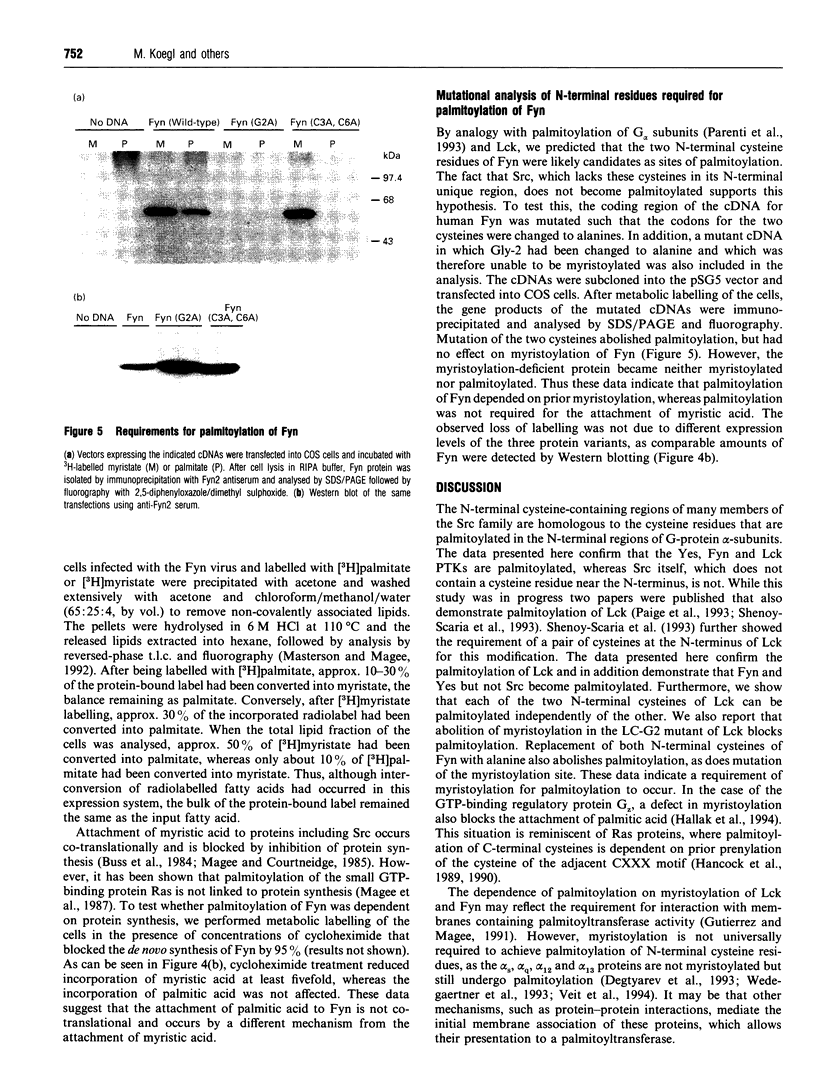
Abstract
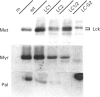
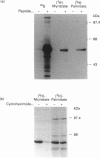
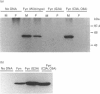
We have recently identified a novel N-terminal cysteine-containing motif which specifies the palmitoylation of several G-protein alpha-subunits [Parenti, Viganó, Newman, Milligan and Magee (1993) Biochem. J. 291, 349-353]. A related motif occurs at the N-terminus of members of the Src family of protein tyrosine kinases except for Src itself and Blk. We have investigated whether the Src, Fyn, Yes and Lck gene products are palmitoylated. Src was not labelled with [3H]palmitate when endogenously expressed in COS cells. In contrast, endogenous Yes immunoprecipitated from COS cells was palmitoylated. Fyn was palmitoylated in insect cells infected with a recombinant baculovirus and the palmitoylation was independent of protein synthesis, suggesting a dynamic turnover of this lipid. Fatty acid analysis indicated that most of the label was incorporated as palmitate. Lck was palmitoylated when expressed by transfection in COS cells. All of these protein tyrosine kinases were also detectably myristoylated in each of the systems tested. Experiments performed with mutants of Lck expressed by transfection in COS cells indicated that cysteines at positions 3 and 5 were both palmitoylation sites and that myristoylation was required for palmitoylation. To confirm that palmitoylation was occurring on cysteines in the N-terminal region of Fyn, site-directed mutagenesis was used to replace the cysteines at positions 3 and 6 with alanine. The resulting protein was not palmitoylated but was still myristoylated when expressed in COS cells. A glycine to alanine mutant at position 2 was also not palmitoylated, showing that myristoylation is a prerequisite for palmitoylation. Our data indicate that Src family members containing the N-terminal cysteine motif are indeed palmitoylated. By analogy with Ras, it is possible that palmitoylation may play an important role in the localization and function of Src family protein tyrosine kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B. Nonreceptor tyrosine protein kinases. Oncogene. 1993 Aug;8(8):2025–2031. [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Degtyarev M. Y., Spiegel A. M., Jones T. L. The G protein alpha s subunit incorporates [3H]palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry. 1993 Aug 17;32(32):8057–8061. doi: 10.1021/bi00083a001. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I. Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochim Biophys Acta. 1991 Jun 24;1078(2):147–154. doi: 10.1016/0167-4838(91)99003-b. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak H., Brass L. F., Manning D. R. Failure to myristoylate the alpha subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein kinase C. J Biol Chem. 1994 Feb 11;269(6):4571–4576. [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Paterson H., Marshall C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991 Dec;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Kypta R. M., Hemming A., Courtneidge S. A. Identification and characterization of p59fyn (a src-like protein tyrosine kinase) in normal and polyoma virus transformed cells. EMBO J. 1988 Dec 1;7(12):3837–3844. doi: 10.1002/j.1460-2075.1988.tb03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder M. E., Middleton P., Hepler J. R., Taussig R., Gilman A. G., Mumby S. M. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. M., Giannakouros T., Hancock J. F., Fawell E. H., Armstrong J., Magee A. I. Post-translational processing of Schizosaccharomyces pombe YPT proteins. J Biol Chem. 1992 Jun 5;267(16):11329–11336. [PubMed] [Google Scholar]
- Paige L. A., Nadler M. J., Harrison M. L., Cassady J. M., Geahlen R. L. Reversible palmitoylation of the protein-tyrosine kinase p56lck. J Biol Chem. 1993 Apr 25;268(12):8669–8674. [PubMed] [Google Scholar]
- Parenti M., Viganó M. A., Newman C. M., Milligan G., Magee A. I. A novel N-terminal motif for palmitoylation of G-protein alpha subunits. Biochem J. 1993 Apr 15;291(Pt 2):349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Williams N. G., Cheng S. H., Roberts T. M. Regulation of pp60c-src and its interaction with polyomavirus middle T antigen in insect cells. J Virol. 1990 Jan;64(1):61–68. doi: 10.1128/jvi.64.1.61-68.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochim Biophys Acta. 1993 Dec 23;1155(3):307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Prasad K. V., Raab M., da Silva A., Telfer J. C., Yamamoto M. src-related protein tyrosine kinases and their surface receptors. Biochim Biophys Acta. 1993 Aug 23;1155(2):239–266. doi: 10.1016/0304-419x(93)90007-y. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Gauen L. K., Kwong J., Shaw A. S., Lublin D. M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993 Oct;13(10):6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Twamley G. M., Kypta R. M., Hall B., Courtneidge S. A. Association of Fyn with the activated platelet-derived growth factor receptor: requirements for binding and phosphorylation. Oncogene. 1992 Oct;7(10):1893–1901. [PubMed] [Google Scholar]
- Veit M., Nürnberg B., Spicher K., Harteneck C., Ponimaskin E., Schultz G., Schmidt M. F. The alpha-subunits of G-proteins G12 and G13 are palmitoylated, but not amidically myristoylated. FEBS Lett. 1994 Feb 14;339(1-2):160–164. doi: 10.1016/0014-5793(94)80406-0. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P. B., Chu D. H., Wilson P. T., Levis M. J., Bourne H. R. Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J Biol Chem. 1993 Nov 25;268(33):25001–25008. [PubMed] [Google Scholar]