Abstract
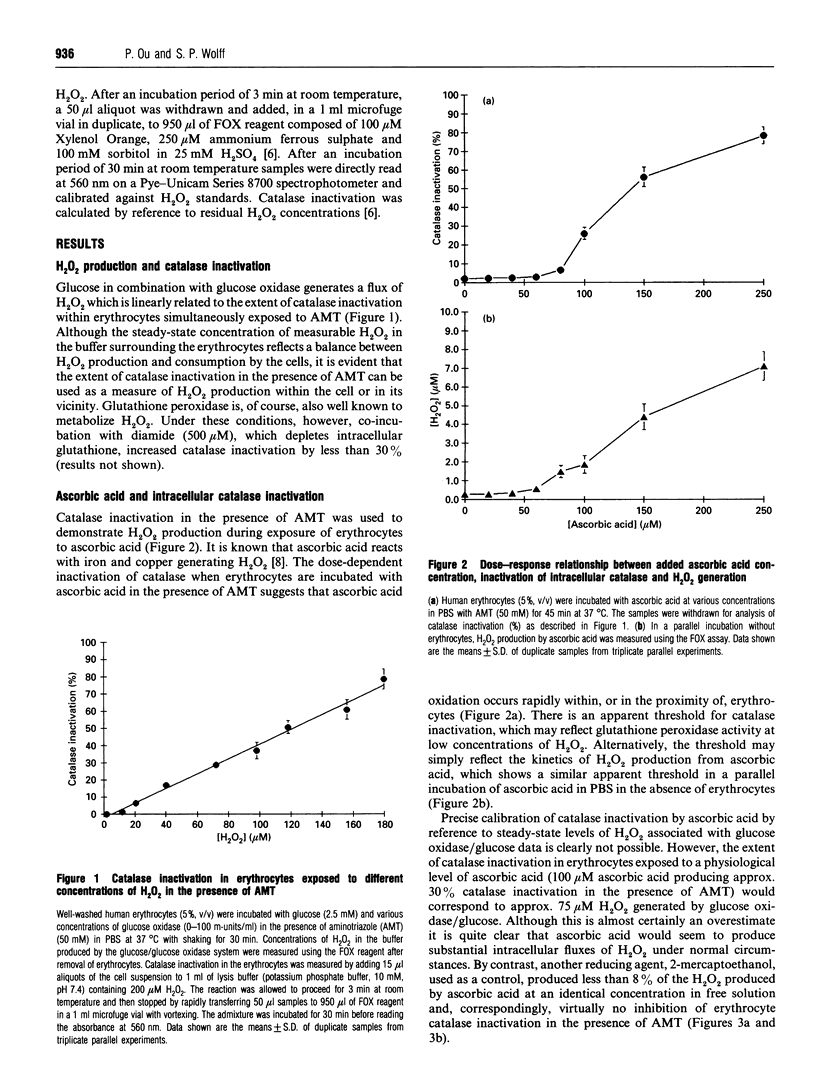
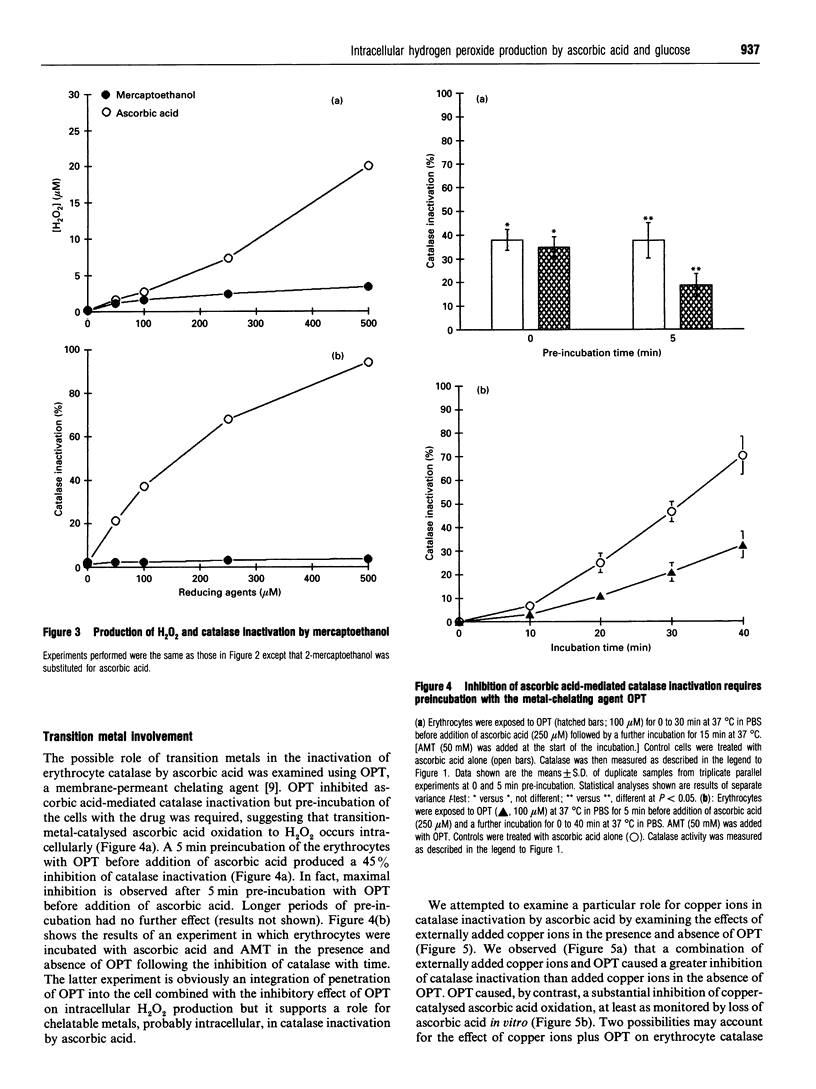
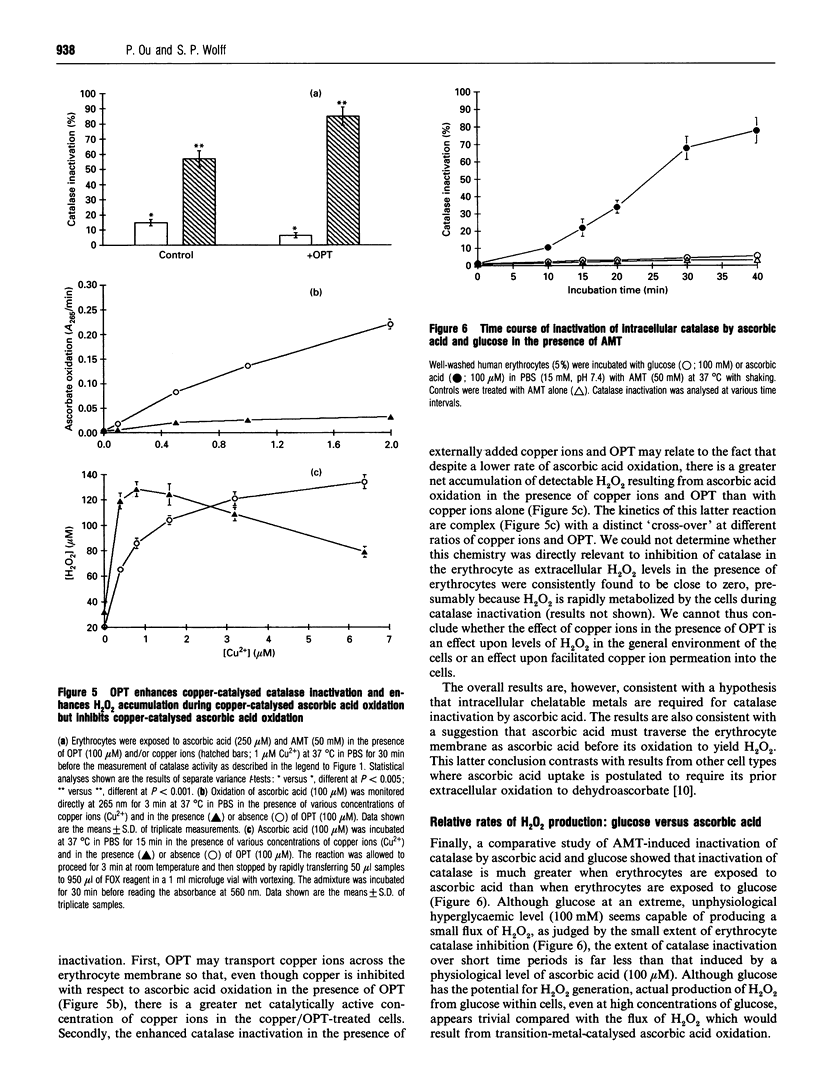
Erythrocytes exposed to ascorbic acid in the presence of aminotriazole undergo a dose- and time-dependent inactivation of endogenous catalase which is proportional to environmental hydrogen peroxide (H2O2) concentrations. The production of H2O2 seems to be dependent upon the availability of transition metal chelatable by o-phenanthroline (OPT), although the kinetics of catalase inactivation and H2O2 production by externally added copper ions in the presence of OPT is complex. Furthermore, although glucose is also able to undergo a transition-metal-catalysed oxidation yielding H2O2, the production of H2O2 by glucose seems to be a minor process by comparison with ascorbic acid oxidation. Indeed, on the basis of these data, transition-metal-catalysed ascorbic acid oxidation is likely to be a more important source of oxidative stress in the diabetic state than hyperglycaemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. U., Thorpe S. R., Baynes J. W. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986 Apr 15;261(11):4889–4894. [PubMed] [Google Scholar]
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Ahmed M. U., Murtiashaw M. H., Richardson J. M., Walla M. D., Thorpe S. R., Baynes J. W. Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990 Dec 11;29(49):10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- Hunt J. V., Bottoms M. A., Mitchinson M. J. Ascorbic acid oxidation: a potential cause of the elevated severity of atherosclerosis in diabetes mellitus? FEBS Lett. 1992 Oct 19;311(2):161–164. doi: 10.1016/0014-5793(92)81389-4. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Zhou Q. L., Eaton J. W., Koppenol W. H., Hunt J. V., Wolff S. P. Spirohydantoin inhibitors of aldose reductase inhibit iron- and copper-catalysed ascorbate oxidation in vitro. Biochem Pharmacol. 1991 Aug 22;42(6):1273–1278. doi: 10.1016/0006-2952(91)90265-7. [DOI] [PubMed] [Google Scholar]
- Krishnamurti C., Saryan L. A., Petering D. H. Effects of ethylenediaminetetraacetic acid and 1,10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells. Cancer Res. 1980 Nov;40(11):4092–4099. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou P., Wolff S. P. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem Pharmacol. 1993 Oct 5;46(7):1139–1144. doi: 10.1016/0006-2952(93)90461-5. [DOI] [PubMed] [Google Scholar]
- Sinclair A. J., Girling A. J., Gray L., Le Guen C., Lunec J., Barnett A. H. Disturbed handling of ascorbic acid in diabetic patients with and without microangiopathy during high dose ascorbate supplementation. Diabetologia. 1991 Mar;34(3):171–175. doi: 10.1007/BF00418271. [DOI] [PubMed] [Google Scholar]
- Washko P. W., Wang Y., Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993 Jul 25;268(21):15531–15535. [PubMed] [Google Scholar]
- Wolff S. P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993 Jul;49(3):642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Jiang Z. Y., Hunt J. V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5):339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- Wolff S. P., Wang G. M., Spector A. Pro-oxidant activation of ocular reductants. 1. Copper and riboflavin stimulate ascorbate oxidation causing lens epithelial cytotoxicity in vitro. Exp Eye Res. 1987 Dec;45(6):777–789. doi: 10.1016/s0014-4835(87)80095-7. [DOI] [PubMed] [Google Scholar]
- Young I. S., Torney J. J., Trimble E. R. The effect of ascorbate supplementation on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 1992;13(1):41–46. doi: 10.1016/0891-5849(92)90164-c. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Fisher E., Heffernan S., Capogreco C., Ross G. R., Turtle J. R. Ascorbic acid metabolism and polyol pathway in diabetes. Diabetes. 1989 Feb;38(2):257–261. doi: 10.2337/diab.38.2.257. [DOI] [PubMed] [Google Scholar]


