Syntheses [32]. The following data were extracted for each included systematic review: study details (title and reference; authors and year of publication; type of review; objective(s); participants; setting; factors explored), search details (number of databases and sources searched; date range of included studies; number of studies included; type of studies included; country of origin of included studies), quality appraisal (appraisal instruments used; appraisal rating), frailty assessment (frailty assessment tools used; result of the assessment), main findings (factors associated with frailty; significance/direction/ effect size), heterogeneity (if applicable) and limitations reported by the authors. To address missing data related to study or research details, we used information from the primary studies and also contacted some authors for supplementary material. Any discrepancies between the two reviewers were resolved through discussion and rechecking the data.
Quality and risk of bias assessment
The quality assessment of the reviews included in our umbrella review was independently performed by two reviewers (MB and AS) using the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses [32]. This appraisal tool consists of 11 questions, each with four possible answers: yes, no, unclear, or not applicable. The overall score is calculated by considering only the "yes" answers, which receive one point each, while all other answers receive zero points.
According to the final assessment score, the quality of the systematic reviews was rated as “high” (9–11), “moderate” (5–8) or “low” (0–4).
An additional assessment of the risk of bias in the included reviews was conducted using the ROBIS tool [33]. This tool is specifically designed to evaluate the risk of bias in systematic reviews and consists of three phases. The first phase, relevance assessment, is optional. The other required phases, 2 and 3, involve the identification of concerns with the review process and judging the risk of bias, respectively. It is important to note that phase 2 is composed of four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. Each domain includes questions that can be answered with "Yes", "Probably Yes", "Probably No", "No", or "No Information", with "Yes" indicating low concerns. The judgment for each domain, as well as for the final phase of risk of bias assessment, will be classified as "Low", "High" or "Unclear".
Data synthesis and certainty assessment
We conducted a qualitative synthesis, which included narrative tables of the main factors associated with frailty among older people. Additionally, we have developed an illustrative socio-ecological model of these factors.
The results obtained from the reviews were based on various effect measures, such as Relative Risk (RR), Odds Ratio (OR), Mean Difference (MD) or Standardized Mean Difference (SMD), with significance determined by a p-value < 0.05.
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach [34, 35] was then used to assess the certainty of the evidence in the systematic reviews for our patient-relevant outcomes. These factors were selected based on their relevance to clinical practice, the potential for making recommendations or implementing public health actions, and the availability of data. The quality of evidence for each outcome initially started as "Low" because it was based on observational studies. Risk of bias, inconsistency, indirectness, imprecision, and publication bias were the five reasons that could downgrade the quality of the evidence. However, there were three other considerations that could upgrade the quality of evidence: large effect size, dose response, and all plausible residual confounding factors or biases. The quality of a body of evidence will be rated as "High", "Moderate", "Low" or "Very Low".
Results
Study selection
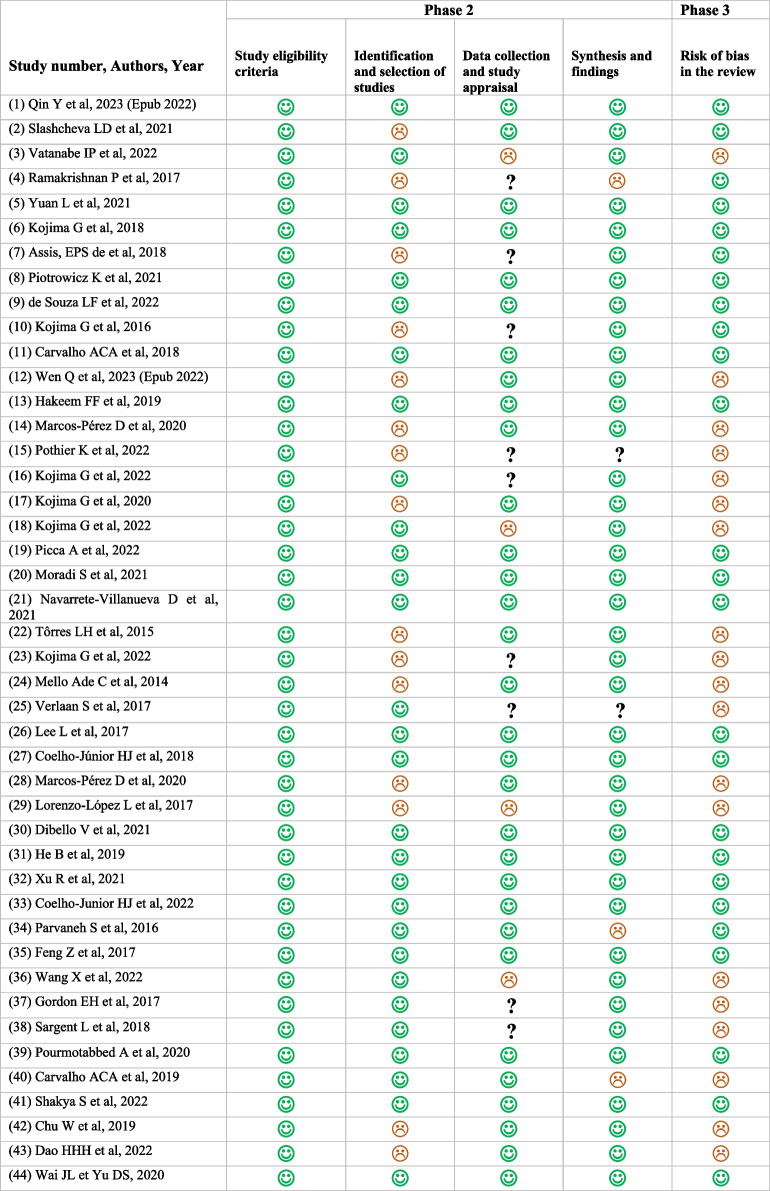
A total of 11,975 studies were identified by searching all databases. After removing duplicates, the remaining 3,689 articles were screened by title and abstract according to our inclusion criteria. Of these, 3,545 were excluded and only 144 full-text articles were assessed for eligibility. Subsequently, 103 studies were also excluded, leaving 41 studies. The additional search retrieved four studies and only one was selected after assessment for inclusion. No additional articles were found in the alerts of the databases, while two additional studies were retrieved by re-running our search strategy. Also, checking the reference lists of the last two included articles as well as a final checking of the citations of all included papers did not generate any new eligible articles. Thus, a total of 44 studies were retained in our umbrella review [28–30, 36–76]. Full details of the selection process for the systematic reviews included in our qualitative synthesis are presented in the PRISMA flow diagram (Fig. 1). Additionally, the list of full-texts that were excluded after evaluation is provided in the supplementary material (Additional file 2), along with the reasons for their exclusion.
Fig. 1.
PRISMA flow diagram for study selection. * Integration of two studies from the re-run of the research strategy
Study characteristics
The main characteristics of the included systematic reviews are summarized in Table 1.
Table 1.
Summary of the characteristics of the systematic reviews included in the umbrella reviewa
| Authors/year of publication/type of review | Factors explored | Date range of included studies | Number of studies included | Quality appraisal of the review | Risk of bias in the review |
|---|---|---|---|---|---|
| (1) Qin Y et al. 2023 (Epub 2022) [36] Systematic review with meta-analysis | Sociodemographic factors (age, sex, education, Income, living alone); Lifestyle-related factors; Health related factors | 2008–2021 | 62 | High | Low |
|
(2) Slashcheva LD et al. 2021 [37] Systematic review |
Oral health characteristics | 2012–2019 | 26 | High | Low |
|
(3) Vatanabe IP et al. 2022 [38] Systematic review with meta-analysis |
Sociodemographic factors (age, gender, marital status, rural residence, schooling,) Health-related factors (Chronic diseases, Nutrition, Physical activity, walking speed, psychological disorder, depression, polypharmacy, falls and related fractures, disability, Incident physical limitation, calf circumference, Hearing impairment, quality of life, potential social support) Blood and brain alterations factors (glycated hemoglobin A1c, eGFR/estimated glomerular filtration rate, levels of cryptoxanthin and zeaxanthin, inflammation, White matter) | 2009–2020 | 28 | Moderate | High |
|
(4) Ramakrishnan P et al. 2017 [39] Systematic review |
Biomarkers | 2010–2017 | 40 | High | Low |
| (5) Yuan L et al. 2021 [40] Systematic review with meta-analysis | Abdominal obesity, body mass index (BMI) | 2005–2020 | 17 | High | Low |
| (6) Kojima G et al. 2018 [41] Systematic review with meta-analysis | Mediterranean Diet | 2012–2017 | 4 | High | Low |
|
(7) Assis, EPS de et al. 2018 [42] Systematic review |
Anemia | 2007–2015 | 7 | Moderate | Low |
| (8) Piotrowicz K et al. 2021 [43] Systematic review with meta-analysis | Arterial stiffness | 2016–2019 | 5 | High | Low |
| (9) de Souza LF et al. 2022 [44] Systematic review | Fear of falling | 1994–2021 | 10 | High | Low |
| (10) Kojima G et al. 2016 [45] Systematic review with meta-analysis | Quality of life | 2009–2015 | 13 | Moderate | Low |
| (11) Carvalho ACA et al. 2018 [46] Systematic review with meta-analysis | Human herpes virus seropositivity [cytomegalovirus (CMV), Epstein-Barr virus (EBV), Varicella zoster virus (VZV), and Herpes simplex virus (HSV)] | 2005–2017 | 6 | High | Low |
| (12) Wen Q et al. 2023 (Epub 2022) [47] Systematic review with meta-analysis | Insomnia | 2009–2022 | 12 | High | High |
| (13) Hakeem FF et al. 2019 [48] Systematic review | Oral health | 2017–2018 | 5 | High | Low |
| (14) Marcos-Pérez D et al. 2020 [49] Systematic review with meta-analysis | Inflammatory mediators | 2002–2018 | 49 | Moderate | High |
|
(15) Pothier K et al. 2022 [50] Systematic review |
Inflammation (Interleukin (mostly IL-6) levels, C-reactive protein (CRP), or high sensitive CRP (hsCRP) levels, white blood cell (WBC) count, tumor necrosis factors (TNFs; mostly TNF-α), hemostatic factors (fibrinogen, Factor VII, Factor VIII, transferrin, and haptoglobin); vascular adhesion protein-1 (VAP-1); erythropoietin (EPO); inflammation index score). Physical health (biochemical measurement, anthropometric measures, comorbidities, smoking and alcohol status, medications, physical performance, blood pressure, past medical history, nutritional status, falls or the risk of falls, overnight hospital admissions) Psycho-Social Health (lifestyle characteristics (years of education, marital status, and capital income), measured cognition (Mini-Mental State Examination (MMSE), memory loss, and subjective cognitive decline); Behavioral disorders and autonomy) |
2002–2020 | 22 | Moderate | High |
| (16) Kojima G et al. 2022 [51] Systematic review with meta-analysis | Loneliness | 2012–2022 | 16 | High | High |
|
(17) Kojima G et al. 2020 [52] Systematic review |
Pet ownership | 2018–2019 | 3 | Moderate | High |
| (18) Kojima G et al. 2022 [53] Systematic review with meta-analysis | Masticatory dysfunction | 2012–2021 | 5 | Moderate | High |
| (19) Picca A et al. 2022 [54] Systematic review with meta-analysis | Biomarkers (metabolic, inflammatory, hormonal and hematologic markers) | 2002–2020 | 80 | High | Low |
| (20) Moradi S et al. 2021 [55] Systematic review with meta-analysis | Dietary Inflammatory Index | 2018–2019 | 5 | High | Low |
| (21) Navarrete-Villanueva D et al. 2021 [56] Systematic review with meta-analysis | Physical fitness components | 2009–2019 | 20 | High | Low |
|
(22) Tôrres LH et al. 2015 [57] Systematic review |
Oral Health | 2000–2013 | 12 | Moderate | High |
| (23) Kojima G et al. 2022 [58] Systematic review with meta-analysis | Fruit and vegetable consumption | 2016–2020 | 3 | Moderate | High |
|
(24) Mello Ade C et al. 2014 [29] Systematic review |
Health-related and socio-demographic factors | 2001–2012 | 35 | High | High |
| (25) Verlaan S et al. 2017 [59] Systematic review with meta-analysis | Malnutrition | 2012–2016 | 28 | Moderate | High |
| (26) Lee L et al. 2017 [60] Systematic review | Biomarkers; Health related factors (depression, comorbidities); health risk factors; sociodemographic factors | 2005–2015 | 12 | High | Low |
|
(27) Coelho-Júnior HJ et al. 2018 [61] Systematic review with meta-analysis |
Protein intake | 2006–2018 | 10 | High | Low |
|
(28) Marcos-Pérez D et al. 2020 [62] Systematic review with meta-analysis |
Vitamin D Levels | 2005–2018 | 26 | High | High |
|
(29) Lorenzo-López L et al. 2017 [63] Systematic review |
Nutritional determinants | 2006–2017 | 19 | Moderate | High |
|
(30) Dibello V et al. 2021 [64] Systematic review |
Oral health factors | 2012–2021 | 39 | High | Low |
|
(31) He B et al. 2019 [65] Systematic review with meta-analysis |
Sociodemographic factors (age, gender) and health factors (comorbidities, disability) | 2010–2018 | 14 | High | Low |
| (32) Xu R et al. 2021 [66] Systematic review with meta-analysis | Sociodemographic factors and health factors | 2014–2020 | 23 | High | Low |
|
(33) Coelho-Junior HJ et al. 2022 [67] Systematic review with meta-analysis |
Protein intake | 2006–2021 | 17 | High | Low |
| (34) Parvaneh S et al. 2016 [68] Systematic review | Activity of the cardiac autonomic nervous system | 2008–2015 | 6 | High | Low |
| (35) Feng Z et al. 2017 [30] Systematic review | Sociodemographic factors; lifestyle factors; physical factors; psychological factors; biological factors | 2005–2016 | 23 | High | Low |
| (36) Wang X et al. 2022 [69] Systematic review with meta-analysis | Characteristics and lifestyles of people, Comorbidities (age, body mass index (BMI), sex (female), living alone, low levels of exercise, polypharmacy, recorded number of drugs, education, smoking, drinking, malnutrition, vitamin D levels, comorbidity and ten comorbid diseases: stroke, cardiac disease, diabetes, vision dysfunction, hearing dysfunction, cognitive impairment, poor sleep, fall history, pain, and depression) | 2014–2021 | 36 | Moderate | High |
|
(37) Gordon EH et al. 2017 [70] Systematic review with meta-analysis |
Sex differences | 2005–2015 | 7 | Moderate | High |
|
(38) Sargent L et al. 2018 [71] Systematic review |
Biological protein marker (n = 287); genetic factors (n = 46); medication risk (n = 9) | 1995–2017 | 342 | Moderate | High |
|
(39) Pourmotabbed A et al. 2020 [72] Systematic review with meta-analysis |
Sleep parameters | 2009–2019 | 10 | High | Low |
|
(40) Carvalho ACA et al. 2019 [73] Systematic review with meta-analysis |
Telomere length | 2008–2019 | 9 | High | High |
| (41) Shakya S et al. 2022 [74] Systematic review | Cardiometabolic Risk Factors (elevated waist circumference, blood glucose, blood pressure, triglycerides, total cholesterol (TC), low-density lipoprotein (LDL), and reduced high-density lipoprotein (HDL)) | 2007–2021 | 12 | High | Low |
| (42) Chu W et al. 2019 [28] Systematic review with meta-analysis | Depression | 2010–2015 | 14 | High | High |
|
(43) Dao HHH et al. 2022 [75] Systematic review with meta-analysis |
Metabolic syndrome (presence of at least 3 out of the five constitutive components: abdominal obesity, high fasting blood glucose, hypertension, hypertriglyceridaemia, and low high-density lipoprotein level) | 2007–2021 | 11 | High | High |
|
(44) Wai JL et Yu DS, 2020 [76] Systematic review |
Sleep–wake disturbances | 2009–2017 | 7 | High | Low |
aThe complete version of this table is available in the Additional file 3
Of the 44 studies included in our review, 27 were systematic reviews with meta-analysis and 17 had only qualitative synthesis. The publication dates of these reviews ranged from 2014 to 2023, with the highest number of publications in 2022, with 11 studies (25%). It is worth noting that the two studies considered in 2023 were already first published online in 2022.
Regarding the number of databases searched it varied from one to eight databases with PubMed, Embase and MEDLINE as the main data sources. A total of 1,150 primary studies were included in our systematic reviews, with a range of three to 342 studies published from 1994 to 2022. The total number of participants per review ranged from 1,757 to 889,880 with approximately 2,687,911 participants overall. Cross-sectional studies or data dominated in comparison to longitudinal studies and in some papers, they have been combined.
Most primary studies collected information from both sexes and depending on the available data, the proportion of females ranged from 15.3% to 88.3%. Some studies involved only women while a few studies included only men.
The study setting was primarily at the community level and in terms of the countries of origin of included primary studies, 61 countries were reported, distributed as follows: 29 countries in Europe (47%); 17 countries in Asia (28%); 10 countries in the Americas (16%); four countries in Africa (7%) and one country in Oceania (2%). Furthermore, the countries of origin were not specified in eight cases.
As for the distribution of the primary studies around the world, the European continent was also leading, followed by the American continent, especially North America, and by Asia, Oceania and Africa respectively. In addition, seven countries accounted for more than 65% of the studies, namely the United States of America (USA), China, Japan, Spain, the United Kingdom (UK), Italy and Brazil.
Frailty assessment
Several tools have been used to assess frailty, and some of them have been adapted for specific countries, settings or components of frailty.
The Fried frailty phenotype [8] was the most commonly used tool in the included systematic reviews, whether used alone, in combination or in a modified version. It was followed by the Frailty Index [77, 78] and the FRAIL scale [79, 80], respectively.
According to the available data, the prevalence of frailty in older people ranged from 0.9% (Germany) using Fried’s criteria [75] to 88.4% (China) with Comprehensive Geriatric Assessment (CGA) combined with the Frailty Index [36].
Only two reviews have examined the factors associated with frailty and cognitive impairment or cognitive frailty [38, 71]. However, cognitive function or cognitive impairment has been explored as factors associated with frailty in several reviews [29, 30, 36, 50, 66, 69].
Quality appraisal and risk of bias in the included systematic reviews
Using the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses, the overall score ranged from seven to 11 out of 11 (Table 2). Therefore, 30 studies (68%) were rated as "high" quality, including 19 systematic reviews with meta-analyses and 11 systematic reviews with qualitative synthesis. There were no studies of low quality, and the remaining studies were rated as "moderate", with eight systematic reviews with meta-analyses and six systematic reviews with qualitative synthesis.
Table 2.
Quality assessment of included studies using the JBI critical appraisal checklist for systematic reviews and research syntheses
| Study number, authors, year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Qin Y et al. 2023 (Epub 2022) [36] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11/11 |
| (2) Slashcheva LD et al. 2021 [37] | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | 10/11 |
| (3) Vatanabe IP et al. 2022 [38] | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | 8/11 |
| (4) Ramakrishnan P et al. 2017 [39] | Y | Y | Y | Y | Y | U | U | Y | Y | Y | Y | 9/11 |
| (5) Yuan L et al. 2021 [40] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11/11 |
| (6) Kojima G et al. 2018 [41] | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | 9/11 |
| (7) Assis, EPS de et al. 2018 [42] | Y | Y | Y | Y | Y | U | U | Y | N | Y | Y | 8/11 |
| (8) Piotrowicz K et al. 2021 [43] | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | 9/11 |
| (9) de Souza LF et al. 2022 [44] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
| (10) Kojima G et al. 2016 [45] | Y | Y | Y | Y | Y | U | U | Y | Y | U | Y | 8/11 |
| (11) Carvalho ACA et al. 2018 [46] | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | 9/11 |
| (12) Wen Q et al. 2023 (Epub 2022) [47] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11/11 |
| (13) Hakeem FF et al. 2019 [48] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
| (14) Marcos-Pérez D et al. 2020 [49] | Y | Y | Y | N | N | Y | Y | Y | Y | U | Y | 8/11 |
| (15) Pothier K et al. 2022 [50] | Y | Y | Y | Y | U | U | Y | Y | N | U | Y | 7/11 |
| (16) Kojima G et al. 2022 [51] | Y | Y | Y | Y | Y | U | U | Y | Y | Y | Y | 9/11 |
| (17) Kojima G et al. 2020 [52] | Y | Y | Y | N | Y | Y | U | Y | N | U | Y | 7/11 |
| (18) Kojima G et al. 2022 [53] | Y | Y | Y | Y | Y | N | N | Y | Y | U | Y | 8/11 |
| (19) Picca A et al. 2022 [54] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
| (20) Moradi S et al. 2021 [55] | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | 10/11 |
| (21) Navarrete-Villanueva D et al. 2021 [56] | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | 9/11 |
| (22) Tôrres LH et al. 2015 [57] | Y | Y | Y | Y | N | U | Y | Y | N | Y | Y | 8/11 |
| (23) Kojima G et al. 2022 [58] | Y | Y | Y | N | Y | U | U | Y | Y | Y | Y | 8/11 |
| (24) Mello Ade C et al. 2014 [29] | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | 9/11 |
| (25) Verlaan S et al. 2017 [59] | Y | Y | Y | Y | U | U | Y | Y | N | Y | U | 7/11 |
| (26) Lee L et al. 2017 [60] | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | 9/11 |
| (27) Coelho-Júnior HJ et al. 2018 [61] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11/11 |
| (28) Marcos-Pérez D et al. 2020 [62] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | 9/11 |
| (29) Lorenzo-López L et al. 2017 [63] | Y | Y | Y | Y | N | U | U | Y | N | Y | Y | 7/11 |
| (30) Dibello V et al. 2021 [64] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
| (31) He B et al. 2019 [65] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
| (32) Xu R et al. 2021 [66] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | 10/11 |
| (33) Coelho-Junior HJ et al. 2022 [67] | Y | Y | Y | Y | Y | Y | Y | Y | N | U | Y | 9/11 |
| (34) Parvaneh S et al. 2016 [68] | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | 9/11 |
| (35) Feng Z et al. 2017 [30] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 9/11 |
| (36) Wang X et al. 2022 [69] | Y | Y | Y | Y | U | U | Y | Y | N | Y | Y | 8/11 |
| (37) Gordon EH et al. 2017 [70] | Y | Y | Y | Y | Y | U | U | Y | N | U | Y | 7/11 |
| (38) Sargent L et al. 2018 [71] | Y | Y | Y | Y | U | U | Y | Y | N | Y | Y | 8/11 |
| (39) Pourmotabbed A et al. 2020 [72] | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | 10/11 |
| (40) Carvalho ACA et al. 2019 [73] | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | 9/11 |
| (41) Shakya S et al. 2022 [74] | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y | 9/11 |
| (42) Chu W et al. 2019 [28] | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | U | 9/11 |
| (43) Dao HHH et al. 2022 [75] | Y | Y | Y | Y | Y | Y | U | Y | N | Y | Y | 9/11 |
| (44) Wai JL et Yu DS, 2020 [76] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 10/11 |
Y: Yes N: No U: Unclear
Q1: Is the review question clearly and explicitly stated?
Q2: Were the inclusion criteria appropriate for the review question?
Q3: Was the search strategy appropriate?
Q4: Were the sources and resources used to search for studies adequate?
Q5: Were the criteria for appraising studies appropriate?
Q6: Was critical appraisal conducted by two or more reviewers independently?
Q7: Were there methods to minimize errors in data extraction?
Q8: Were the methods used to combine studies appropriate?
Q9: Was the likelihood of publication bias assessed?
Q10: Were recommendations for policy and/or practice supported by the reported data?
Q11: Were the specific directives for new research appropriate?
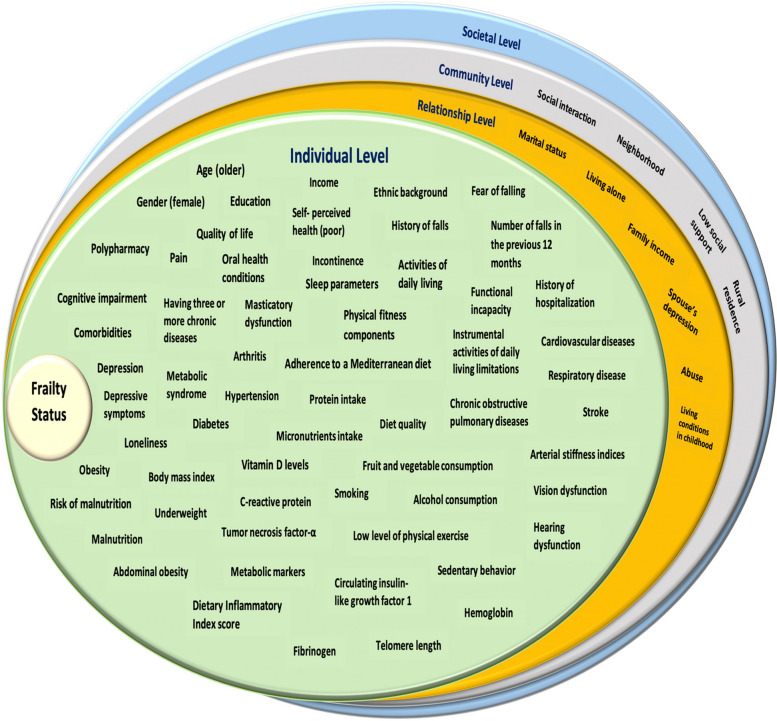
Based on the ROBIS tool [33] for assessing the risk of bias in systematic reviews, the final judgment of the risk of bias in the reviews was rated as "Low" for 25 studies (57%). This corresponds to 14 systematic reviews with meta-analyses and 11 systematic reviews with qualitative synthesis. The remaining 19 studies were rated as having a "High" risk of bias, including 13 systematic reviews with meta-analyses and six systematic reviews with qualitative synthesis. Table 3 presents the details of the assessment of the four domains of the second phase of the ROBIS tool and the final judgment of the risk of bias in the reviews.
Table 3.
Assessment of Risk of Bias in Included Systematic Reviews Using ROBIS Tool
 Low
Low
 High
High
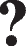 Unclear
Unclear
Main factors associated with frailty in older people
Several factors were considered in the reviews, corresponding to different levels of influence in the adopted socio-ecological model. These factors mainly included individual factors that were explored in all reviews, including demographic and personal factors (11 reviews), clinical and health-related factors (30 reviews), lifestyle factors (13 reviews) and biological factors or biomarkers (11 reviews). Relationship and community factors were explored in ten and four reviews, respectively. Only ten reviews have combined the previous levels of influence in the socio-ecological model, and there were no reviews that addressed the societal or policy level. However, six reviews associated the individual and relational levels and four reviews combined the three levels explored. In addition, 24 reviews (55%) were able to explore multiple factors or parameters related to frailty, while the remaining 20 reviews focused only on one or two factors. In total, 75 factors were explored in several specific meta-analyses, revealing 67 significant associations with frailty in the older population, with 49 positive associations and 18 negative associations.
A summary of the main factors identified by the level of influence of the socio-ecological model and their associations with frailty is available in the Additional files (Additional file 4).
Individual level
Demographic factors and personal characteristics
Older age [29, 30, 36, 38, 60, 65, 66, 69] and female gender [29, 30, 36, 65, 69, 70] were the most frequently identified risk factors for frailty in the older population, according to various systematic reviews and meta-analyses.
A lower level of education was often found to be significantly associated with frailty status [30, 36, 50, 60], but non-significant associations were also reported [30]. The number of years of education was inversely associated with frailty [29], while the association between higher education level and frailty was controversial based on the results of meta-analyses [69]. Income was found to be inversely associated with frailty [29, 30], and a consistent inverse association was identified between frailty and quality of life [45]. Ethnic background was reported to be associated with frailty in a few studies [29, 30].
Clinical and health-related factors
Among the clinical and health-related factors explored, seventeen factors were identified as risk factors for frailty based on the results of meta-analyses. These factors included pain [69], loneliness [51], having three or more chronic diseases [65], metabolic syndrome [75], incontinence [36], respiratory disease [69], arterial stiffness indices [43] and sleep parameters [47, 69, 72] such as insomnia [47], difficulty falling asleep [47], difficulty in maintaining sleep [47], non-restorative sleep [47], poor sleep [69], short sleep duration (< 6 h) [72], long sleep duration (> 8 h) [72], daytime drowsiness [72], sleep-disordered breathing [72], and prolonged sleep latency [72].
According to both qualitative syntheses and meta-analyses, significant positive associations with frailty were also found with the following factors: poor self-perceived health [29, 30, 36, 66], polypharmacy [36, 69], history of falls [29, 36, 38, 69], cognitive impairment [30, 36, 50, 66, 69], risk of malnutrition [29, 36, 63, 66], malnutrition [29, 59, 63, 69], masticatory dysfunction [53, 64], limitations in activities of daily living (ADL) [36, 60, 65, 66], abdominal obesity [29, 40, 60], obesity [30, 40, 60], underweight [29, 40], comorbidities [29, 36, 69], diabetes [29, 36, 60, 69], hypertension [36, 60], arthritis [29, 36, 60], stroke [29, 36], chronic obstructive pulmonary diseases (COPD) [29, 36], hearing dysfunction [60, 69], depression [28, 29, 36, 60, 69], and depressive symptoms [29, 30, 50, 60, 66]. However, non-significant associations with frailty were reported from quantitative analyses, particularly for early morning awakening [47], overweight [40], stroke [69], cardiovascular diseases [69], vision dysfunction [69], and human herpes virus seropositivity [46]. The five components of physical fitness [56] and the body mass index (BMI) [69] were found to have a significant negative association with frailty in the older adults.
Abdominal obesity, hyperglycemia and multiple concomitant cardiometabolic risk factors have been associated with an increased likelihood of frailty in older people with inconsistency between studies concerning the associations between dyslipidemia, elevated blood pressure, and frailty [74].
Oral health characteristics were specifically addressed in four systematic reviews [37, 48, 57, 64]. The first review, published in 2015 [57], suggested a possible association between poor oral health and frailty. However, this review was not strongly conclusive due to limitations and concerns related to the included studies, and it also presented a high risk of bias. The most recent reviews with high quality and low risk of bias have identified different oral health indicators [37, 48, 64], which have been classified into four categories: "oral health status deterioration; deterioration of oral motor skills; chewing, swallowing, and saliva disorders; and oral pain" [64]. These factors include the number of teeth, decreased masticatory function, difficulty chewing, deterioration of oral health, oral diadochokinesis, reduced occlusal force, reduced tongue pressure, dry mouth, periodontal disease, difficulty swallowing, oral dysbiosis, and tooth or mouth pain [64]. In addition, non-use of dental services, the need for dentures, the need for preventive dental care, and worse self-perceived or self-reported oral health factors have also been associated with frailty in older persons [37]. Thus, oral frailty and the accumulation of oral health problems have been found to be associated with an increased incidence of frailty, while good oral health and good oral hygiene have been identified as protective factors [37, 48, 64].
Despite indicating positive associations with frailty, the exploration of anemia [42], sleep and wake disturbances [76], and cardiac autonomic nervous system impairment [68] were inconclusive or should be interpreted with caution.
Other factors that have been cited as contributing to frailty in systematic reviews include the number of falls in the last 12 months [30, 60], fear of falling [44], a history of hospitalization [36], functional incapacity [29], and limitations in instrumental activities of daily living (IADL) [36].
Lifestyle factors and behaviors
Greater adherence to a Mediterranean diet [30, 41], consumption of fruits and vegetables [30, 58], higher protein intake [30, 61, 63, 67], better diet quality and higher intake of α-carotene, β-carotene equivalent, vitamin D, α-tocopherol, vitamin B6, folate and vitamin C were found to be inversely associated with frailty [63]. Additionally, a higher Dietary Inflammatory Index score [55] and a low intake of specific micronutrients (vitamin D, E, C, folate, carotenoids, α-tocopherol) [63] showed a significant increased risk of frailty.
Sedentary behavior [60], a low level of physical exercise [36, 69] and smoking [29, 30, 60, 69] were considered to be significantly associated with the risk of frailty in many reviews. However, there was some inconsistency regarding alcohol consumption [29, 30, 36, 60], with one meta-analysis showing a significant inverse association with frailty [69].
Biological factors
When considering biological factors, C-reactive protein (CRP) [30, 39, 49, 50, 54, 60, 71], Interleukin-6 (IL-6) [39, 49, 50, 54, 71], and Tumor necrosis factor alpha (TNF-α) [39, 54, 71] have been frequently studied and often found to have a positive and significant association with frailty. Low levels of vitamin D (25(OH)D) have also commonly been found in frail older people [30, 39, 50, 62, 69, 71]. Various inflammatory, hematological, protein, endocrine, and nutritional markers have been explored, but with a limited number of primary studies [30, 38, 39, 71]. Indeed, some reviews have suggested that frailty is associated with high levels of glycated hemoglobin [60, 71], fibrinogen [39, 71], and low levels of albumin [50, 54, 71], hemoglobin [50, 54, 71] and circulating insulin-like growth factor 1 (IGF-1) [54].
However, several genetic factors have been identified as associated with the frailty phenotype and, 13 correlations have been identified between serum and genetic markers [71]. For serum biomarkers, they included: myostatin, Klotho and IL-18, which were associated with frailty; vitamin D, cystatin C, IL-6, TNF-alpha, IL-6R, CRP and IL-1 βeta were related to frailty associated with cognitive decline; and chemokine receptor 2, IL-12p70, and brain-derived neurotrophic factor were associated with cognitive decline only [71]. In addition, the following genes, specified by their "single nucleotide polymorphism", were associated with the frailty phenotype: IL-18 rs360722, IL-6 rs1800796, COMT (catechol-O-methyltransferase) rs464316, TNF rs1800629 linked to chromosomes 11, 7, 22, and 6, respectively [71].
As for the association between frailty and telomere length, frail older people had shorter telomeres and this appears to be ethnic dependent [73].
Relationship level
Living alone [29, 36, 60, 69] and marital status (single or widowed) [29, 36] have been identified as risk factors for frailty in older adults, based on qualitative and quantitative syntheses. Lower family income [36, 60], abuse [29], and spouse’s depression [30] were also cited as having a significant positive association with frailty in some systematic reviews based on a few primary studies. Living conditions during childhood, monthly family income and being married appear to be associated with a decreased risk of frailty [29]. One systematic review also suggested a possible association between pet ownership and a lower risk of frailty [52]. Older people taking care of their grandchildren or pets had a 40% lower risk of being frail [52].
Community and society levels
While no societal-level factors were studied, very few community-level factors were addressed or identified. It was reported that neighborhood conditions [30], rural residence [38], and low social support [38] were associated with higher frailty in older people. Additionally, social interaction was mentioned to have an inverse effect on frailty in older individuals [29].
An illustrative socio-ecological model of the main factors contributing to frailty in older people is provided in Fig. 2.
Fig. 2.
Socio-ecological model of the main factors associated with frailty in older people
Certainty of the evidence
A total of 37 relevant factors for older people were assessed for certainty of the evidence using the GRADE approach. According to 18 systematic reviews with meta-analyses [28, 36, 38, 40, 41, 47, 51, 53, 55, 56, 58, 61, 62, 65, 66, 69, 72, 75] and 51 specific evaluations, the certainty of the evidence was rated as "Very low" or "Low" for 15 and 12 factors, respectively. Additionally, seven factors were rated as moderate or reached a moderate level of evidence. These include the Dietary Inflammatory Index score (OR 1.24, 95% CI 1.16 to 1.33) [55], maximum walking speed (SMD -0.97, 95% CI -1.25 to -0.68) [56], self-reported masticatory dysfunction (OR 1.83, 95% CI 1.55 to 2.18) [53], depression (OR 4.66, 95% CI 4.07 to 5.34) [36], loneliness (OR 3.51, 95% CI 2.70 to 4.56) [51], ADL limitations (OR 2.59, 95% CI 1.71 to 3.48) [66], and risk of malnutrition (OR 3.52, 95% CI 2.96 to 4.17) [36]. For the three remaining factors, adherence to a Mediterranean diet showed a "High" level of evidence, while the certainty of evidence varied for vitamin D levels and diabetes with "Very low" and "Low" ratings.
The main reasons for downgrading the level of evidence were inconsistency with significant heterogeneity (31 evaluations) and publication bias (20 evaluations). However, all plausible residual confounding or bias and large effect allowed us to upgrade the level of evidence in 27 and 20 evaluations, respectively. Details of these assessments are provided in the Additional files (Additional file 5).
Discussion
The aim of this umbrella review was to identify factors associated with frailty in the older population, using a socioecological approach. Several risk factors, protective factors and biomarkers were found to be related to frailty, especially in community-dwelling older people, with 67 significant associations from meta-analyses. Individual-level factors were predominant, followed by relationship and community factors, while no society-level factors were explored in the included systematic reviews.
Our umbrella review highlighted the wide variability in frailty prevalence reported in the literature [3–6, 81, 82]. This variability can be attributed to a number of factors, including the frailty assessment tool used, differences between countries, regions, and study settings, as well as age and gender. Frailty increases with age [3, 4, 6], and although women are more frail than men [2, 4–6], they paradoxically experience a lower mortality rate [83]. Some authors have identified biological, behavioral, and social hypotheses that could explain sex differences in mortality, morbidity, and frailty, and have consequently suggested specific interventions for frailty prevention [84].
The majority of our relevant factors for the older population showed very low or low levels of evidence, with little or limited confidence in the effect estimate. Among these factors, co-morbidities, having three or more chronic diseases, diabetes, hypertension, and metabolic syndrome were identified as risk factors for frailty in older people. All of these conditions lead to multiple drug prescriptions and polypharmacy was also identified as a factor that increases the probability of becoming frail in our review. According to a recent overview, medication reviews can reduce inappropriate use of medications, but there is insufficient evidence to improve frailty [85]. Despite the lack of hindsight, a new and more comprehensive model, "the polypharmacy stewardship model", has recently been proposed to promote the appropriate use of medicines, particularly in frail older people with multimorbidity and polymedication [86]. This model is based on five stages: patient identification, medication review, personalized deprescribing, support and engagement with patient-centered intervention, and collaboration between all stakeholders.
When considering relevant nutritional, lifestyle, behavioral and psychosocial factors, our review has identified several factors significantly associated with frailty, namely: malnutrition, risk of malnutrition, high Dietary Inflammatory Index score, low vitamin D levels, protein intake, fruit and vegetable consumption, adherence to Mediterranean diet, abdominal obesity, obesity, underweight, sleep parameters, smoking, alcohol use, low level of physical exercise, physical fitness components, ADL limitations, depression or depressive symptoms, cognitive impairment, loneliness, and living alone. Of note, some of these factors achieved a moderate certainty of evidence (depression, ADL limitations, loneliness, risk of malnutrition, dietary inflammatory index score, maximal walking speed), while only greater adherence to a Mediterranean diet showed a high level of evidence for its protective effect against frailty.
In addition, systematic reviews, mainly based on randomized controlled trials (RCTs) support our findings by revealing the benefits of physical activity and nutrition-based interventions on frailty and its related risk factors or components [87–91]. There is moderate certainty of evidence that physical activity interventions have significant effects on mobility, ADL, cognitive function, quality of life and frailty [87]. Also, interventions based on a combined approach, including physical activity and a nutritional intervention with protein and vitamin D supplementation, dietary counseling, education or cooking classes showed significant effects on physical function, mobility and measures of frailty [88]. Another recently published overview suggested that diets, physical activity and digital technologies can also improve loneliness, social isolation and frailty among older people [92]. Resistance training alone or multi-component exercise interventions have been recommended for frail and pre-frail older persons to improve their muscle strength, walking speed, balance, and physical performance [93]. The average frequency of exercise reported was two to three times a week, for ten to 90 min per session, with a total duration of five to 72 weeks, and a minimum of 2.5 months for the majority [93].
It is important to report that there is limited but interesting evidence of the anti-inflammatory effect of physical activity, diet supplementation and the Mediterranean diet, with a reduction in inflammatory biomarkers, which may contribute to the effect on frailty [94–96].
According to our findings, self-reported masticatory dysfunction is another relevant factor associated with frailty, with moderate certainty of evidence. Poor oral health status is associated with the onset of frailty in the older population, with nutritional, inflammatory, psychological and neuronal mechanisms suggested to explain this relationship [97].
Even with low certainty of evidence, a history of falls is also an important factor related to frailty in our review. It is one of the major risk factors for falls in older people, along with balance and gait disorders, polypharmacy, female gender, fear of falling, visual impairment, cognitive decline, depression, and environmental factors [98, 99]. Among older adults living in the community, multifactorial interventions, including exercise, may decrease the falls rate [100], and with high certainty of evidence, home fall-hazard interventions were also effective in reducing falls in high-risk individuals [101].
Strengths and limitations of this umbrella review
To our knowledge, there is no previous umbrella review summarizing the evidence from published systematic reviews and meta-analyses on factors associated with frailty in older persons. Our current review followed the PRISMA 2020 guidelines and the Joanna Briggs Institute recommendations with a pre-registered protocol on PROSPERO.
A comprehensive search strategy was adopted and all details on study selection and data collection were provided. The quality and risk of bias assessment of the included systematic reviews were conducted using two relevant tools. In addition, the GRADE approach was used to assess the certainty of the evidence for outcomes relevant to older people.
However, our review has certain limitations. Some of these limitations are common to umbrella reviews, namely: the influence of possible errors in the conduct of the included systematic reviews or meta-analyses; the reliance on syntheses of the results of systematic reviews, which are based on the primary studies included in these reviews; and the coverage of existing systematic reviews, which means that several factors are not exploited or have not yet been addressed by systematic reviews. Although the quality of the included reviews was rated as high or moderate, some reviews had a high risk of bias. Furthermore, inconsistency with significant heterogeneity and publication bias were the main reasons for downgrading the level of certainty of the evidence for outcomes relevant to older people. Finally, since the included systematic reviews were solely based on observational studies, only associations, rather than causations, can be identified.
Implications for practice, policy and future research
Various clinical practice guidelines have focused on the identification and management of frailty, providing recommendations primarily in one or more of the following areas: prevention, frailty screening, comprehensive assessment, physical activity, nutrition, medication management and fall prevention [102–106]. These recommendations were often based on scientific evidence or expert consensus, but some gaps have been identified. These gaps include a lack of evidence-building and detailed strategies for effective implementation and monitoring of these recommendations.
Our umbrella review will help to update these clinical practice guidelines and guide health policies and research towards the importance of adopting a holistic approach that considers all the influences of the different levels of the socio-ecological model and also considers the individual as a whole with a person-centered approach. There is a need to involve older people in the whole process, from identifying their needs to studying the feasibility, acceptability and cost-effectiveness of the actions.
Existing primary research data on factors associated with frailty in older people should be better exploited in future systematic reviews and meta-analyses. In addition to examining the individual level, future research should also consider other relational, community, and societal or policy levels. It is important to promote community-based research in order to tailor interventions to local specificities and resources, in collaboration with all stakeholders.
Conclusion
Frailty in older individuals is a dynamic and multifactorial syndrome that requires a holistic approach to its prevention and management. Our umbrella review highlighted several factors that are associated with frailty, particularly in community-dwelling older people. These factors seem to interact to either increase or decrease the likelihood of the occurrence of frailty. Individual factors were predominant, followed by relationship and community factors, suggesting the possibility of interventions on all modifiable factors at the different levels of influence of the socio-ecological model. Currently, lifestyle interventions based on physical activity and nutrition are the most effective in reducing frailty and a number of its risk factors or components, while other interventions remain promising, particularly those based on digital health and innovation.
Supplementary Information
Additional file 1. Detailed Research Strategy.
Additional file 2. List of Excluded Full-Text Articles and Reasons for Exclusion.
Additional file 3. Detailed Narrative Table of Data from Systematic Reviews Included in the Umbrella Review.
Additional file 4. Summary of the Main Factors Identified by Level of Influence of the Socio-Ecological Model and Their Associations with Frailty.
Additional file 5. Grading of Recommendations, Assessment, Development, and Evaluations for Frailty-Related Factors Relevant to the Patient.
Acknowledgements
We would like to thank all the authors who kindly shared their full-text articles with us during the study inclusion process.
Abbreviations
- ADL
Activities of daily living
- BMI
Body mass index
- CRP
C-reactive protein
- GRADE
Grading of Recommendations, Assessment, Development and Evaluations
- IADL
Instrumental activities of daily living limitations
- IL-6
Interleukin-6
- JBI
Joanna Briggs Institute
- MD
Mean Difference
- OR
Odds Ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International prospective register of systematic reviews
- ROBIS
Risk of bias in systematic reviews
- RR
Relative Risk
- SMD
Standardized Mean Difference
- TNF-α
Tumor necrosis factor alpha
Authors’ contributions
MB and MK: conceptualization and design. MB and GYG: preliminary research. MB and MK: methodology and search strategy. MB and AS: study selection and quality assessment. MB and NEH: data extraction. MB: assessment of risk of bias and certainty of evidence. MB: writing the first draft of the manuscript. LB and MK: review and supervision. All authors reviewed and approved the manuscript.
Funding
No funding was received for this research.
Availability of data and materials
All data relevant to our review are included in the article and also available as additional files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Ageing and health. 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 2.Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults. JAMA Netw Open. 2019;2:e198398. 10.1001/jamanetworkopen.2019.8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To T-L, Doan T-N, Ho W-C, Liao W-C. Prevalence of frailty among community-dwelling older adults in asian countries: a systematic review and meta-analysis. Healthcare (Basel). 2022;10:895. 10.3390/healthcare10050895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open. 2018;8:e018195. 10.1136/bmjopen-2017-018195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96–104. 10.1093/ageing/afaa219 [DOI] [PubMed] [Google Scholar]
- 6.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO clinical consortium on healthy ageing: topic focus: frailty and intrinsic capacity: report of consortium meeting, 1–2 December 2016 in Geneva. Switzerland: World Health Organization; 2017. [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 9.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue Q-L, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs. 2012;68:2047–60. 10.1111/j.1365-2648.2011.05896.x [DOI] [PubMed] [Google Scholar]
- 11.Pilotto A, Custodero C, Maggi S, Polidori MC, Veronese N, Ferrucci L. A multidimensional approach to frailty in older people. Ageing Res Rev. 2020;60:101047. 10.1016/j.arr.2020.101047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levers M-J, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56:282–91. 10.1111/j.1365-2648.2006.04021.x [DOI] [PubMed] [Google Scholar]
- 13.Junius-Walker U, Onder G, Soleymani D, Wiese B, Albaina O, Bernabei R, et al. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10. 10.1016/j.ejim.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 14.Cheng M-H, Chang S-F. Frailty as a risk factor for falls among community dwelling people: evidence from a meta-analysis. J Nurs Scholarsh. 2017;49:529–36. 10.1111/jnu.12322 [DOI] [PubMed] [Google Scholar]
- 15.Castro-Rodríguez M, Carnicero JA, Garcia-Garcia FJ, Walter S, Morley JE, Rodríguez-Artalejo F, et al. Frailty as a major factor in the increased risk of death and disability in older people with diabetes. J Am Med Dir Assoc. 2016;17:949–55. 10.1016/j.jamda.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 16.Kojima G. Frailty as a predictor of nursing home placement among community-dwelling older adults: a systematic review and meta-analysis. J Geriatr Phys Ther. 2018;41:42–8. 10.1519/JPT.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Chang S, Lin P. Frailty as a predictor of future fracture in older adults: a systematic review and meta-analysis. Worldviews Evid Based Nurs. 2017;14:282–93. 10.1111/wvn.12222 [DOI] [PubMed] [Google Scholar]
- 18.Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:722–9. 10.1136/jech-2015-206978 [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Hu P, Sun W, Wu W, Zhang J, Deng H, et al. Effect of physical activity on the risk of frailty: a systematic review and meta-analysis. PLoS One. 2022;17:e0278226. 10.1371/journal.pone.0278226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128:1225-1236.e1. 10.1016/j.amjmed.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 22.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351–77. 10.1177/109019818801500401 [DOI] [PubMed] [Google Scholar]
- 23.The Social-Ecological Model: A Framework for Prevention |Violence Prevention|Injury Center|CDC. 2022. https://www.cdc.gov/violence-prevention/about/index.html.
- 24.Hayajneh A, Rababa M. The association of frailty with poverty in older adults: a systematic review. Dementia Geriatr Cogn Disord. 2022;50:407–13. 10.1159/000520486 [DOI] [PubMed] [Google Scholar]
- 25.Lavicoli I, Leso V, Cesari M. The contribution of occupational factors on frailty. Arch Gerontol Geriatr. 2018;75:51–8. 10.1016/j.archger.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 26.Duppen D, Van der Elst MCJ, Dury S, Lambotte D, De Donder L, D-SCOPE. The social environment’s relationship with frailty: evidence from existing studies. J Appl Gerontol. 2019;38:3–26. 10.1177/0733464816688310 [DOI] [PubMed] [Google Scholar]
- 27.Ellwood A, Quinn C, Mountain G. Psychological and social factors associated with coexisting frailty and cognitive impairment: a systematic review. Res Aging. 2022;44:448–64. 10.1177/01640275211045603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu W, Chang S, Ho H, Lin H. The relationship between depression and frailty in community-dwelling older people: a systematic review and meta-analysis of 84,351 older adults. J Nurs Scholarsh. 2019;51:547–59. 10.1111/jnu.12501 [DOI] [PubMed] [Google Scholar]
- 29.de Mello AC, Engstrom EM, Alves LC. Health-related and socio-demographic factors associated with frailty in the elderly: a systematic literature review. Cadernos Saude Publica. 2014;30:1143–68. 10.1590/0102-311X00148213 [DOI] [PubMed] [Google Scholar]
- 30.Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12:e0178383. 10.1371/journal.pone.0178383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, PRISMA, et al. explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2020;2021:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Umbrella Reviews (2020). Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, editors. JBI Manual for Evidence Synthesis. JBI; 2024. https://jbi-global-wiki.refined.site/space/MANUAL/355829653/9.+Umbrella+reviews.
- 33.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 35.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 36.Qin Y, Hao X, Lv M, Zhao X, Wu S, Li K. A global perspective on risk factors for frailty in community-dwelling older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2023;105:104844. 10.1016/j.archger.2022.104844 [DOI] [PubMed] [Google Scholar]
- 37.Slashcheva LD, Karjalahti E, Hassett LC, Smith B, Chamberlain AM. A systematic review and gap analysis of frailty and oral health characteristics in older adults: a call for clinical translation. Gerodontology. 2021;38:338–50. 10.1111/ger.12577 [DOI] [PubMed] [Google Scholar]
- 38.Vatanabe IP, Pedroso RV, Teles RHG, Ribeiro JC, Manzine PR, Pott-Junior H, et al. A systematic review and meta-analysis on cognitive frailty in community-dwelling older adults: risk and associated factors. Aging Ment Health. 2022;26:464–76. 10.1080/13607863.2021.1884844 [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan P, Alyousefi N, Abdul-Rahman P, Kamaruzzaman S, Chin A, Tan M. A systematic review of studies comparing potential biochemical biomarkers of frailty with frailty assessments. Eur Geriatr Med. 2017;8:397–407. 10.1016/j.eurger.2017.07.010 [DOI] [Google Scholar]
- 40.Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50:1118–28. 10.1093/ageing/afab039 [DOI] [PubMed] [Google Scholar]
- 41.Kojima G, Avgerinou C, Iliffe S, Walters K. Adherence to mediterranean diet reduces incident frailty risk: systematic review and meta-analysis. J Am Geriatr Soc. 2018;66:783–8. 10.1111/jgs.15251 [DOI] [PubMed] [Google Scholar]
- 42.de Assis EPS, de Macêdo BG, de Oliveira HSC, de Rezende PD, Antunes CMF. Anemia and the frailty syndrome amongst the elderly living in the community: a systematic review. Rev Br Geriatr Gerontol. 2018;21:223–31. 10.1590/1981-22562018021.170100 [DOI] [Google Scholar]
- 43.Piotrowicz K, Gryglewska B, Grodzicki T, Gąsowski J. Arterial stiffness and frailty - A systematic review and metaanalysis. Exp Gerontol. 2021;153:111480. 10.1016/j.exger.2021.111480 [DOI] [PubMed] [Google Scholar]
- 44.de Souza L, Canever J, Moreira B, Danielewicz A, de Avelar N. Association between fear of falling and frailty in community-dwelling older adults: a systematic review. Clin Interv Aging. 2022;17:129–40. 10.2147/CIA.S328423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:716–21. 10.1136/jech-2015-206717 [DOI] [PubMed] [Google Scholar]
- 46.Araújo Carvalho AC, Tavares Mendes ML, Santos VS, Tanajura DM, Prado Nunes MA, Martins-Filho PRS. Association between human herpes virus seropositivity and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2018;48:145–52. 10.1016/j.arr.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Wen Q, Yan X, Ren Z, Wang B, Liu Y, Jin X. Association between insomnia and frailty in older population: a meta-analytic evaluation of the observational studies. Brain Behav. 2023;13:e2793. 10.1002/brb3.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hakeem F, Bernabe E, Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36:205–15. 10.1111/ger.12406 [DOI] [PubMed] [Google Scholar]
- 49.Marcos-Pérez D, Sánchez-Flores M, Proietti S, Bonassi S, Costa S, Teixeira JP, et al. Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta-analysis. GeroScience. 2020;42:1451–73. 10.1007/s11357-020-00247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pothier K, Gana W, Bailly N, Fougère B. Associations between frailty and inflammation, physical, and psycho-social health in older adults: a systematic review. Front Psychol. 2022;13:805501. 10.3389/fpsyg.2022.805501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kojima G, Taniguchi Y, Aoyama R, Tanabe M. Associations between loneliness and physical frailty in community-dwelling older adults: a systematic review and meta-analysis. Ageing Res Rev. 2022;81:101705. 10.1016/j.arr.2022.101705 [DOI] [PubMed] [Google Scholar]
- 52.Kojima G, Aoyama R, Taniguchi Y. Associations between pet ownership and frailty: a systematic review. Geriatrics (Switzerland). 2020;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima G, Taniguchi Y, Iwasaki M, Aoyama R, Urano T. Associations between self-reported masticatory dysfunction and frailty: a systematic review and meta-analysis. PLoS One. 2022;17:e0273812. 10.1371/journal.pone.0273812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picca A, Coelho-Junior HJ, Calvani R, Marzetti E, Vetrano DL. Biomarkers shared by frailty and sarcopenia in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2022;73:101530. 10.1016/j.arr.2021.101530 [DOI] [PubMed] [Google Scholar]
- 55.Moradi S, Hadi A, Mohammadi H, Asbaghi O, Zobeiri M, Marx W, et al. Dietary inflammatory index and the risk of frailty among older adults: a systematic review and meta-analysis. Res Aging. 2021;43:323–31. 10.1177/0164027520948176 [DOI] [PubMed] [Google Scholar]
- 56.Navarrete-Villanueva D, Gómez-Cabello A, Marín-Puyalto J, Moreno LA, Vicente-Rodríguez G, Casajús JA. Frailty and physical fitness in elderly people: a systematic review and meta-analysis. Sports Med (Auckland, NZ). 2021;51:143–60. 10.1007/s40279-020-01361-1 [DOI] [PubMed] [Google Scholar]
- 57.Tôrres LHDN, Tellez M, Hilgert JB, Hugo FN, De Sousa MDLR, Ismail AI. Frailty, frailty components, and oral health: a systematic review. J Am Geriatr Soc. 2015;63:2555–62. 10.1111/jgs.13826 [DOI] [PubMed] [Google Scholar]
- 58.Kojima G, Taniguchi Y, Urano T. Fruit and vegetable consumption and incident frailty in older adults: a systematic review and meta-analysis. J Frailty Aging. 2022;11:45–50. [DOI] [PubMed] [Google Scholar]
- 59.Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults–a systematic review and meta-analysis. J Am Med Direct Assoc. 2017;18:374–82. 10.1016/j.jamda.2016.12.074 [DOI] [PubMed] [Google Scholar]
- 60.Lee L, Patel T, Hillier LM, Maulkhan N, Slonim K, Costa A. Identifying frailty in primary care: a systematic review. Geriatr Gerontol Int. 2017;17:1358–77. 10.1111/ggi.12955 [DOI] [PubMed] [Google Scholar]
- 61.Coelho-Júnior HJ, Rodrigues B, Uchida M, Marzetti E. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10:E1334. 10.3390/nu10091334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcos-Pérez D, Sánchez-Flores M, Proietti S, Bonassi S, Costa S, Teixeira JP, et al. Low vitamin D levels and frailty status in older adults: a systematic review and meta-analysis. Nutrients. 2020;12:E2286. 10.3390/nu12082286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17:108. 10.1186/s12877-017-0496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dibello V, Zupo R, Sardone R, Lozupone M, Castellana F, Dibello A, et al. Oral frailty and its determinants in older age: a systematic review. Lancet Healthy Longevity. 2021;2:e507–20. 10.1016/S2666-7568(21)00143-4 [DOI] [PubMed] [Google Scholar]
- 65.He B, Ma Y, Wang C, Jiang M, Geng C, Chang X, et al. Prevalence and risk factors for frailty among community-dwelling older people in china: a systematic review and meta-analysis. J Nutr Health Aging. 2019;23:442–50. 10.1007/s12603-019-1179-9 [DOI] [PubMed] [Google Scholar]
- 66.Xu R, Li Q, Guo F, Zhao M, Zhang L. Prevalence and risk factors of frailty among people in rural areas: a systematic review and meta-analysis. BMJ Open. 2021;11:e043494. 10.1136/bmjopen-2020-043494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coelho-Junior HJ, Calvani R, Picca A, Tosato M, Landi F, Marzetti E. Protein intake and frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2022;14:2767. 10.3390/nu14132767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parvaneh S, Howe C, Toosizadeh N, Honarvar B, Slepian M, Fain M, et al. Regulation of cardiac autonomic nervous system control across frailty statuses: a systematic review. Gerontology. 2016;62:3–15. 10.1159/000431285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Hu J, Wu D. Risk factors for frailty in older adults. Medicine (Baltimore). 2022;101:e30169. 10.1097/MD.0000000000030169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon E, Peel N, Samanta M, Theou O, Howlett S, Hubbard R. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 71.Sargent L, Nalls M, Starkweather A, Hobgood S, Thompson H, Amella EJ, et al. Shared biological pathways for frailty and cognitive impairment: a systematic review. Ageing Res Rev. 2018;47:149–58. 10.1016/j.arr.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pourmotabbed A, Boozari B, Babaei A, Asbaghi O, Campbell M, Mohammadi H, et al. Sleep and frailty risk: a systematic review and meta-analysis. Sleep Breath. 2020;24:1187–97. 10.1007/s11325-020-02061-w [DOI] [PubMed] [Google Scholar]
- 73.Araújo Carvalho AC, Tavares Mendes ML, da Silva Reis MC, Santos VS, Tanajura DM, Martins-Filho PRS. Telomere length and frailty in older adults—A systematic review and meta-analysis. Ageing Res Rev. 2019;54:100914. 10.1016/j.arr.2019.100914 [DOI] [PubMed] [Google Scholar]
- 74.Shakya S, Bajracharya R, Ledbetter L, Cary M. The association between cardiometabolic risk factors and frailty in older adults: a systematic review. Innov Aging. 2022;6:032. 10.1093/geroni/igac032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dao HHH, Burns MJ, Kha R, Chow CK, Nguyen TN. The relationship between metabolic syndrome and frailty in older people: a systematic review and meta-analysis. Geriatrics (Switzerland). 2022;7(4):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wai JL, Yu DS. The relationship between sleep–wake disturbances and frailty among older adults: a systematic review. J Adv Nurs. 2020;76:96–108. 10.1111/jan.14231 [DOI] [PubMed] [Google Scholar]
- 77.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scie World J. 2001;1:323–36. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Ser A. 2007;62:722–7. 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 79.van Abellan Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–2. 10.1016/j.jamda.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 80.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged african americans. J Nutr Health Aging. 2012;16:601–8. 10.1007/s12603-012-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alqahtani BA, Alshehri MM, Elnaggar RK, Alsaad SM, Alsayer AA, Almadani N, et al. Prevalence of frailty in the middle east: systematic review and meta-analysis. Healthcare (Switzerland). 2022;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veronese N, Custodero C, Cella A, Demurtas J, Zora S, Maggi S, et al. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: a systematic review and meta-analysis. Ageing Res Rev. 2021;72:101498. 10.1016/j.arr.2021.101498 [DOI] [PubMed] [Google Scholar]
- 83.Alberts SC, Archie EA, Gesquiere LR, et al. The Male-Female Health-Survival Paradox: A Comparative Perspective on Sex Differences in Aging and Mortality. In: Committee on Population; Division of Behavioral and Social Sciences and Education; National Research Council; Weinstein M, Lane MA, editors. Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers. Washington (DC): National Academies Press (US); 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK242444/. [PubMed]
- 84.Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust. 2020;212:183–8. 10.5694/mja2.50466 [DOI] [PubMed] [Google Scholar]
- 85.Verma A, Saha S, Jarl J, Conlon E, McGuinness B, Trépel D. An overview of systematic reviews and meta-analyses on the effect of medication interventions targeting polypharmacy for frail older adults. J Clin Med. 2023;12:1379. 10.3390/jcm12041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daunt R, Curtin D, O’Mahony D. Polypharmacy stewardship: a novel approach to tackle a major public health crisis. Lancet Healthy Longevity. 2023;4:e228–35. 10.1016/S2666-7568(23)00036-3 [DOI] [PubMed] [Google Scholar]
- 87.Racey M, Ali MU, Sherifali D, Fitzpatrick-Lewis D, Lewis R, Jovkovic M, et al. Effectiveness of physical activity interventions in older adults with frailty or prefrailty: a systematic review and meta-analysis. CMAJ Open. 2021;9:E728–43. 10.9778/cmajo.20200222 [DOI] [Google Scholar]
- 88.Racey M, Ali MU, Sherifali D, Fitzpatrick-Lewis D, Lewis R, Jovkovic M, et al. Effectiveness of nutrition interventions and combined nutrition and physical activity interventions in older adults with frailty or prefrailty: a systematic review and meta-analysis. CMAJ Open. 2021;9:E744. 10.9778/cmajo.20200248 [DOI] [Google Scholar]
- 89.Kidd T, Mold F, Jones C, Ream E, Grosvenor W, Sund-Levander M, et al. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019;19:184. 10.1186/s12877-019-1196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Negm AM, Kennedy CC, Thabane L, Veroniki A-A, Adachi JD, Richardson J, et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2019;20:1190–8. 10.1016/j.jamda.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 91.Morciano L, Cerone G, Cerutti F, Di Gaspare F, Alessandroni C, Lucaroni F, et al. Physical activity and nutritional supplementation to reduce frailty in community- dwelling older adults, searching for evidence: a systematic review of randomized controlled trials. 2018. 10.19252/000000074.
- 92.Adekpedjou R, Léon P, Dewidar O, Al-Zubaidi A, Jbilou J, Kaczorowski J, et al. Effectiveness of interventions to address different types of vulnerabilities in community-dwelling older adults: an umbrella review. Campbell Syst Rev. 2023;19:e1323. 10.1002/cl2.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16:752–75. 10.11124/JBISRIR-2017-003551 [DOI] [PubMed] [Google Scholar]
- 94.Zheng G, Qiu P, Xia R, Lin H, Ye B, Tao J, et al. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci. 2019;11:98. 10.3389/fnagi.2019.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hernández-Lepe MA, Ortiz-Ortiz M, Hernández-Ontiveros DA, Mejía-Rangel MJ. Inflammatory profile of older adults in response to physical activity and diet supplementation: a systematic review. Int J Environ Res Public Health. 2023;20:4111. 10.3390/ijerph20054111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koelman L, Egea Rodrigues C, Aleksandrova K. Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2022;13:101–15. 10.1093/advances/nmab086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Konstantopoulou K, Kossioni A. Mechanisms linking oral health and frailty in older adults: a narrative review. 2021. [Google Scholar]
- 98.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. 10.1016/j.maturitas.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Hou L, Zhao H, Xie R, Yi Y, Ding X. Risk factors for falls among community-dwelling older adults: a systematic review and meta-analysis. Front Med. 2023;9:1019094. 10.3389/fmed.2022.1019094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hopewell S, Copsey B, Nicolson P, Adedire B, Boniface G, Lamb S. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340–50. 10.1136/bjsports-2019-100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clemson L, Stark S, Pighills AC, Fairhall NJ, Lamb SE, Ali J, et al. Environmental interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2023. 10.1002/14651858.CD013258.pub2. [DOI] [PMC free article] [PubMed]
- 102.Lorbergs AL, Prorok JC, Holroyd-Leduc J, Bouchard DR, Giguere A, Gramlich L, et al. Nutrition and physical activity clinical practice guidelines for older adults living with frailty. J Frailty Aging. 2022;11:3–11. [DOI] [PubMed] [Google Scholar]
- 103.Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23:771–87. 10.1007/s12603-019-1273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Izquierdo M, Duque G, Morley JE. Physical activity guidelines for older people: knowledge gaps and future directions. Lancet Healthy Longevity. 2021;2:e380–3. 10.1016/S2666-7568(21)00079-9 [DOI] [PubMed] [Google Scholar]
- 105.Liau SJ, Lalic S, Sluggett JK, Cesari M, Onder G, Vetrano DL, et al. Medication management in frail older people: consensus principles for clinical practice, research, and education. J Am Med Dir Assoc. 2021;22:43–9. 10.1016/j.jamda.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 106.Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, et al. World guidelines for falls prevention and management for older adults: a global initiative. Age and Ageing. 2022;51:afac205. 10.1093/ageing/afac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed Research Strategy.
Additional file 2. List of Excluded Full-Text Articles and Reasons for Exclusion.
Additional file 3. Detailed Narrative Table of Data from Systematic Reviews Included in the Umbrella Review.
Additional file 4. Summary of the Main Factors Identified by Level of Influence of the Socio-Ecological Model and Their Associations with Frailty.
Additional file 5. Grading of Recommendations, Assessment, Development, and Evaluations for Frailty-Related Factors Relevant to the Patient.
Data Availability Statement
All data relevant to our review are included in the article and also available as additional files.