Abstract
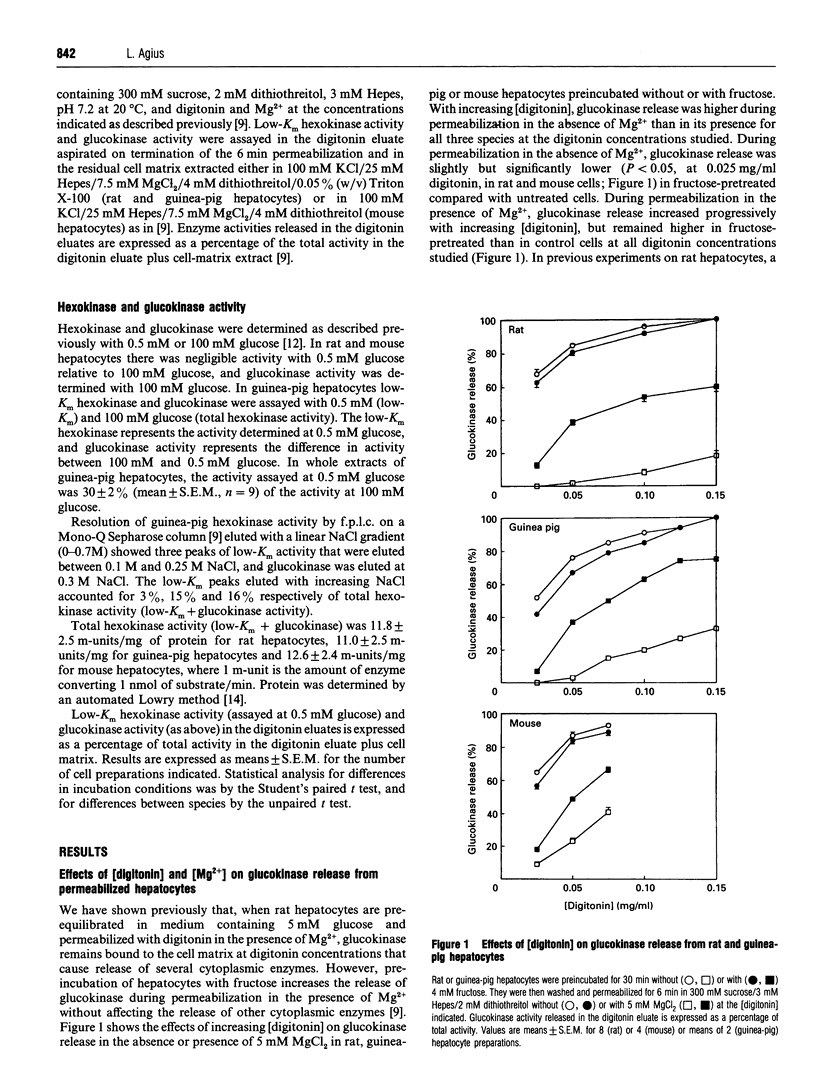
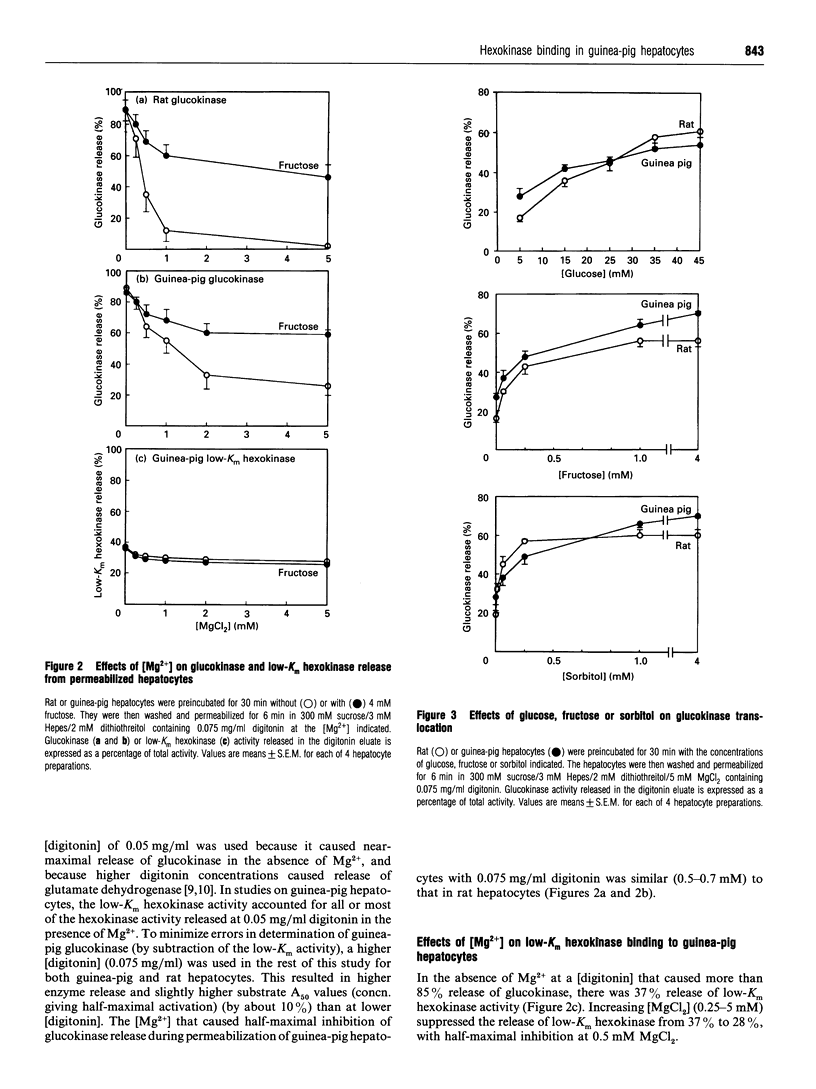
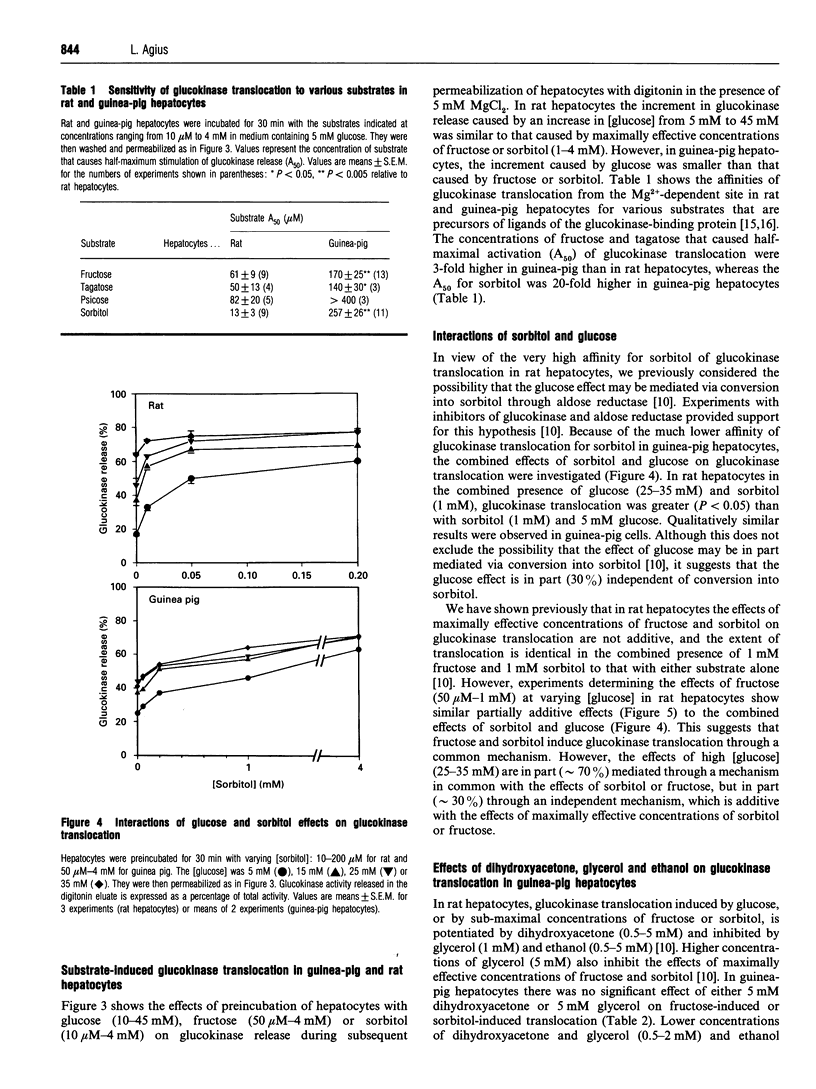
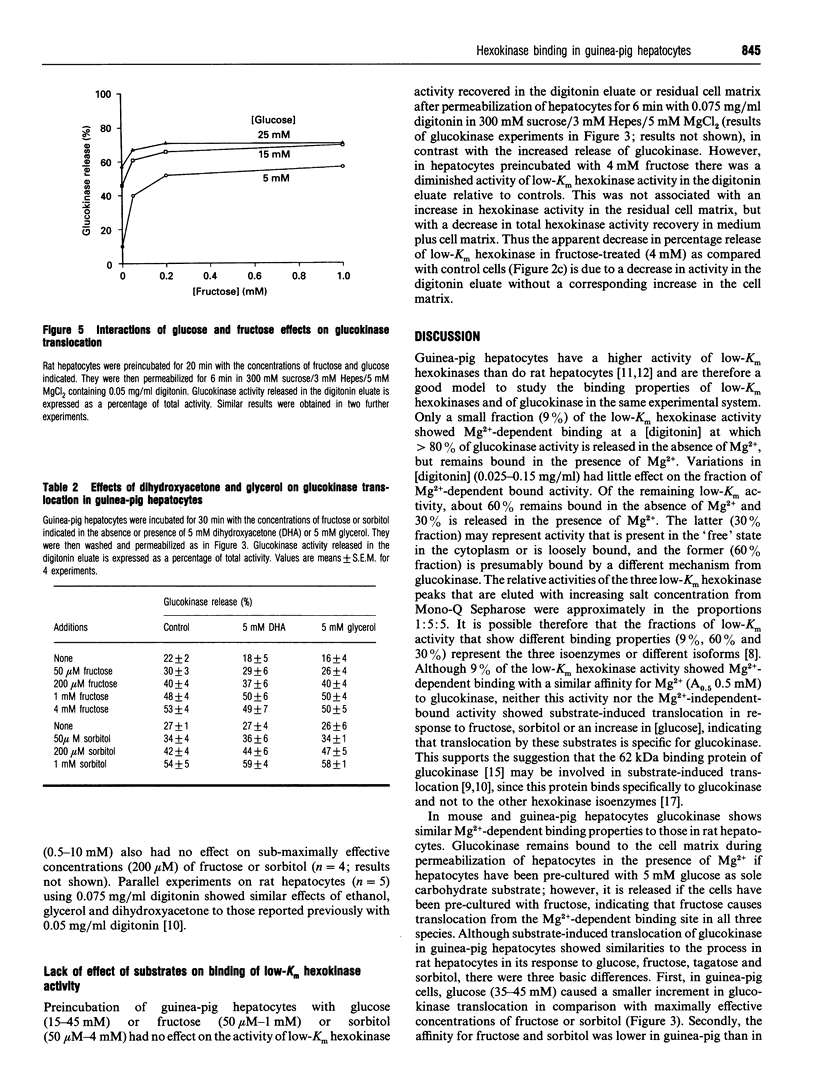
The release of glucokinase (hexokinase IV) from digitonin-permeabilized hepatocytes from rat, guinea pig or mouse liver is inhibited by physiological concentrations of Mg2+ (> 0.25 mM). Preincubation of hepatocytes with fructose increases glucokinase release during permeabilization in the presence of Mg2+ but decreases glucokinase release in the absence of Mg2+, suggesting that fructose causes translocation of glucokinase from the Mg(2+)-dependent site. Glucose (25 mM) and sorbitol (1 mM) also induce translocation of glucokinase from the Mg(2+)-dependent site in guinea-pig, as in rat hepatocytes, but glucose is less effective than fructose or sorbitol, and the concentrations of fructose and sorbitol that cause half-maximal activation (A50) are 3-fold and 20-fold higher, respectively, in guinea-pig than in rat hepatocytes (170 microM and 257 microM, compared with 61 microM and 13 microM). Dihydroxyacetone and glycerol have no effect on fructose-induced or sorbitol-induced translocation in guinea-pig hepatocytes, in contrast with the potentiation and inhibition, respectively, by these substrates in rat hepatocytes. Some, but not all, of the differences between rat and guinea-pig hepatocytes could be due to the more reduced cytoplasmic NADH/NAD+ redox state in guinea-pig cells. The activity of low-Km hexokinases accounts for 30% of total hexokinase activity (low-Km hexokinases + glucokinase) in guinea-pig hepatocytes. Of the low-Km hexokinase activity, approx. 30% is released in the presence of Mg2+, 9% shows Mg(2+)-dependent binding and 60% shows Mg(2+)-independent binding. There was no substrate-induced translocation of low-Km hexokinase activity, indicating that translocation is specific for hexokinase IV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams V., Griffin L., Towbin J., Gelb B., Worley K., McCabe E. R. Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem Med Metab Biol. 1991 Jun;45(3):271–291. doi: 10.1016/0885-4505(91)90032-g. [DOI] [PubMed] [Google Scholar]
- Agius L. Control of glucokinase translocation in rat hepatocytes by sorbitol and the cytosolic redox state. Biochem J. 1994 Feb 15;298(Pt 1):237–243. doi: 10.1042/bj2980237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Peak M., Alberti K. G. Regulation of glycogen synthesis from glucose and gluconeogenic precursors by insulin in periportal and perivenous rat hepatocytes. Biochem J. 1990 Feb 15;266(1):91–102. doi: 10.1042/bj2660091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Peak M. Intracellular binding of glucokinase in hepatocytes and translocation by glucose, fructose and insulin. Biochem J. 1993 Dec 15;296(Pt 3):785–796. doi: 10.1042/bj2960785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Tosh D. Acinar zonation of cytosolic but not organelle-bound activities of phosphoenolpyruvate carboxykinase and aspartate aminotransferase in guinea-pig liver. Biochem J. 1990 Oct 15;271(2):387–391. doi: 10.1042/bj2710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K. K., Fanciulli M., Pedersen P. L. Glucose phosphorylation in tumor cells. Cloning, sequencing, and overexpression in active form of a full-length cDNA encoding a mitochondrial bindable form of hexokinase. J Biol Chem. 1990 Apr 15;265(11):6481–6488. [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Compartmentation of hexokinase and creatine phosphokinase, cellular regulation, and insulin action. Curr Top Cell Regul. 1980;16:55–86. doi: 10.1016/b978-0-12-152816-4.50007-8. [DOI] [PubMed] [Google Scholar]
- Clifton P. M., Chang L., Mackinnon A. M. Development of an automated Lowry protein assay for the Cobas-Bio centrifugal analyzer. Anal Biochem. 1988 Jul;172(1):165–168. doi: 10.1016/0003-2697(88)90426-5. [DOI] [PubMed] [Google Scholar]
- Detheux M., Vandercammen A., Van Schaftingen E. Effectors of the regulatory protein acting on liver glucokinase: a kinetic investigation. Eur J Biochem. 1991 Sep 1;200(2):553–561. doi: 10.1111/j.1432-1033.1991.tb16218.x. [DOI] [PubMed] [Google Scholar]
- Gaynes B. I., Watkins J. B., 3rd Comparison of glucose, sorbitol and fructose accumulation in lens and liver of diabetic and insulin-treated rats and mice. Comp Biochem Physiol B. 1989;92(4):685–690. doi: 10.1016/0305-0491(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Heath H., Hamlett Y. C. The sorbitol pathway: effect of streptozotocin induced diabetes and the feeding of a sucrose-rich diet on glucose, sorbitol and fructose in the retina, blood and liver of rats. Diabetologia. 1976 Mar;12(1):43–46. doi: 10.1007/BF01221963. [DOI] [PubMed] [Google Scholar]
- Parry D. M., Pedersen P. L. Intracellular localization of rat kidney hexokinase. Evidence for an association with low density mitochondria. J Biol Chem. 1984 Jul 25;259(14):8917–8923. [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J., Rudolph F. B. The hexokinases: kinetic, physical, and regulatory properties. Adv Enzymol Relat Areas Mol Biol. 1973;39:249–326. doi: 10.1002/9780470122846.ch4. [DOI] [PubMed] [Google Scholar]
- Reyes A., Cárdenas M. L. All hexokinase isoenzymes coexist in rat hepatocytes. Biochem J. 1984 Jul 15;221(2):303–309. doi: 10.1042/bj2210303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Interactions of glycolytic enzymes with cellular membranes. Curr Top Cell Regul. 1992;33:31–46. doi: 10.1016/b978-0-12-152833-1.50008-3. [DOI] [PubMed] [Google Scholar]
- Vandercammen A., Van Schaftingen E. Competitive inhibition of liver glucokinase by its regulatory protein. Eur J Biochem. 1991 Sep 1;200(2):545–551. doi: 10.1111/j.1432-1033.1991.tb16217.x. [DOI] [PubMed] [Google Scholar]
- Vandercammen A., Van Schaftingen E. Species and tissue distribution of the regulatory protein of glucokinase. Biochem J. 1993 Sep 1;294(Pt 2):551–556. doi: 10.1042/bj2940551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaftingen E., Vandercammen A., Detheux M., Davies D. R. The regulatory protein of liver glucokinase. Adv Enzyme Regul. 1992;32:133–148. doi: 10.1016/0065-2571(92)90013-p. [DOI] [PubMed] [Google Scholar]


