Abstract
Traumatic brain injury (TBI) ultimately leads to a reduction in the cerebral metabolic rate for oxygen due to ischemia. Previously, we showed that 2 ppm i.v. of drag-reducing polymers (DRP) improve hemodynamic and oxygen delivery to tissue in a rat model of mild-to-moderate TBI. Here we evaluated sex-specific and dose-dependent effects of DRP on microvascular CBF (mvCBF) and tissue oxygenation in rats after moderate TBI. In-vivo two-photon laser scanning microscopy over the rat parietal cortex was used to monitor the effects of DRP on microvascular perfusion, tissue oxygenation, and blood-brain barrier (BBB) permeability. Lateral fluid-percussion TBI (1.5 ATA, 100 ms) was induced after baseline imaging and followed by 4 hours of monitoring. DRP was injected at 1, 2, or 4 ppm within 30 minutes after TBI. Differences between groups were determined using a two-way ANOVA analysis for multiple comparisons and post hoc testing using the Mann-Whitney U test. Moderate TBI progressively decreased mvCBF, leading to tissue hypoxia and BBB degradation in the pericontusion zone (p<0.05). The i.v. injection of DRP increased near-wall flow velocity and flow rate in arterioles, leading to an increase in the number of erythrocytes entering capillaries, enhancing capillary perfusion and tissue oxygenation while protecting BBB in a dose-dependent manner without significant difference between males and females (p<0.01). TBI resulted in an increase in intracranial pressure (20.1±3.2 mmHg, p<0.05), microcirculatory redistribution to non-nutritive microvascular shunt flow, and stagnation of capillary flow, all of which were dose-dependently mitigated by DRP. DRP at 4 ppm was most effective, with a non-significant trend to better outcomes in female rats.
Keywords: Cerebral blood flow, Drag-reducing polymers, Microvascular flow, NADH autofluorescence, Intracranial pressure, Traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is a significant health problem, responsible for a third of all injury-related deaths and 70% of disabilities [1]. Decades of TBI research focused almost exclusively on neuroprotective strategies have failed to develop any therapeutics for clinical treatment. One less explored potential target is cerebral microcirculation (mvCBF). Recent studies suggest that the peri-contusional and diffuse injury areas represent salvageable tissue where diffusional hypoxia and nutrient deprivation occur at the microcirculation level, much like the penumbra in stroke [2]. We recently proposed a modulation of hemodynamics by blood-soluble drag-reducing polymers (DRP) as a novel treatment modality for TBI that specifically targets cerebral microcirculation based on physical but not pharmacological principles. Nanomolar concentrations of intravascular blood soluble drag-reducing polymers (DRP) were shown to increase tissue perfusion and oxygenation and decrease peripheral vascular resistance by rheological modulation of hemodynamics. The greatest impact of the DRP-enhanced flow is at the level of the microcirculation and capillaries, where the shear rate is highest [3]. We hypothesized that drag-reducing polymers (DRPs) would improve mvCBF and reduce tissue hypoxia in TBI and have proven it experimentally [4]. In this work, we evaluated the dose-dependent efficacy of DRP (1, 2, and 4 ppm) in the treatment of post-TBI ischemia using a rat lateral fluid percussion injury model of moderate TBI. We have shown that at 4 ppm, the drag-reducing effect is near the plateau (unpublished data). Since an increasing body of evidence suggests that cerebral blood flow differs between males and females in the intact and injured brain, we dissected possible sex-dependent effects.
2. Methods
2.1. Study Design
The procedures used in the study have already been described [3] and were conducted according to the approval granted by the Institutional Animal Care and Use Committee of the Lovelace Biomedical Research Institute under Protocol #20034. In-vivo two-photon laser scanning microscopy over the rat parietal cortex was used to monitor the dose-dependent effects of DRP on microvascular perfusion, tissue oxygenation (NADH) and blood-brain barrier permeability in male and female rats subjected to moderate TBI. Lateral fluid-percussion TBI was induced after baseline imaging and followed by 4 hours of monitoring. DRP was injected at 1, 2, or 4 ppm within 30 minutes after TBI induction. Brain and rectal temperatures, mean arterial (MAP) and intracranial pressures (ICP), blood gases and electrolytes were monitored.
For DRP preparation, polyethylene oxide (PEO, MW ~4000 kDa) was dissolved in saline to 0.1% (1000 ppm), dialyzed against saline using a 50 kD cutoff membrane, diluted in saline to 50 ppm, slow rocked for ~2 hours and sterilized using a 0.22 μm filter [4].
2.2. Surgical preparation
Acclimatized male Sprague-Dawley rats (250–300 g) were mechanically ventilated on isoflurane (2%) in a mix of nitrous oxide (69%) and oxygen (29%) anesthesia. The femoral artery and venous, and intracranial catheters were inserted. For TBI and imaging, a craniotomy (5 mm) over the left parietal cortex was filled with agarose in saline (2%) and sealed by a cover glass. The fluid percussion TBI was induced by a pulse from the Pneumatic Impactor connected to the brain through a transducer filled with artificial cerebrospinal fluid (1.5 ATA, 100 ms).
2.3. Two-Photon Laser Scanning Microscopy
Fluorescent serum (tetramethylrhodamine isothiocyanate (TAMRA) dextran, 500 kDa in physiological saline, 5% wt/vol) was visualized using an Olympus BX 51WI upright microscope and water-immersion LUMPlan FL/IR 20X/0.50 W objective. Excitation was provided by a PrairieView Ultima multiphoton microscopy laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: Sapphire laser (Spectra-Physics, Mountain View, CA, USA) tuned to 750 nm center wavelength. Band-pass-filtered epifluorescence (560–660 nm for TAMRA and 425–475 nm for NADH) was collected by photomultiplier tubes of the Prairie View Ultima system. Images (512 X 512 pixels, 0.15 um/pixel in the x- and y-axes) or line scans were acquired using Prairie View software. Red blood cell flow velocity was measured in microvessels ranging from 3–50 μm diameter up to 500 μm below the surface of the parietal cortex, as previously described [4]. Tissue hypoxia was assessed by measurement of NADH autofluorescence and BBB permeability by TAMRA transcapillary extravasation. In offline analyses using NIH ImageJ software, three-dimensional anatomy of the vasculature in areas of interest was reconstructed from two-dimensional (planar) scans of the fluorescence intensity obtained at successive focal depths in the cortex (XYZ stack).
2.4. Statistical Analysis
Statistical analyses were done by Student’s t-test or Kolgomorov-Smirnov test where appropriate using GraphPad Prism 9 (GraphPad Software, Inc, San Diego, CA). Differences between groups were determined using a two-way ANOVA analysis for multiple comparisons and post hoc testing with the significance level was preset to p < 0.05.
3. Results
At a baseline, we observed intact microcirculation with a capillary flow velocity of 0.81 ± 0.04 and 0.82 ± 0.05 mm/s and the number of perfused capillaries per 0.075 mm3 of 211± 17 and 215 ± 18 in male and female rats, respectively (Fig. 1 a, b). ICP was also within the normal range without differences between male and female rats, 8.1 ± 0.7 and 8.5 ± 0.6 mm Hg, respectively (Fig. 1 c).
Fig. 1.
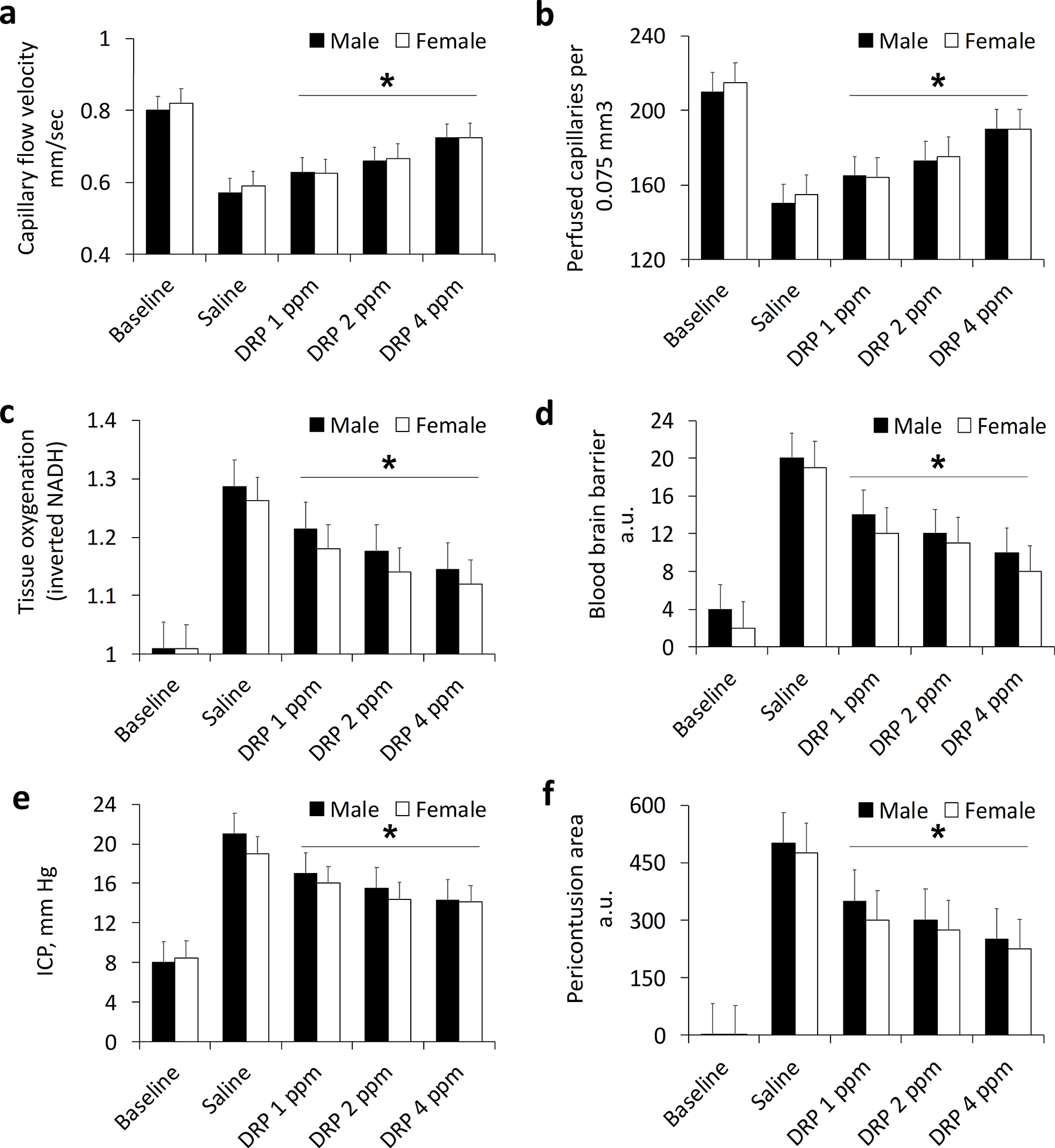
Sex-specific and dose-dependent effects of DRP on a) Capillary flow velocity; b) Number of perfused capillaries; c) Tissue oxygenation status (NADH); d) Blood-brain barrier; e) Intracranial pressure; and f) Pericontusion area. Mean ± SEM, N=10 rats per group, *P < 0.05.
Moderate TBI progressively decreased microvascular circulation (capillary flow velocity and the number of perfused capillaries), leading to tissue hypoxia in the pericontusion zone (Fig. 1 a–c, p<0.05). The i.v. injection of DRP increased near-wall flow velocity and flow rate in arterioles, leading to an increase in the number of erythrocytes entering capillaries, enhancing capillary perfusion and restoring perfusion in collapsed capillaries in a dose-dependent manner without significant differences between males and females (Fig. 1 a–c, p<0.05). At the end of the monitoring period, capillary flow velocity was 0.57 ± 0.06, 0.63 ± 0.05, 0.66 ± 0.06, and 0.72 ± 0.06 mm/sec in male rats and 0.59 ± 0.04, 0.63 ± 0.05, 0.67 ± 0.06 and 0.73 ± 0.06 mm/s in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 a, p<0.05 between DRP and saline treatments). At the same time, the number of perfused capillaries per 0.075 mm3 was 150 ± 18, 165 ± 14, 173 ± 15 and 190 ± 19 in male rats and 155 ± 14, 164 ± 15, 175 ± 15 and 190 ± 18 in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 b, p<0.05 between DRP and saline treatments). Microcirculation improvement after DRP treatment led to tissue oxygen supply enhancement. By the end of the monitoring period, NADH, inversely reflecting mitochondrial respiration and tissue oxygenation, was 1.29 ± 0.11, 1.21 ± 0.12, 1.18 ± 0.12 and 1.15 ± 0.13 a.u. in male rats and 1.26 ± 0.14, 1.18 ± 0.11, 1.14 ± 0.10 and 1.12 ± 0.11 a.u. in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 c, p<0.05 between DRP and saline treatments).
TBI led to BBB breakdown, reflected by TAMRA extravasation, which was reduced by DRP in a dose-dependent manner (Fig. 1 d, p<0.05). At the end of the monitoring period, the extravascular fluorescence was 20.1 ± 1.1, 14.0 ± 1.2, 12.2 ± 1.3 and 10.1 ± 1.3 a.u. in male rats and 19.0 ± 1. 4, 12.3 ± 1.3, 11.2 ± 1.1 and 8.4 ± 1.0 a.u. in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 d, p<0.05 between DRP and saline treatments). Increased BBB permeability presumably led to vasogenic edema, reflected by an increase in ICP, mitigated by DRP in a dose-dependent manner (Fig 1 e, p<0.05). The ICP was 21.1 ± 1.4, 17.2 ± 1.3, 15.5 ± 1.4 and 14.3 ± 1.2 mm Hg in male rats and 19.1 ± 1. 4, 16.2 ± 1.2, 14.4 ± 1.3 and 14.1 ± 1.1 mm Hg in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 e, p<0.05 between DRP and saline treatments). Reduced by DRP vasogenic and, probably, cytogenic edema, reflected by a decrease in BBB breakdown and ICP build-up, led to a reduction in a pericontusion area, which by the end of the study was 504 ± 36.2, 352 ± 21.5, 313 ± 33.4 and 254 ± 18.9 a.u. in male rats and 475 ± 34. 4, 302 ± 31.3, 274 ± 28.4 and 226 ± 21.1 a.u in female rats with saline, DRP 1 ppm, 2 ppm and 4 ppm treatment, respectively (Fig. 1 f, p<0.05 between DRP and saline treatments).
4. Discussion
We demonstrated a dose-dependent efficacy of DRP (1, 2 and 4 ppm) in the treatment of post-TBI pericontusion ischemia in a rat lateral fluid percussion injury model of TBI. The i.v. injection of DRP increased near-wall flow velocity and flow rate in arterioles, leading to an increase in the number of erythrocytes entering capillaries, enhancing capillary perfusion and tissue oxygen supply in a dose-dependent manner. Treatment with DRPs effectively mitigated an increase in ICP, and protected BBB from degradation.
We did not find a statistically significant sex-specific difference in response to to TBI or DRP, which does not necessarily contradict several studies observing significantly better outcomes in female animals as this is an acute study without long term survival [5–8]. However, there was a clear trend in better-preserved tissue oxygen supply, BBB permeability, intracranial pressure and pericontusion area in females (Fig. 1 c–f), which corresponds to works, also reporting notable but insignificant sex-dependent difference suggesting better outcomes in female animals [7]. Considering the current body of research on sex differences in TBI, the overall picture is not a straightforward one that points to better outcomes in one sex versus the other, and the findings are complicated and often contradictory [8]. A closer examination of the sex-dependent responses to TBI and TBI treatment will provide new insights that will move the field forward in a meaningful way.
Acknowledgments:
Supported by NIH R01NS112808.
References
- 1.Faul M, Xu L, Wald MM et al. (2010) Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths; Atlanta, GA: 2010. Available from: http://braininjury.blogs.com/braininjury/2010/03/cdc-releases-latest-statistics-on-traumatic-brain-injury.html. [Google Scholar]
- 2.Veenith TV, Carter EL, Geeraerts T et al. (2016) Pathophysiologic Mechanisms of Cerebral Ischemia and Diffusion Hypoxia in Traumatic Brain Injury. JAMA Neurol. 73(5), 542–550. [DOI] [PubMed] [Google Scholar]
- 3.Papaioannou TG, Stefanadis C (2005) Vascular wall shear stress: Basic principles and methods. Hellenic J Cardiol. 2005; 46, 9–15. [PubMed] [Google Scholar]
- 4.Bragin DE, Kameneva MV, Bragina OA et al. (2017) Rheological effects of drag-reducing polymers improve cerebral blood flow and oxygenation after traumatic brain injury in rats. J Cereb Blood Flow Metab. 37(3), 762–775. doi: 10.1177/0271678X16684153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor CA, Cernak I, Vink R (2006) The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir Suppl. 96, 121–124. doi: 10.1007/3-211-30714-1_27. [DOI] [PubMed] [Google Scholar]
- 6.Rubin TG, Lipton ML (2019) Sex Differences in Animal Models of Traumatic Brain Injury. J Exp Neurosci. 13, 13:1179069519844020. doi: 10.1177/1179069519844020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott MC, Prabhakara KS, Walters AJ, et al. (2022) Determining Sex-Based Differences in Inflammatory Response in an Experimental Traumatic Brain Injury Model. Front Immunol. 9, 13:753570. doi: 10.3389/fimmu.2022.753570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupte R, Brooks W, Vukas R, et al. (2018) Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J Neurotrauma. 15;36(22), 3063–3091. doi: 10.1089/neu.2018.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]


