Abstract
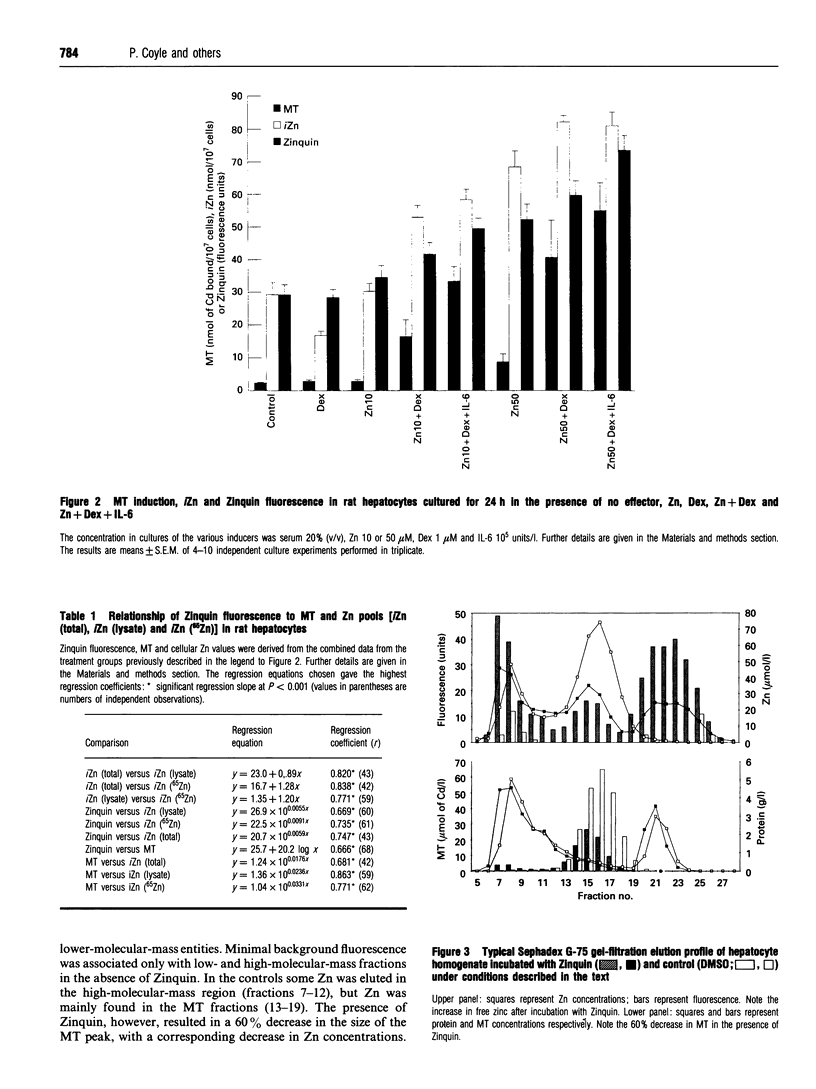
Zinquin [ethyl (2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetate], a new intracellular zinc fluorophore, was used to reveal and to measure Zn in cultured rat hepatocytes before and after metallothionein (MT) induction. Hepatocytes labelled with an intense extranuclear fluorescence. Culture with combinations of Zn, dexamethasone and interleukin-6, increased intracellular MT by 24-fold, Zn 3-fold, and Zinquin fluorescence by approx. 2-fold above control values. Zinquin fluorescence correlated in descending order with the total cellular Zn (r = 0.747), exchangeable Zn (r = 0.735), soluble cytosolic Zn (r = 0.669) and MT (r = 0.666). When Zinquin was incubated with a cytosolic fraction of liver proteins before Sephadex G-75 column chromatography, it fluoresced with free, MT-incorporated and protein-bound Zn. Although only a slight attenuation of fluorescence was seen with high-molecular-mass protein-bound Zn, MT was degraded by 60% in the presence of Zinquin. The undegraded Zn-MT fluoresced at about 20% of the expected intensity. Although Zinquin fluoresces with all cytosolic Zn, caution is required when comparisons are made between samples with different concentrations of MT. This limitation was demonstrated by staining liver slices from adjuvant-treated rats where MT was increased 24-fold, intracellular Zn by 77%, but Zinquin fluorescence by only 19% above controls. Nevertheless, Zinquin should prove to be a useful tool for studying the distribution of Zn in living cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaf S. Y., Chung S. H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984 Apr 19;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- Bettger W. J., O'Dell B. L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981 Mar 30;28(13):1425–1438. doi: 10.1016/0024-3205(81)90374-x. [DOI] [PubMed] [Google Scholar]
- Bremner I., Beattie J. H. Metallothionein and the trace minerals. Annu Rev Nutr. 1990;10:63–83. doi: 10.1146/annurev.nu.10.070190.000431. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., Lee-Ambrose L. M. Nuclear zinc uptake and interactions and metallothionein gene expression are influenced by dietary zinc in rats. J Nutr. 1992 Jan;122(1):56–64. doi: 10.1093/jn/122.1.56. [DOI] [PubMed] [Google Scholar]
- Coyle P., Philcox J. C., Rofe A. M. Corticosterone enhances the zinc and interleukin-6-mediated induction of metallothionein in cultured rat hepatocytes. J Nutr. 1993 Sep;123(9):1464–1470. doi: 10.1093/jn/123.9.1464. [DOI] [PubMed] [Google Scholar]
- Coyle P., Philcox J. C., Rofe A. M. Metallothionein induction in freshly isolated rat hepatocytes. Biol Trace Elem Res. 1993 Jan;36(1):35–49. doi: 10.1007/BF02783778. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Toal B. F. Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol. 1982 Oct;66(1):134–142. doi: 10.1016/0041-008x(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Elmes M. E. Apoptosis in the small intestine of zinc-deficient and fasted rats. J Pathol. 1977 Dec;123(4):219–223. doi: 10.1002/path.1711230404. [DOI] [PubMed] [Google Scholar]
- Fliss H., Ménard M., Desai M. Hypochlorous acid mobilizes cellular zinc. Can J Physiol Pharmacol. 1991 Nov;69(11):1686–1691. doi: 10.1139/y91-250. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Kasarskis E. J., Ringo D., Frederickson R. E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987 Jun;20(2):91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Giannakis C., Forbes I. J., Zalewski P. D. Ca2+/Mg(2+)-dependent nuclease: tissue distribution, relationship to inter-nucleosomal DNA fragmentation and inhibition by Zn2+. Biochem Biophys Res Commun. 1991 Dec 16;181(2):915–920. doi: 10.1016/0006-291x(91)91278-k. [DOI] [PubMed] [Google Scholar]
- Goode H. F., Kelleher J., Walker B. E. Zinc concentrations in pure populations of peripheral blood neutrophils, lymphocytes and monocytes. Ann Clin Biochem. 1989 Jan;26(Pt 1):89–95. doi: 10.1177/000456328902600114. [DOI] [PubMed] [Google Scholar]
- Grummt F., Weinmann-Dorsch C., Schneider-Schaulies J., Lux A. Zinc as a second messenger of mitogenic induction. Effects on diadenosine tetraphosphate (Ap4A) and DNA synthesis. Exp Cell Res. 1986 Mar;163(1):191–200. doi: 10.1016/0014-4827(86)90572-0. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E. Microtubule assembly in rat brain extracts. Further characterization of the effects of zinc on assembly and cold stability. Int J Biochem. 1984;16(12):1331–1339. doi: 10.1016/0020-711x(84)90236-2. [DOI] [PubMed] [Google Scholar]
- Kobusch A. B., Bock K. W. Zinc increases EGF-stimulated DNA synthesis in primary mouse hepatocytes. Studies in tumor promoter-treated cell cultures. Biochem Pharmacol. 1990 Feb 1;39(3):555–558. doi: 10.1016/0006-2952(90)90063-q. [DOI] [PubMed] [Google Scholar]
- Li T. Y., Kraker A. J., Shaw C. F., 3rd, Petering D. H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Mazdai G., Strain J. J., Cotter T. G., Hannigan B. M. Programmed cell death (apoptosis) in lymphoid and myeloid cell lines during zinc deficiency. Clin Exp Immunol. 1991 Feb;83(2):338–343. doi: 10.1111/j.1365-2249.1991.tb05639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R., Kambadur R., Gulati S., Paliwal V. K., Sharma M. Molecular aspects, physiological function, and clinical significance of metallothioneins. Crit Rev Food Sci Nutr. 1988;27(1):41–85. doi: 10.1080/10408398809527477. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., James H. M., Bais R., Edwards J. B., Conyers R. A. The production of (14C) oxalate during the metabolism of (14C) carbohydrates in isolated rat hepatocytes. Aust J Exp Biol Med Sci. 1980 Apr;58(2):103–116. doi: 10.1038/icb.1980.10. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Philcox J. C., Haynes D. R., Whitehouse M. W., Coyle P. Changes in plasma zinc, copper, iron, and hepatic metallothionein in adjuvant-induced arthritis treated with cyclosporin. Biol Trace Elem Res. 1992 Sep;34(3):237–248. doi: 10.1007/BF02783679. [DOI] [PubMed] [Google Scholar]
- Savage D. D., Montano C. Y., Kasarskis E. J. Quantitative histofluorescence of hippocampal mossy fiber zinc. Brain Res. 1989 Sep 4;496(1-2):257–267. doi: 10.1016/0006-8993(89)91073-1. [DOI] [PubMed] [Google Scholar]
- Schroeder J. J., Cousins R. J. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3137–3141. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J. Zinc: what is its role in biology? Endeavour. 1984;8(2):65–70. doi: 10.1016/0160-9327(84)90040-1. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Differences in the polymorphic forms of metallothionein. Arch Biochem Biophys. 1982 Mar;214(1):80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Betts W. H. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II). Biochem J. 1993 Dec 1;296(Pt 2):403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Giannakis C. Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem Int. 1991 Aug;24(6):1093–1101. [PubMed] [Google Scholar]