Abstract
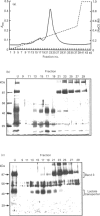
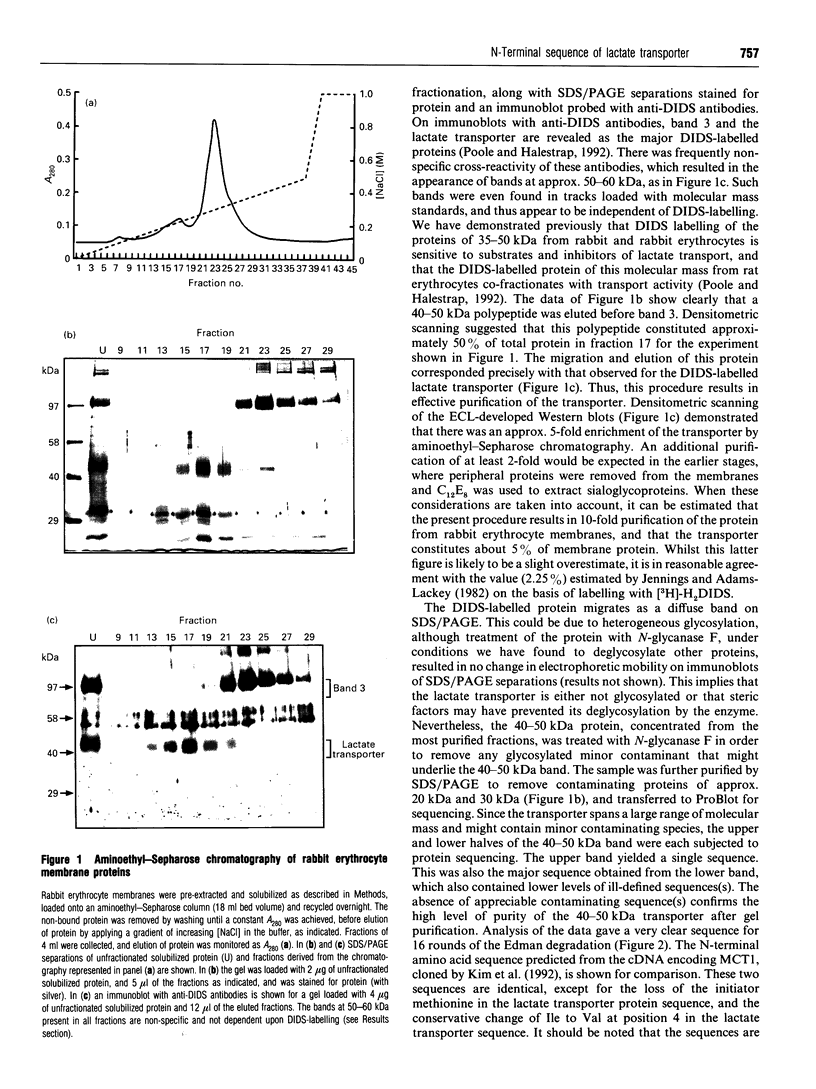
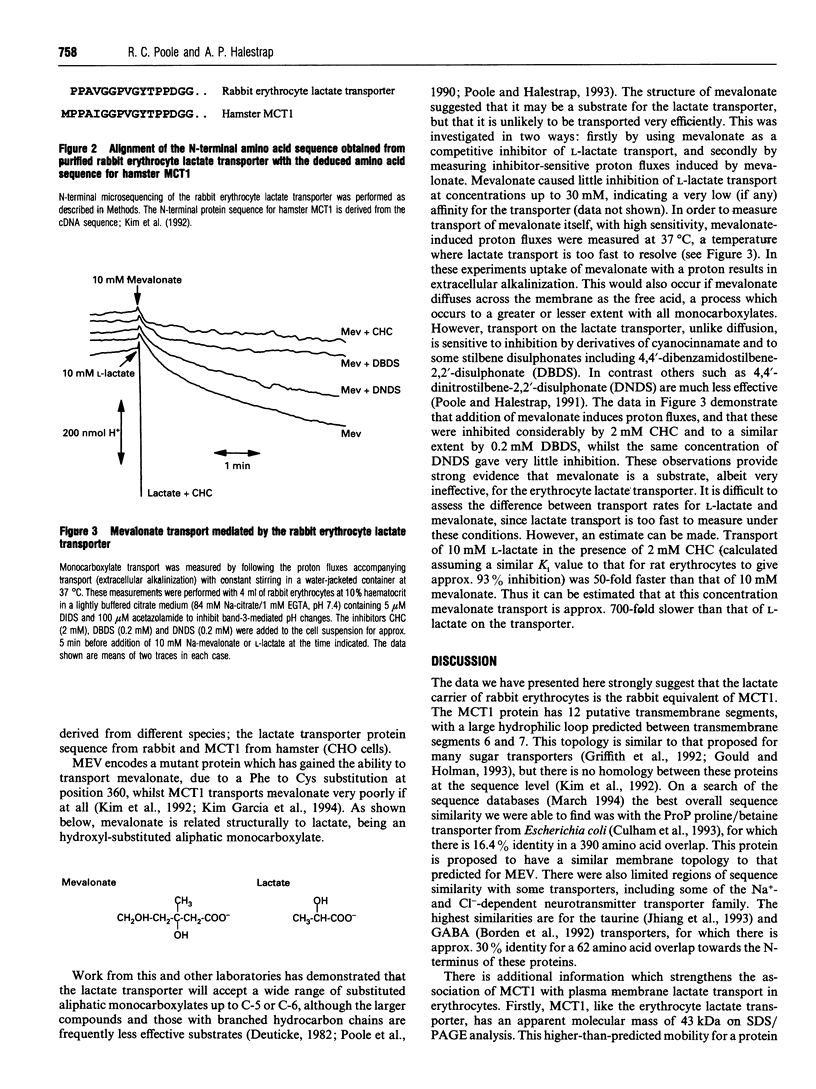
An improved purification for the rabbit erythrocyte lactate transporter, using aminoethyl-Sepharose chromatography, is described. The process of purification of the 40-50 kDa transporter, labelled with 4,4'-diisothiocyanostilbene-2,2'-disulphonate (DIDS), was followed by Western blotting with anti-DIDS antibodies [Poole, R. C. and Halestrap, A. P. (1992) Biochem. J. 283, 855-862]. Fractions highly-enriched in transporter were further purified by SDS/PAGE and the 40-50 kDa DIDS-labelled polypeptide was subjected to N-terminal protein sequencing. This analysis identified the first 16 amino acids of the protein. With the exception of one conservative substitution, this protein sequence is identical to the N-terminal protein sequence predicted from a cDNA isolated from Chinese hamster ovary cells that encode a monocarboxylate transporter, MCT1 [Kim Garcia, C., Goldstein, J. L., Pathak, R. K., Anderson, R. G. W. and Brown, M. S. (1994) Cell 76, 865-873]. This observation, along with similarities in functional properties, leads us to conclude that lactate transport in rabbit erythrocytes is mediated by the MCT1 monocarboxylate transporter isoform.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borden L. A., Smith K. E., Hartig P. R., Branchek T. A., Weinshank R. L. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992 Oct 15;267(29):21098–21104. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Casey J. R., Lieberman D. M., Reithmeier R. A. Purification and characterization of band 3 protein. Methods Enzymol. 1989;173:494–512. doi: 10.1016/s0076-6879(89)73034-2. [DOI] [PubMed] [Google Scholar]
- Casey J. R., Reithmeier R. A. Detergent interaction with band 3, a model polytopic membrane protein. Biochemistry. 1993 Feb 2;32(4):1172–1179. doi: 10.1021/bi00055a023. [DOI] [PubMed] [Google Scholar]
- Culham D. E., Lasby B., Marangoni A. G., Milner J. L., Steer B. A., van Nues R. W., Wood J. M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993 Jan 5;229(1):268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70(2):89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Rickert I., Beyer E. Stereoselective, SH-dependent transfer of lactate in mammalian erythrocytes. Biochim Biophys Acta. 1978 Feb 2;507(1):137–155. doi: 10.1016/0005-2736(78)90381-4. [DOI] [PubMed] [Google Scholar]
- Faust J., Krieger M. Expression of specific high capacity mevalonate transport in a Chinese hamster cell variant. J Biol Chem. 1987 Feb 15;262(5):1996–2004. [PubMed] [Google Scholar]
- Garcia C. K., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994 Mar 11;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Holman G. D. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993 Oct 15;295(Pt 2):329–341. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. K., Baker M. E., Rouch D. A., Page M. G., Skurray R. A., Paulsen I. T., Chater K. F., Baldwin S. A., Henderson P. J. Membrane transport proteins: implications of sequence comparisons. Curr Opin Cell Biol. 1992 Aug;4(4):684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L., Adams-Lackey M. A rabbit erythrocyte membrane protein associated with L-lactate transport. J Biol Chem. 1982 Nov 10;257(21):12866–12871. [PubMed] [Google Scholar]
- Jhiang S. M., Fithian L., Smanik P., McGill J., Tong Q., Mazzaferri E. L. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 1993 Mar 1;318(2):139–144. doi: 10.1016/0014-5793(93)80008-i. [DOI] [PubMed] [Google Scholar]
- Kim C. M., Goldstein J. L., Brown M. S. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J Biol Chem. 1992 Nov 15;267(32):23113–23121. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nielsen B. L., Brown L. R. The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem. 1984 Sep;141(2):311–315. doi: 10.1016/0003-2697(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Cranmer S. L., Halestrap A. P., Levi A. J. Substrate and inhibitor specificity of monocarboxylate transport into heart cells and erythrocytes. Further evidence for the existence of two distinct carriers. Biochem J. 1990 Aug 1;269(3):827–829. doi: 10.1042/bj2690827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Identification and partial purification of the erythrocyte L-lactate transporter. Biochem J. 1992 May 1;283(Pt 3):855–862. doi: 10.1042/bj2830855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Reconstitution of the L-lactate carrier from rat and rabbit erythrocyte plasma membranes. Biochem J. 1988 Sep 1;254(2):385–390. doi: 10.1042/bj2540385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Reversible and irreversible inhibition, by stilbenedisulphonates, of lactate transport into rat erythrocytes. Identification of some new high-affinity inhibitors. Biochem J. 1991 Apr 15;275(Pt 2):307–312. doi: 10.1042/bj2750307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]