Abstract
Using pressure-tuning Fourier transform infrared spectroscopy to study an in vitro system consisting of actin and distearoyl-phosphatidylcholine (DSPC) liposomes, we have determined the mechanism of interaction between actin and membrane lipids. This interaction results in a significant conformational change in actin molecules. Analysis of the amide I band of actin shows an increase in the beta-sheets to alpha-helix ratio, in random turns, and in interactions between actin monomers. In the absence of lipids, the actin molecules are denatured by pressures of 8 x 10(8) Pa and more, which give rise to a random organization of the peptide chain. However, in the presence of DSPC liposomes, pressure greater than 2 x 10(8) Pa induces a change in actin conformation, which is dominated by strongly interacting beta-sheets. As the spectra of the lipid molecules are not changed by the presence of actin, the organization of the lipid molecules in the bilayer is not affected by the protein. It is concluded from these results that this interaction of actin with membrane lipids involves very few lipid molecules. These lipid molecules may interact with actin at a few specific sites on the protein.
Full text
PDF

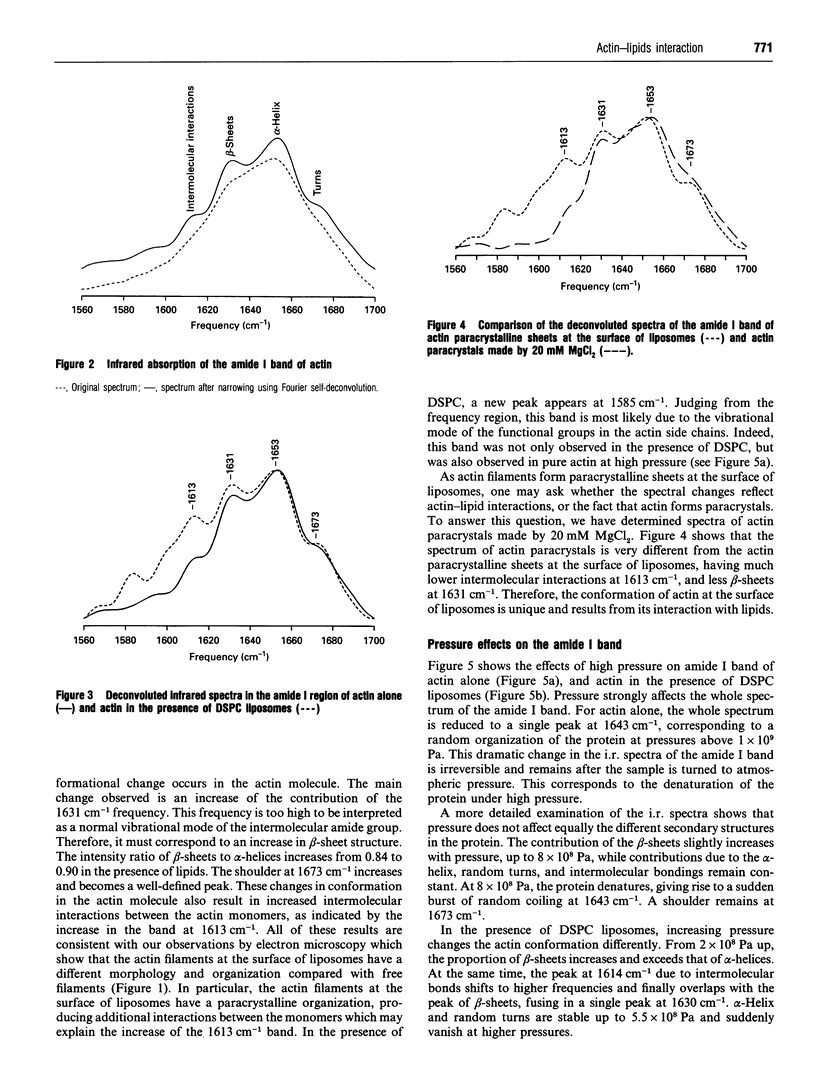

Images in this article
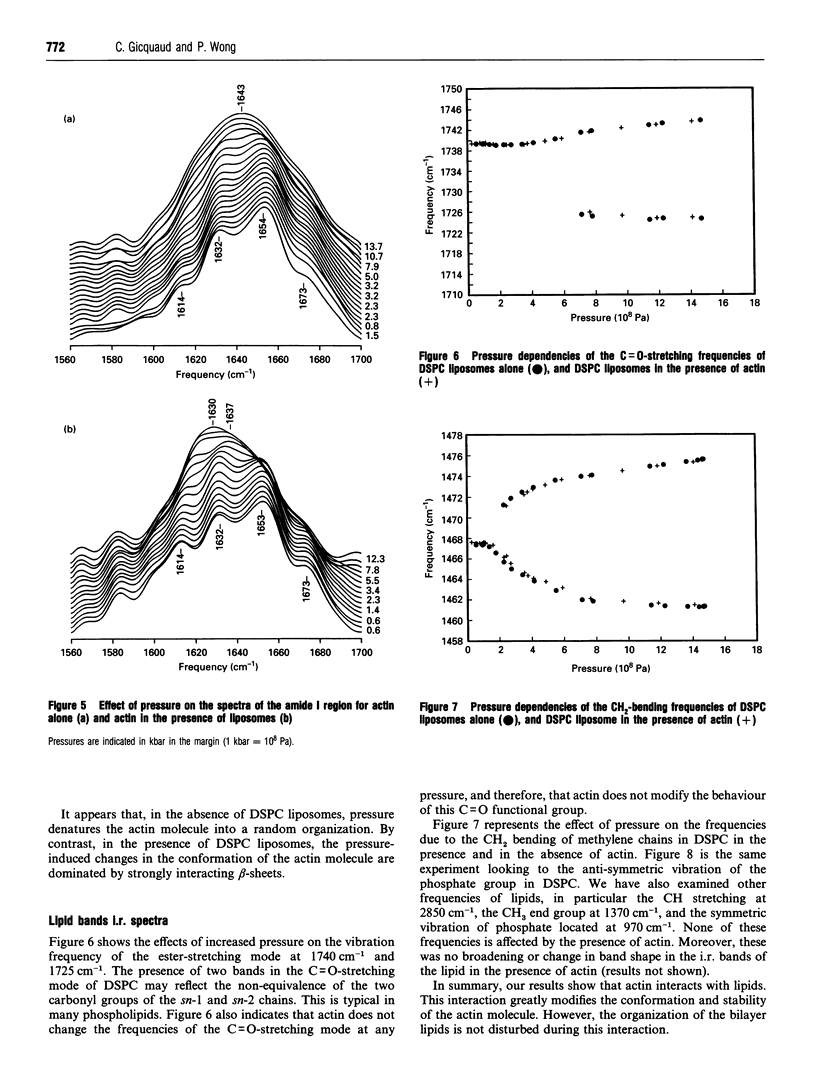
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger M., Jarrell H. C., Smith I. C., Wong P. T., Siminovitch D. J., Mantsch H. H. Pressure-induced exclusion of a local anesthetic from model and nerve membranes. Biochemistry. 1987 Dec 29;26(26):8513–8516. doi: 10.1021/bi00400a003. [DOI] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
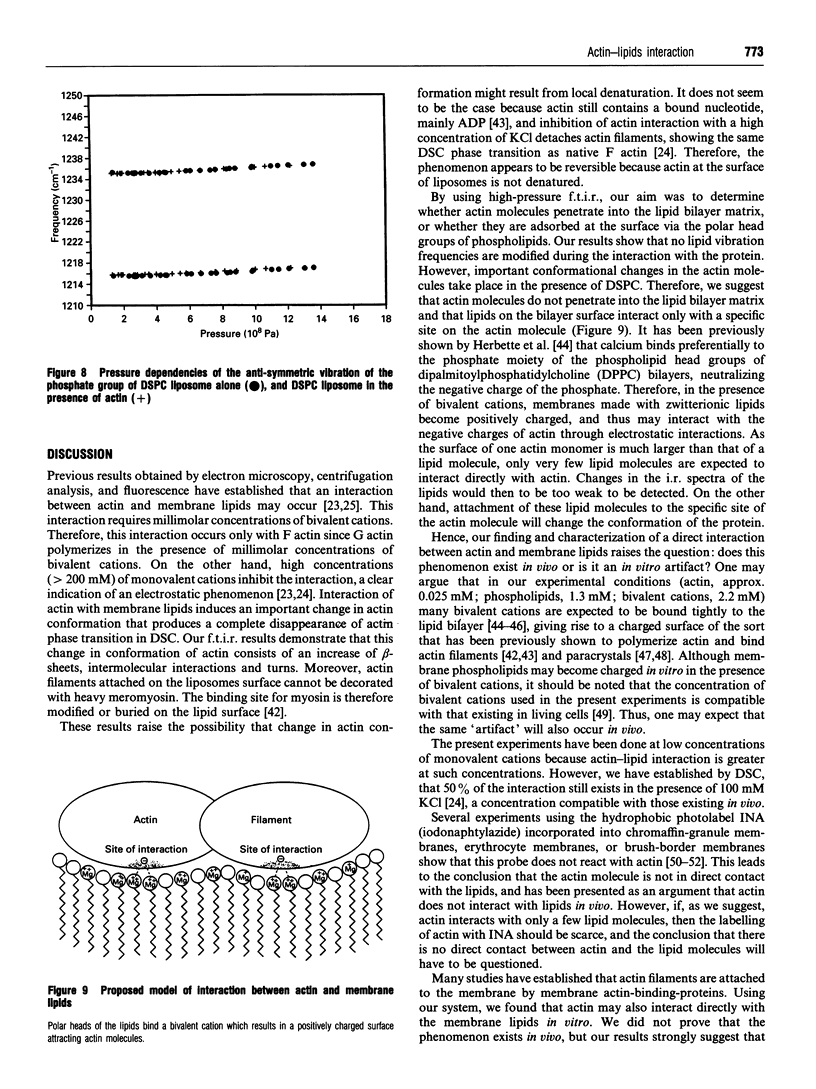
- Bercovici T., Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978 Apr 18;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Malinoff H. L., Wicha M. S. Connectin: cell surface protein that binds both laminin and actin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5927–5930. doi: 10.1073/pnas.80.19.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3(5-6):405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Carothers Carraway C. A., Fang H., Ye X. H., Juang S. H., Liu Y. C., Carvajal M. E., Carraway K. L. Membrane-microfilament interactions in ascites tumor cell microvilli. Identification and isolation of a large microfilament-associated membrane glycoprotein complex. J Biol Chem. 1991 Aug 25;266(24):16238–16246. [PubMed] [Google Scholar]
- Carraway K. L., Carraway C. A. Membrane-cytoskeleton interactions in animal cells. Biochim Biophys Acta. 1989 May 9;988(2):147–171. doi: 10.1016/0304-4157(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Kerjaschki D., Louvard D. Cytoskeleton organization and submembranous interactions in intestinal and renal brush borders. Kidney Int. 1988 Sep;34(3):309–320. doi: 10.1038/ki.1988.183. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. Treadmilling, diffusional exchange and cytoplasmic structures. J Muscle Res Cell Motil. 1985 Jun;6(3):263–273. doi: 10.1007/BF00713169. [DOI] [PubMed] [Google Scholar]
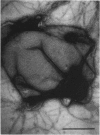
- Gicquaud C. Actin conformation is drastically altered by direct interaction with membrane lipids: a differential scanning calorimetry study. Biochemistry. 1993 Nov 9;32(44):11873–11877. doi: 10.1021/bi00095a016. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Krebs K. E., Whitfield C. F., Riederer B. M., Zagon I. S. Spectrin and related molecules. CRC Crit Rev Biochem. 1988;23(2):171–234. doi: 10.3109/10409238809088319. [DOI] [PubMed] [Google Scholar]
- Herbette L., Napolitano C. A., McDaniel R. V. Direct determination of the calcium profile structure for dipalmitoyllecithin multilayers using neutron diffraction. Biophys J. 1984 Dec;46(6):677–685. doi: 10.1016/S0006-3495(84)84066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Actin binding proteins--lipid interactions. J Muscle Res Cell Motil. 1991 Apr;12(2):136–144. doi: 10.1007/BF01774032. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosin I. Curr Opin Cell Biol. 1990 Feb;2(1):57–61. doi: 10.1016/s0955-0674(05)80031-6. [DOI] [PubMed] [Google Scholar]
- Laliberte A., Gicquaud C. Polymerization of actin by positively charged liposomes. J Cell Biol. 1988 Apr;106(4):1221–1227. doi: 10.1083/jcb.106.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tilly V., Sire O., Alpert B., Wong P. T. An infrared study of 2H-bond variation in myoglobin revealed by high pressure. Eur J Biochem. 1992 May 1;205(3):1061–1065. doi: 10.1111/j.1432-1033.1992.tb16874.x. [DOI] [PubMed] [Google Scholar]
- Lis L. J., Lis W. T., Parsegian V. A., Rand R. P. Adsorption of divalent cations to a variety of phosphatidylcholine bilayers. Biochemistry. 1981 Mar 31;20(7):1771–1777. doi: 10.1021/bi00510a010. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Hitt A. L. Cytoskeleton--plasma membrane interactions. Science. 1992 Nov 6;258(5084):955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- McLaughlin A., Grathwohl C., McLaughlin S. The adsorption of divalent cations to phosphatidylcholine bilayer membranes. Biochim Biophys Acta. 1978 Nov 16;513(3):338–357. doi: 10.1016/0005-2736(78)90203-1. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Burger M. M. The chromaffin granule surface: the presence of actin and the nature of its interaction with the membrane. FEBS Lett. 1979 May 1;101(1):129–133. doi: 10.1016/0014-5793(79)81310-1. [DOI] [PubMed] [Google Scholar]
- Muga A., Surewicz W. K., Wong P. T., Mantsch H. H. Structural studies with the uveopathogenic peptide M derived from retinal S-antigen. Biochemistry. 1990 Mar 27;29(12):2925–2930. doi: 10.1021/bi00464a006. [DOI] [PubMed] [Google Scholar]
- Niggli V., Burger M. M. Interaction of the cytoskeleton with the plasma membrane. J Membr Biol. 1987;100(2):97–121. doi: 10.1007/BF02209144. [DOI] [PubMed] [Google Scholar]
- Nonomura Y., Katayama E., Ebashi S. Effect of phosphates on the structure of the actin filament. J Biochem. 1975 Nov;78(5):1101–1104. doi: 10.1093/oxfordjournals.jbchem.a130988. [DOI] [PubMed] [Google Scholar]
- Otto J. J. Vinculin. Cell Motil Cytoskeleton. 1990;16(1):1–6. doi: 10.1002/cm.970160102. [DOI] [PubMed] [Google Scholar]
- Parise L. V., Phillips D. R. Reconstitution of the purified platelet fibrinogen receptor. Fibrinogen binding properties of the glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 5;260(19):10698–10707. [PubMed] [Google Scholar]
- Pollard T. D., Doberstein S. K., Zot H. G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- Rioux L., Gicquaud C. Actin paracrystalline sheets formed at the surface of positively charged liposomes. J Ultrastruct Res. 1985 Oct-Nov;93(1-2):42–49. doi: 10.1016/0889-1605(85)90084-9. [DOI] [PubMed] [Google Scholar]
- Scheel J., Ziegelbauer K., Kupke T., Humbel B. M., Noegel A. A., Gerisch G., Schleicher M. Hisactophilin, a histidine-rich actin-binding protein from Dictyostelium discoideum. J Biol Chem. 1989 Feb 15;264(5):2832–2839. [PubMed] [Google Scholar]
- Sigrist-Nelson K., Sigrist H., Bercovici T., Gitler C. Intrinsic proteins of the intestinal microvillus membrane. Iodonaphthylazide labeling studies. Biochim Biophys Acta. 1977 Jul 14;468(2):163–176. doi: 10.1016/0005-2736(77)90111-0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- St-Onge D., Gicquaud C. Evidence of direct interaction between actin and membrane lipids. Biochem Cell Biol. 1989 Jun;67(6):297–300. doi: 10.1139/o89-045. [DOI] [PubMed] [Google Scholar]
- St-Onge D., Gicquaud C. Research on the mechanism of interaction between actin and membrane lipids. Biochem Biophys Res Commun. 1990 Feb 28;167(1):40–47. doi: 10.1016/0006-291x(90)91727-a. [DOI] [PubMed] [Google Scholar]
- Susi H., Timasheff S. N., Stevens L. Infrared spectra and protein conformations in aqueous solutions. I. The amide I band in H2O and D2O solutions. J Biol Chem. 1967 Dec 10;242(23):5460–5466. [PubMed] [Google Scholar]
- Takahashi H., French S. W., Wong P. T. Alterations in hepatic lipids and proteins by chronic ethanol intake: a high-pressure Fourier transform infrared spectroscopic study on alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1991 Mar;15(2):219–223. doi: 10.1111/j.1530-0277.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Taylor D. W. Formation of 2-D paracrystals of F-actin on phospholipid layers mixed with quaternary ammonium surfactants. J Struct Biol. 1992 Mar-Apr;108(2):140–147. doi: 10.1016/1047-8477(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Ward R. J., Menetret J. F., Pattus F., Leonard K. Method for forming two-dimensional paracrystals of biological filaments on lipid monolayers. J Electron Microsc Tech. 1990 Apr;14(4):335–341. doi: 10.1002/jemt.1060140408. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Heremans K. Pressure effects on protein secondary structure and hydrogen deuterium exchange in chymotrypsinogen: a Fourier transform infrared spectroscopic study. Biochim Biophys Acta. 1988 Aug 31;956(1):1–9. doi: 10.1016/0167-4838(88)90291-9. [DOI] [PubMed] [Google Scholar]
- Wuestehube L. J., Luna E. J. F-actin binds to the cytoplasmic surface of ponticulin, a 17-kD integral glycoprotein from Dictyostelium discoideum plasma membranes. J Cell Biol. 1987 Oct;105(4):1741–1751. doi: 10.1083/jcb.105.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zot H. G., Doberstein S. K., Pollard T. D. Myosin-I moves actin filaments on a phospholipid substrate: implications for membrane targeting. J Cell Biol. 1992 Jan;116(2):367–376. doi: 10.1083/jcb.116.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]