Abstract
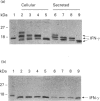
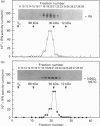
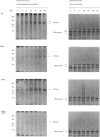
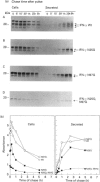
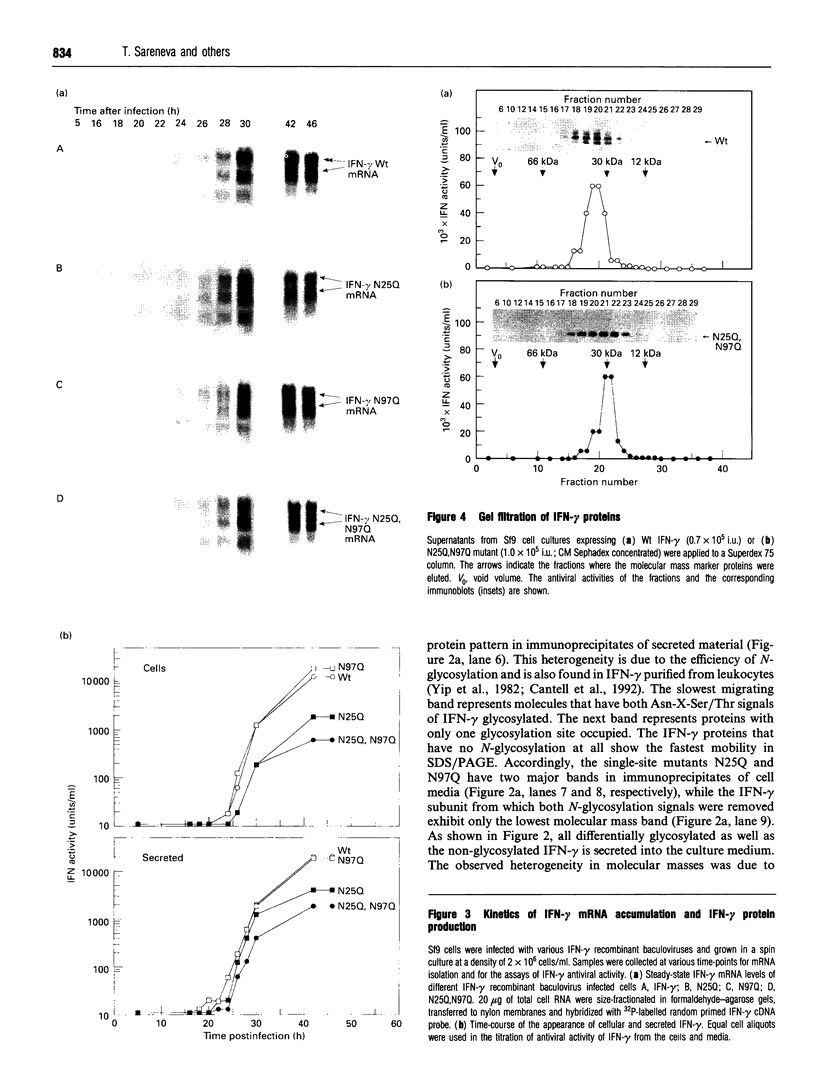
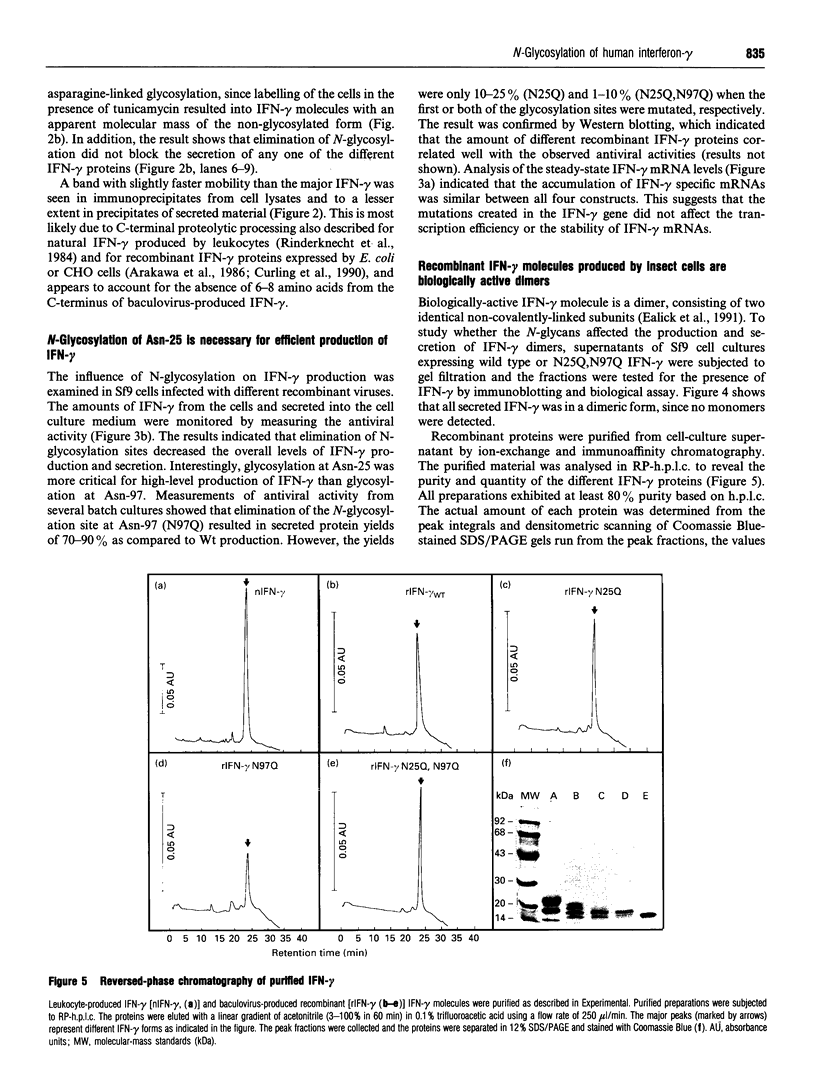
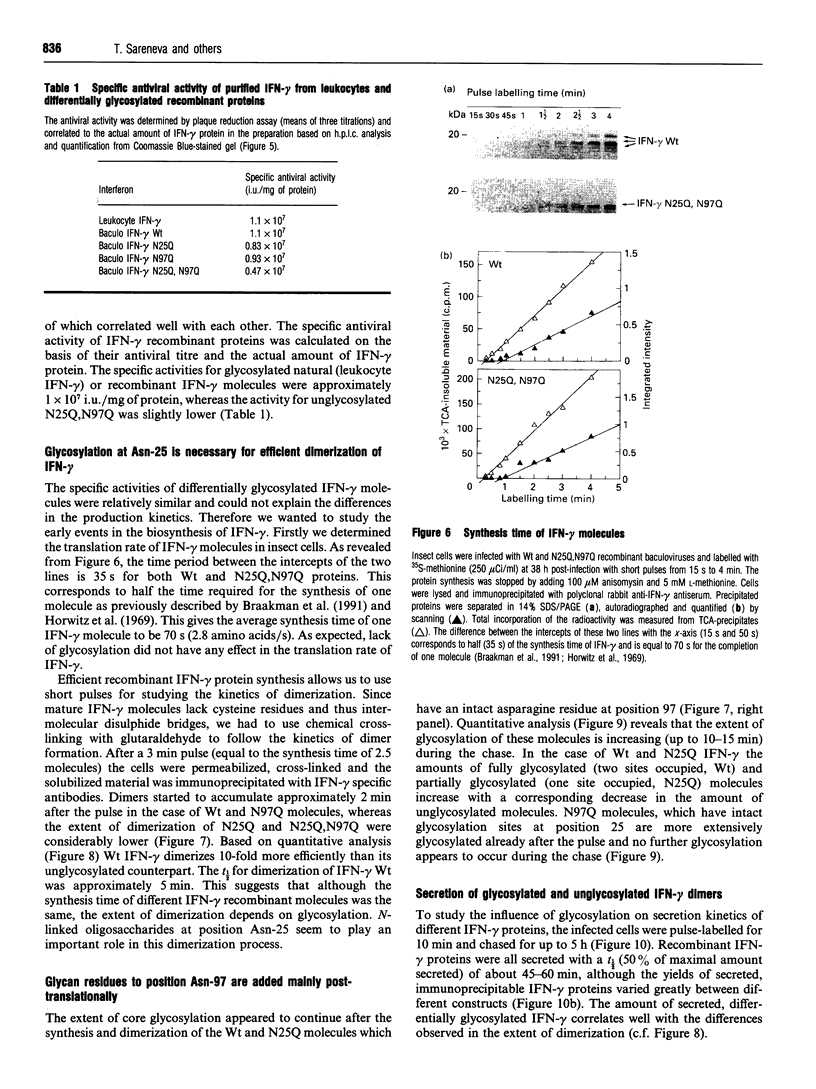
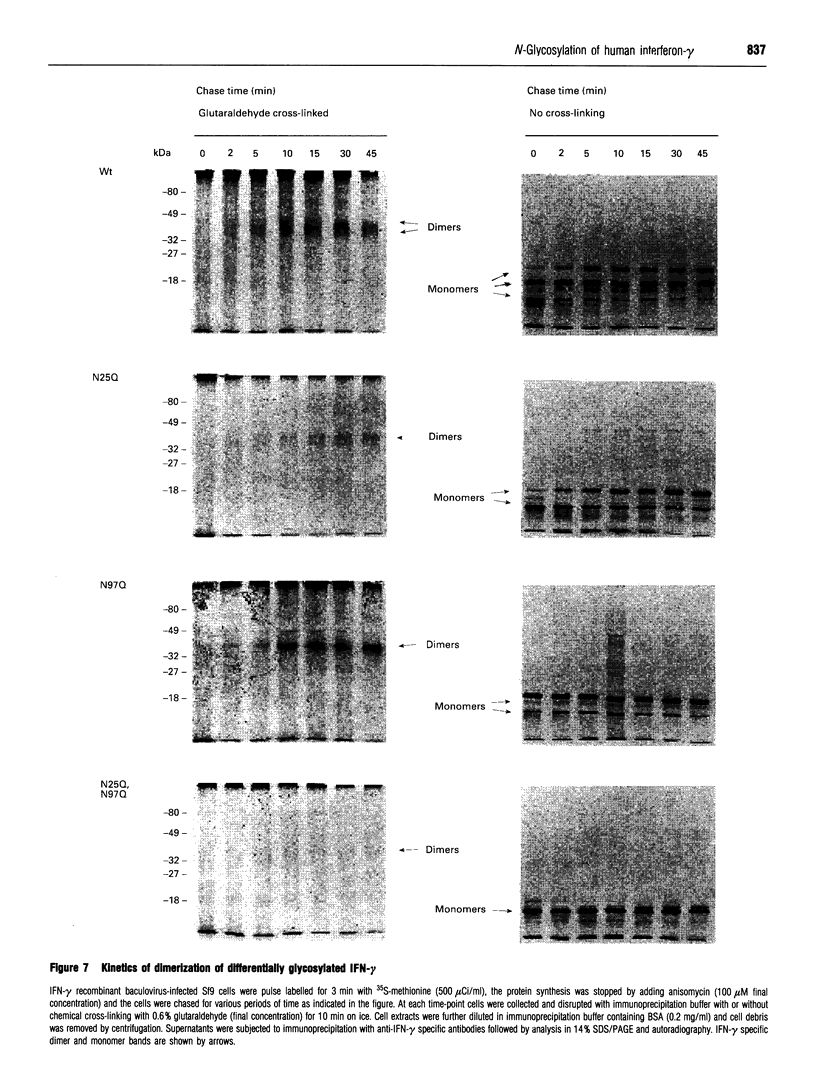
Human interferon-gamma (IFN-gamma) is a secretory glycoprotein, which has two potential N-linked glycosylation sites at positions Asn-25 and Asn-97 of its 143 amino acid long mature polypeptide chain. In order to understand the role of glycan residues in the synthesis and secretion of human IFN-gamma, both or either one of the potential N-linked glycosylation sites were mutated to Gln. The mutant and the wild-type (Wt) polypeptides were expressed in insect cells using a baculovirus vector. Elimination of the N-glycosylation site at position Asn-97 (N97Q) resulted in secreted protein yields of 70-90% as compared with the Wt production, whereas only 10-25% (N25Q) and 1-10% (N25Q,N97Q) levels of protein production was observed when the first or both sites were mutated, respectively. Although there was a difference between extracellular levels of produced protein, the kinetics of secretion was similar for all different IFN-gamma molecules. The Wt and the N-glycosylation site mutants were all secreted as dimers. The formation of biologically active dimers was more efficient for IFN-gamma polypeptides that had the intact glycosylation site at Asn-25 as compared with the other two mutant forms of IFN-gamma. The extent of dimerization correlated well with the observed secretion. The specific antiviral activity was of the same order (1 x 10(7) i.u./mg of protein) for the glycosylated IFN-gamma molecules, whereas it was slightly lower (0.5 x 10(7) i.u./mg of protein) for the unglycosylated mutant form.
Full text
PDF


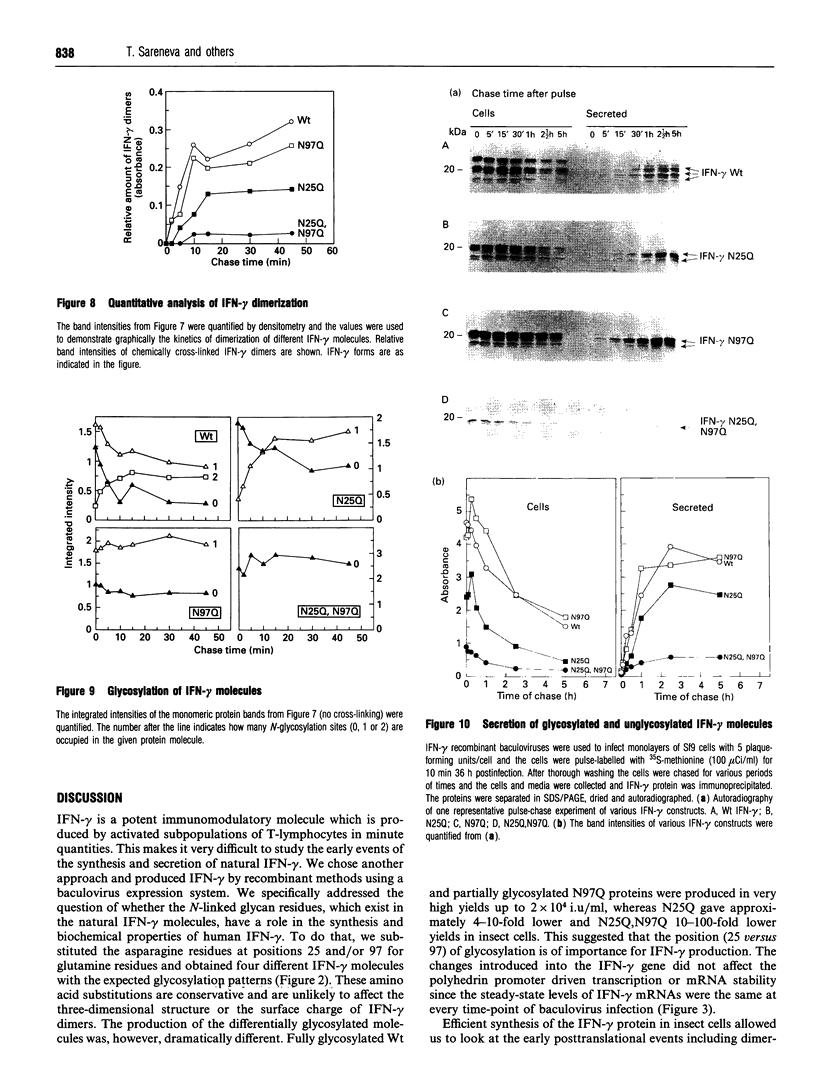


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Hsu Y. R., Parker C. G., Lai P. H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986 Jun 25;261(18):8534–8539. [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991 Aug;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S., Kauppinen H. L., Kalkkinen N. Rapid production of interferon-gamma in uninduced human leukocyte suspensions. J Interferon Res. 1991 Aug;11(4):231–236. doi: 10.1089/jir.1991.11.231. [DOI] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S., Kauppinen H. L. Production and partial purification of human immune interferon. Methods Enzymol. 1986;119:54–63. doi: 10.1016/0076-6879(86)19009-4. [DOI] [PubMed] [Google Scholar]
- Curling E. M., Hayter P. M., Baines A. J., Bull A. T., Gull K., Strange P. G., Jenkins N. Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem J. 1990 Dec 1;272(2):333–337. doi: 10.1042/bj2720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Ealick S. E., Cook W. J., Vijay-Kumar S., Carson M., Nagabhushan T. L., Trotta P. P., Bugg C. E. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Birchenall-Sparks M. C., Young H. B. Interleukin 2 induction of interferon-gamma mRNA synthesis. J Immunol. 1986 Dec 15;137(12):3836–3840. [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Greenlund A. C., Schreiber R. D., Goeddel D. V., Pennica D. Interferon-gamma induces receptor dimerization in solution and on cells. J Biol Chem. 1993 Aug 25;268(24):18103–18110. [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Scharff M. D., Maizel J. V., Jr Synthesis and assembly of adenovirus 2. I. Polypeptide synthesis, assembly of capsomeres, and morphogenesis of the virion. Virology. 1969 Dec;39(4):682–694. doi: 10.1016/0042-6822(69)90006-3. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Oker-Blom C., Summers M. D. Role of glycosylation in the transport of recombinant glycoproteins through the secretory pathway of lepidopteran insect cells. J Cell Biochem. 1990 Apr;42(4):181–191. doi: 10.1002/jcb.240420402. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Geyer H., Geyer R., Doerfler W., Klenk H. D. The oligosaccharides of influenza virus hemagglutinin expressed in insect cells by a baculovirus vector. Virology. 1990 Feb;174(2):418–429. doi: 10.1016/0042-6822(90)90095-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Samudzi C. T., Burton L. E., Rubin J. R. Crystal structure of recombinant rabbit interferon-gamma at 2.7-A resolution. J Biol Chem. 1991 Nov 15;266(32):21791–21797. [PubMed] [Google Scholar]
- Sareneva T., Cantell K., Pyhälä L., Pirhonen J., Julkunen I. Effect of carbohydrates on the pharmacokinetics of human interferon-gamma. J Interferon Res. 1993 Aug;13(4):267–269. doi: 10.1089/jir.1993.13.267. [DOI] [PubMed] [Google Scholar]
- Senda T., Shimazu T., Matsuda S., Kawano G., Shimizu H., Nakamura K. T., Mitsui Y. Three-dimensional crystal structure of recombinant murine interferon-beta. EMBO J. 1992 Sep;11(9):3193–3201. doi: 10.1002/j.1460-2075.1992.tb05396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Weber H. The interferon genes. Prog Nucleic Acid Res Mol Biol. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Hase S., Yamauchi H., Tanimoto T., Ikenaka T. Studies on the sugar chains of interferon-gamma from human peripheral-blood lymphocytes. J Biochem. 1989 Jun;105(6):1034–1039. doi: 10.1093/oxfordjournals.jbchem.a122762. [DOI] [PubMed] [Google Scholar]
- Yip Y. K., Barrowclough B. S., Urban C., Vilcek J. Purification of two subspecies of human gamma (immune) interferon. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1820–1824. doi: 10.1073/pnas.79.6.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]