Abstract
Aim: Assess the time-to-treatment discontinuation (TTD) and overall survival (OS) in a Swedish metastatic renal cell carcinoma (mRCC) nationwide cohort who received second-line axitinib.
Methods: Retrospective analysis of 110 patients with mRCC treated with second-line axitinib in Sweden (2012–2019). Patients included in the study received axitinib after mainly first-line sunitinib or pazopanib.
Results: The median (95% CI) TTD of patients who received second-line axitinib was 5.2 (3.7–6.1) months with 6 (5.5%) patients still receiving treatment at the time of analysis. Median (95% CI) OS was 12.2 (7.7–14.2) months.
Conclusion: The results are consistent with previous findings in mRCC and add to the evidence demonstrating efficacy of second-line axitinib, after failure of a prior anti-angiogenic therapy in a real-world setting.
Clinical Trial Registration: NCT04669366 (ClinicalTrials.gov)
Keywords: : axitinib, metastatic renal cell carcinoma, real-world, second-line axitinib
Plain language summary
Article highlights.
The aim of this study was to assess the time-to-treatment discontinuation (TTD) and overall survival (OS) in a Swedish metastatic renal cell carcinoma (mRCC) national cohort who received second-line axitinib.
A retrospective analysis of patients with mRCC treated with second-line axitinib in Sweden (2012–2019).
A total of 110 patients with mRCC received second-line treatment with axitinib in the full population; 53 patients received first-line sunitinib, 53 patients received first-line pazopanib and the remaining four patients received another permitted first-line treatment.
The median (95% CI) TTD in patients who received second-line axitinib was 5.2 (3.7–6.1) months with 6 (5.5%) patients still receiving treatment at the time of analysis. The median OS (95% CI) for all patients who received second-line axitinib was 12.2 (7.7–14.2) months; 28 (25.5%) patients remained censored at the time of analysis.
The results are consistent with previous findings in mRCC and add to the evidence demonstrating efficacy of second-line axitinib, after failure of a prior anti-angiogenic therapy in a real-world setting.
1. Background
Approximately 2% of all global cancer diagnoses and deaths are attributed to renal cell carcinomas (RCC) [1]. RCC is a heterogenous disease. In the advanced/metastatic stage the most appropriate systemic treatment is largely determined by stratification according to the presence of risk factors such as; performance status, anemia, granulocytosis and thrombocytosis, hypercalcemia, time from diagnosis to treatment start <1 year and histological subtype, in other words, clear cell or non-clear cell [2–6].
The prognosis of metastatic RCC (mRCC) improved with the introduction of targeted therapy [7,8]. More recently, the use of anti-angiogenic and/or immune checkpoint inhibitors in combination therapy marked another substantive step forward in improving clinical outcomes. These include vascular endothelial growth factor-receptor (VEGF-R) tyrosine kinase inhibitors (TKIs; axitinib, cabozantinib, lenvatinib) and immuno-oncological (IO) agents such as immune checkpoint inhibitors (avelumab, ipilimumab, nivolumab and pembrolizumab) [2,3,9]. Depending on the disease setting, the combination of an IO agent and either a TKI (IO/TKI) or another IO (IO/IO) is the standard of care for previously untreated advanced RCC of the clear cell type [3,4]. However, access to IO-based treatments varies globally, particularly in low- and middle-income countries [10]. Consequently, single-agent targeted therapies such as axitinib, play a crucial role when an IO-based regimen is unfeasible or unavailable. Optimizing treatment with these agents remains vital to ensure optimal management of systemic treatment for all patients.
Axitinib, a tyrosine kinase inhibitor of VEGF-R1, 2 and 3, is an anti-angiogenic targeted therapy contained in two of the recently approved IO/TKI combinations, namely pembrolizumab/axitinib and avelumab/axitinib. Axitinib was initially approved in Europe in 2012 for second-line treatment of mRCC as a single agent after failure of prior treatment with sunitinib or a cytokine. The approval of axitinib as a single agent was based on clinical data from the AXIS trial, an open-label, multicenter, randomized, Phase III study that compared axitinib (5 mg twice daily; n = 361) with sorafenib (400 mg twice daily; n = 362) in patients with advanced RCC following failed treatment with one prior systemic therapy [11,12]. Overall, the median progression-free survival (PFS) was significantly longer with axitinib versus sorafenib at 6.7 and 4.7 months, respectively [12]. Median overall survival (OS) did not differ significantly with axitinib versus sorafenib at 20.1 (95% CI: 16.7–23.4) versus 19.2 (95% CI: 17.5–22.3) months, respectively [11]. For patients who previously received sunitinib in the AXIS trial, the median OS was 15.2 (95% CI: 12.8–18.3) months [11].
Outside of the stringent conditions of randomized clinical trials, it is important to describe the clinical outcomes of more heterogeneous patient populations [13–15]. Previous real-world studies have demonstrated the efficacy and safety of second- or later-line axitinib in patients with mRCC after anti-angiogenic targeted therapies [16–20]. Although not the same context as our study, previous studies have investigated the efficacy of TKIs after progression on both IO-doublet and IO-TKI combination treatment in first line [21–23]. This study investigates a comprehensive nationwide real-world cohort of Swedish patients with mRCC using national healthcare registries [24,25]. We expand on the available body of evidence concerning the real-world use of second-line axitinib in this patient population after the failure of anti-angiogenic targeted therapies. The primary objective of this analysis was to investigate the time-to-treatment discontinuation (TTD) and OS in a real-world setting for a nationwide Swedish mRCC cohort who received second-line axitinib.
2. Methods
2.1. Data sources
This retrospective analysis utilized the population-based Swedish health data registers maintained by the National Board of Health and Welfare (NBHW): The Prescribed Drug Register, The National Patient Register, The Swedish Cancer Register and the National Cause of Death Register. Health data for the entire Swedish population are contained in these registries. Individual patient-level data were combined for the 4 registers allowing extraction of the duration of treatment and OS for each patient. Hence, these data included all patients who received axitinib among the entire Swedish RCC population. All data were anonymized prior to delivery from the NBHW. The study was approved by the Swedish ethical review authority (2020-00434).
2.2. Patients & study design
Eligible patients were ≥18 years of age with a confirmed diagnosis of RCC and ≥1 filled prescription of axitinib in the second-line setting from 2012 (marketing approval) until December 2019. Metastatic status was based on the assumption that axitinib is indicated for treatment of metastatic disease and prescribed accordingly. This assumption is supported by the fact that axitinib at the time of data collection was reimbursed and recommended only for treatment in mRCC, and by the strong compliance to reimbursement and treatment guidelines among the Swedish doctors [26]. The second-line axitinib treatment group was identified as having ≥1 filled prescription of axitinib prescription after prescription of any of the available first-line treatments: sunitinib, sorafenib, pazopanib, everolimus, cabozantinib, tivozanib, lenvatinib or interferon. Information on baseline clinical characteristics other than gender, age at RCC diagnosis and age at treatment initiation was not available.
2.3. Outcomes & statistical analysis
The primary goals of this study were to investigate TTD and OS. TTD, as defined by the Medication Compliance Work Group at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), represents the duration from the initial dispensed prescription to the end of the prescribed supply—or initiation of another treatment, or death, if either occurred before the axitinib supply concluded [27]. The duration covered by the dispensed volume was determined based on the prescribing information, including the prescriber's dosing instruction, and the volume of dispensed drug. This calculation allowed for accumulation of medicine, with a grace period of 90 days permitted between filled prescriptions, including accumulated medication from overlapping prescriptions. Notably, the Prescribed Drug Register does not include treatment administered in a hospital setting so any potential immuno-oncology treatment with nivolumab following axitinib could not be accounted for. OS was defined as the time from the date of the initial dispensed axitinib prescription to the date of all-cause death. The analysis did not account for the potential impact on survival of subsequent lines of therapy and due to data availability, safety was not assessed. When no date was recorded the data were censored at latest available date. TTD and OS were described using Kaplan–Meier methods. The data sources and methods used in this study closely resemble those previously documented in another study that specifically examines the first-line setting [28].
Due to the nature of the available data, estimation of PFS was not possible. However, prior research has suggested a patient-level association between TTD and PFS supporting the pragmatic use of TTD as a real-world evidence end point when PFS data are unavailable [29,30]. However, discrepancies may exist between TTD and PFS. Patients can continue treatment beyond objective RECIST progression, both in clinical practice and in randomized clinical trials, prolonging time to clinical progression [31,32] Additionally, TTD might underestimate PFS when discontinuation occurs due to safety, tolerability or other issues unrelated to disease progression [33].
3. Results
3.1. Patients
A total of 110 patients with mRCC received second-line treatment with axitinib in the full population; 53 patients received first-line sunitinib and the remaining patients received either pazopanib (n = 53), cabozantinib (n = 2), everolimus (n = 1) or interferon (n = 1) (Figure 1). At the end of follow-up, 6 patients were still on treatment. Out of the 104 remaining patients who discontinued second-line axitinib treatment during follow-up, 87 (83.7%) reached the end of their dispensed supply, 11 (10.6%) switched to a subsequent treatment and 6 (5.8%) died. Overall, patients were predominantly male (n = 84, 76.4%) with a mean (SD) age of 60.9 (9.6) years at RCC diagnosis and 65.5 (9.9) years at treatment initiation of second-line treatment with axitinib (Table 1). The first-line sunitinib group had a greater number of male patients, a lower age at RCC diagnosis and a lower age at treatment initiation compared with the other first-line group (all p ≤ 0.05; Table 1).
Figure 1.
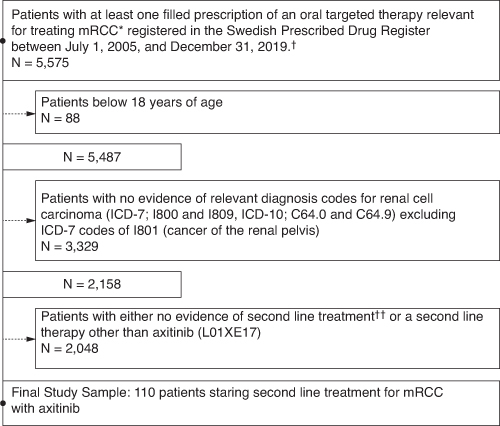
Patient selection.
*Relevant ATC codes for the oral targeted therapy of mRCC: L01XE04 (sunitinib), L01XE05 (sorafenib), L01XE11 (pazopanib), L01XE17 (axitinib), L01XE10 (everolimus), L01XE26 (cabozantinib), L01XE34 (tivozanib), L01XE29 (lenvatinib).
†No data available prior to 1 July 2005
††Reasons include first-line treatment at censoring, death, clinical factors or patient preference.
ATC: Anatomical chemical therapeutic; ICD: International Classification of Diseases; mRCC: Metastatic renal cell carcinoma.
Table 1.
Baseline characteristics, all evaluable patients.
| Total (N = 110) | Sunitinib first-line (n = 53) | Other first-line (n = 57) | p-value | |
|---|---|---|---|---|
| Male, n (%) | 84 (76.4) | 45 (84.9) | 39 (68.4) | 0.042 |
| Mean (SD) age at RCC diagnosis, years | 60.9 (9.6) | 58.4 (9.3) | 63.3 (9.3) | 0.005 |
| Mean (SD) age at treatment initiation, years | 65.5 (9.9) | 63.0 (9.6) | 67.9 (9.6) | 0.005 |
RCC: Renal cell carcinoma; SD: Standard deviation.
3.2. Outcomes
For all patients who received second-line axitinib, the median TTD was 5.2 (95% CI: 3.7–6.1) months with 6 (5.5%) patients still receiving treatment at the time of analysis (Figure 2). The median OS for all patients who received second-line axitinib was 12.2 (95% CI: 7.7–14.2) months; 28 patients (25.5%) remained censored at the time of analysis (Figure 3).
Figure 2.
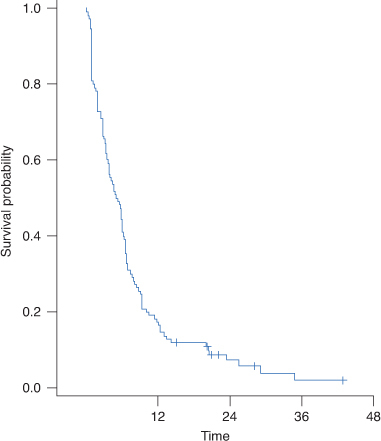
Time-to-treatment discontinuation in evaluable patients. No. of patients at risk: month 0, n = 110; month 12, n = 19; month 24, n = 5; month 36, n = 1; month 44, n = 0.
Figure 3.
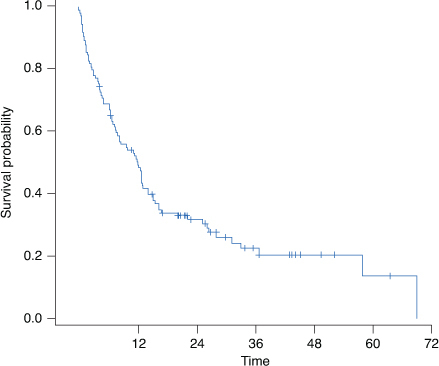
Overall survival in evaluable patients. *No. of patients at risk: month 0, n = 110; month 12, n = 54; month 24, n = 24; month 36, n = 11; month 48, n = 5; month 60, n = 2; month 70, n = 0.
4. Discussion
Real-world evidence for treatment outcomes in heterogeneous populations is particularly valuable, as it can help inform clinical decisions for patient groups not necessarily represented in randomized clinical trials [34]. In this analysis of a real-world Swedish population, OS was generally consistent with that reported in the pivotal AXIS trial for patients who received axitinib after first-line sunitinib. Median OS of patients who received second-line axitinib was 12.2 (95% CI: 7.7–14.2) months. By comparison, median OS of patients in the AXIS trial who previously received sunitinib was 15.2 (95% CI: 12.8–18.3) months. In the full population of the AXIS trial, the median OS was 20.1 months and the median PFS was 8.3 months [11,12]. Similarly, the median TTD of 5.2 (95% CI: 3.7–6.1) months in this Swedish real-world population was consistent with the median PFS of 6.5 (95% CI: 5.7–7.9) months reported in the AXIS trial for patients who received first-line sunitinib [11]. These results further support the effectiveness of second-line axitinib after failure of an anti-angiogenic therapy in an unselected patient population in a real-world setting. The results from this study were also consistent with data from other real-world studies [16–18,20,35,36]. A German study based on data from the STAR-TOR Registry found that 210 patients treated with axitinib in second or later lines had a median PFS of 5.6 months, and median OS of 18.3 months[20]. Sunitinib was the most common previous therapy in 158 patients. In an Italian, multicenter, observational, retrospective real-world study, Facchini et al. evaluated axitinib as second-line treatment of 148 patients with mRCC following first-line sunitinib. The median PFS was 7.14 (95% CI: 5.8–8.5) months and the median OS from the start of axitinib treatment was 15.5 (95% CI: 11–20) months [16]. A prospective real-world study that evaluated 160 patients with mRCC at a large French comprehensive cancer center found that patients treated with second-line or later-line axitinib had a median PFS and OS of 8.3 and 16.4 months, respectively [18].
This study adds to the evidence demonstrating the efficacy of second-line axitinib in patients with mRCC by examining the clinical outcomes of a real-world Swedish population. In a treatment landscape that includes immunotherapy-based combinations as a standard of care, TKI monotherapy maintains an important role in the second-line setting. Anti-angiogenic therapies, including axitinib, are currently recommended by international guidelines after an IO-based combination [9,37]. For patients who received a first-line IO/TKI regimen, it is recommended to offer a different TKI in the second-line [9,37]. This is in line with a consolidated approach in mRCC management that, particularly before IO introduction, has relied on sequencing of anti-angiogenic therapies, including rechallenging strategy [4]. Indeed, despite belonging to the same class, these TKIs present different features and may provide clinical benefit also when used in patients who are pre-exposed to drugs of the same class [4]. Dose optimization at the individual level remains essential to maximize outcome of TKI therapies but the influence of this aspect on efficacy may be difficult to assess in real-world/registry studies and is not captured in the present analysis either. The need to optimize each line of therapy remains key for the individual patient also in the context of a growing number of therapeutic options as it is today in the mRCC space. In prospective studies for second-line axitinib, pazopanib or sunitinib after an IO/TKI combination, responses were observed in approximately 20% of patients [38–40]. This may be compared with response rates ranging from 0 to 43% and a median PFS between 2.9 months to NR, for 2L sunitinib and cabozantinib, respectively, in a retrospective study assessing efficacy of axitinib, pazopanib, sunitinib and cabozantinib post 1L IO/TKI treatment[23]. Other retrospective or exploratory analyses of studies for second-line cabozantinib, tivozanib, lenvatinib–everolimus showed similar response rates [41–43]. However, given the small number and mixed populations in the prospective studies and the exploratory design of the retrospective studies, there is still a need for further robust studies examining the efficacy of second-line TKIs after first-line IO/TKI regimens [3]. Therefore, determining the real-world second-line efficacy of TKI monotherapy, particularly after first-line treatments containing TKIs, is informative for the clinical management of this patient population.
4.1. Strengths & limitations
With the introduction of novel, predominantly IO-based, treatments in mRCC, it may be argued that the relevance of single-agent targeted treatments such as axitinib is limited. However, as access varies and novel treatments may not always be suitable, targeted therapies still remain important treatment options [3,4]. The retrospective design has inherent limitations of risk of bias in the analyses, especially selection and misclassification bias; however, the population-based design offers an advantage of including all patients prescribed with axitinib in Sweden during the investigated time period. Risk group stratification of patients was not included in the dataset, which could influence results for TTD and OS. Estimation of PFS was not possible due to the nature of the available data and the use of TTD is a potential limitation as it could overestimate PFS if patients are maintained on treatment beyond objective RECIST progression. However, TTD could potentially also underestimate PFS when discontinuation occurs for safety, tolerability, or other issues different from disease progression. Detailed baseline demographics and clinical characteristics were not available; therefore, an evaluation of specific populations (e.g., histological subtypes) and potential differences in clinical characteristics (e.g., comorbidities) could not be identified and evaluated. In addition, the potential impact of subsequent treatment on survival was not investigated. This study assumes that patients with an RCC diagnosis and a prescription of axitinib in 2L are diagnosed with mRCC. Although this assumption has support in the Swedish tradition of compliance with treatment guidelines and reimbursement decisions, there is a possibility that the analyzed cohort includes patients who receive axitinib for another malignant diagnosis other than mRCC. However, the impact on the results from potential misclassification is deemed nominal.
In addition, the Prescribed Drug Register does not include drugs that are administered at the hospital. Therefore, for some patients moving on to IO, there is a possibility that TTD was overestimated if treatment with IO was initiated before the end of supply of axitinib. This possibility is considered small since usually one packet of axitinib is dispensed at a time.
5. Conclusion
This study investigates the efficacy of second-line axitinib in patients with mRCC by examining the clinical outcomes of a real-world Swedish population. Despite the approval and use of new, predominantly IO-based, mRCC treatments, axitinib monotherapy remains an important treatment option. The results presented in this paper are consistent with previous findings and add to the evidence demonstrating efficacy of second-line axitinib, after failure of a prior anti-angiogenic therapy in a real-world setting.
Acknowledgments
This study was sponsored by Pfizer. Medical writing support was provided by Steven Moore, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Funding Statement
This study was sponsored by Pfizer. M Jakobsson, F Nilsson and J Arpegård are employees of Pfizer. A Strambi was an employee of Pfizer at the time of writing. J Dalén is an employee of ICON plc and was a paid consultant to Pfizer for this research.
Author contributions
Conception and design: All authors. Performed research: All authors. Contributed new reagents or analytic tools: N/A. Analysis and interpretation of data: All authors. Drafting of the manuscript: All authors.Critical revision of the manuscript for important intellectual content: All authors. Other (specify): N/A
Financial disclosure
This study was sponsored by Pfizer. M Jakobsson, F Nilsson and J Arpegård are employees of Pfizer. A Strambi was an employee of Pfizer at the time of writing. J Dalén is an employee of ICON plc and was a paid consultant to Pfizer for this research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Medical writing support was provided by S Moore of Engage Scientific Solutions and funded by Pfizer.
Ethical conduct of research
The study was approved by the Swedish ethical review authority (2020-00434). Written informed consent was not required for this retrospective analysis.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to applicable laws and regulations pertaining to Swedish administrative and clinical data available for research.
References
Papers of special note have been highlighted as: • of interest
- 1.Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11:79–87. doi: 10.14740/wjon1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ESMO . eUpdate – renal cell carcinoma treatment recommendations 2021 [cited 2023 February 20,]. Available from: www.esmo.org/guidelines/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations-4 ; • European evidence-based guidelines for the management of patients with renal cell cancer.
- 4.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 5.Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16:293–300. doi: 10.1016/S1470-2045(14)71222-7 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530 [DOI] [PubMed] [Google Scholar]
- 7.Coppin C, Le L, Porzsolt F, et al. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev. 2008;2008:CD006017. doi: 10.1002/14651858.CD006017.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496–511. doi: 10.1038/nrneph.2017.82 [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:71–90. doi: 10.6004/jnccn.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of evidence-based recommendations for the management of patients with renal cell carcinoma.
- 10.Noronha V, Abraham G, Patil V, et al. A real-world data of Immune checkpoint inhibitors in solid tumors from India. Cancer Med. 2021;10:1525–1534. doi: 10.1002/cam4.3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised Phase III trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7 [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised Phase III trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9 [DOI] [PubMed] [Google Scholar]
- 13.Dekkers OM, von Elm E, Algra A, et al. How to assess the external validity of therapeutic trials: a conceptual approach. Int J Epidemiol. 2010;39:89–94. doi: 10.1093/ije/dyp174 [DOI] [PubMed] [Google Scholar]
- 14.Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106:2452–2458. doi: 10.1002/cncr.21907 [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM. Commentary: external validity of results of randomized trials: disentangling a complex concept. Int J Epidemiol. 2010;39:94–96. doi: 10.1093/ije/dyp305 [DOI] [PubMed] [Google Scholar]
- 16.Facchini G, Rossetti S, Berretta M, et al. Second line therapy with axitinib after only prior sunitinib in metastatic renal cell cancer: italian multicenter real world SAX study final results. J Transl Med. 2019;17:296. doi: 10.1186/s12967-019-2047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geczi L, Bodoky G, Rokszin G, et al. Survival benefits of second-line axitinib versus everolimus after first line sunitinib treatment in metastatic renal cell carcinoma. Pathol Oncol Res. 2020;26:2201–2207. doi: 10.1007/s12253-020-00809-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matias M, Le Teuff G, Albiges L, et al. Real world prospective experience of axitinib in metastatic renal cell carcinoma in a large comprehensive cancer centre. Eur J Cancer. 2017;79:185–192. doi: 10.1016/j.ejca.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 19.Brown J, Harrow B, Marciniak A, et al. 680P Cabozantinib and axitinib after VEGF therapy in patients with aRCC: a retrospective cohort study. Ann Oncol. 2021;32:S701. doi: 10.1016/j.annonc.2021.08.076 [DOI] [Google Scholar]
- 20.Uhlig A, Uhlig J, Woike M, et al. Axitinib beyond first-line therapy of Metastatic Renal Cell Carcinoma: Real World Data from the STAR-TOR registry. Kidney Cancer. 2023;7:37–48. doi: 10.3233/KCA-220011 [DOI] [Google Scholar]; • Real-world data regarding the utility of axitinib for the treatment of metastatic renal cancer.
- 21.Sazuka T, Matsushita Y, Sato H, et al. Efficacy and safety of second-line cabozantinib after immuno-oncology combination therapy for advanced renal cell carcinoma: japanese multicenter retrospective study. Scientific Reports. 2023;13:20629. doi: 10.1038/s41598-023-48087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzel M, Deuker M, Nocera L, et al. Median time to progression with TKI-based therapy after failure of immuno-oncology therapy in metastatic kidney cancer: a systematic review and meta-analysis. Eur J Cancer. 2021;155:245–255. doi: 10.1016/j.ejca.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 23.Barata PC, De Liano AG, Mendiratta P, et al. The efficacy of VEGFR TKI therapy after progression on immune combination therapy in metastatic renal cell carcinoma. Br J Cancer. 2018;119:160–163. doi: 10.1038/s41416-018-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindskog M, Wahlgren T, Sandin R, et al. Overall survival in Swedish patients with renal cell carcinoma treated in the period 2002 to 2012: update of the RENCOMP study with subgroup analysis of the synchronous metastatic and elderly populations. Urol Oncol. 2017;35(541):e15–e22. doi: 10.1016/j.urolonc.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 25.Wahlgren T, Harmenberg U, Sandstrom P, et al. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000–2008). Br J Cancer. 2013;108:1541–1549. doi: 10.1038/bjc.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agency SDaPB . Inlyta is included in the high-cost protection 2013 [cited 2023]. Available from: www.tlv.se/beslut/beslut-lakemedel/generell-subvention/arkiv/2013-02-08-inlyta-ingar-i-hogkostnadsskyddet.html
- 27.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 28.Jakobsson M, Nilsson F, Strambi A, et al. First-line sunitinib treatment modification in patients with mRCC: nationwide analysis of the Swedish population. Future Oncol. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumenthal GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30:830–838. doi: 10.1093/annonc/mdz060 [DOI] [PubMed] [Google Scholar]
- 30.Gong Y, Kehl KL, Oxnard GR, et al. Time to treatment discontinuation (TTD) as a pragmatic endpoint in metastatic non-small cell lung cancer (mNSCLC): a pooled analysis of 8 trials. J Clin Oncol. 2018;36:9064. doi: 10.1200/JCO.2018.36.15_suppl.9064 [DOI] [Google Scholar]
- 31.Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–312. doi: 10.1001/jamaoncol.2015.4921 [DOI] [PubMed] [Google Scholar]
- 32.Lo PC, Dahlberg SE, Nishino M, et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer. 2015;121:2570–2577. doi: 10.1002/cncr.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker B, Boyd M, Aguilar K, et al. Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Inform. 2021;5:45–46. doi: 10.1200/cci.20.00125 [DOI] [PubMed] [Google Scholar]
- 34.Moran M, Nickens D, Adcock K, et al. Augmenting the randomized controlled trial with real-world data to aid clinical decision making in metastatic renal cell carcinoma: a systematic review and meta-analysis. Future Oncol. 2019;15:3987–4001. doi: 10.2217/fon-2019-0421 [DOI] [PubMed] [Google Scholar]
- 35.Miyake H, Harada KI, Ozono S, et al. Assessment of efficacy, safety, and quality of life of 124 patients treated with axitinib as second-line therapy for metastatic renal-cell carcinoma: experience in real-world clinical practice in Japan. Clin Genitourin Cancer. 2017;15:122–128. doi: 10.1016/j.clgc.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 36.Pinto A, Reig O, Iglesias C, et al. Clinical factors associated with long-term benefit in patients with metastatic renal cell carcinoma treated with axitinib: real-world AXILONG study. Clin Genitourin Cancer. 2022;20:25–34. doi: 10.1016/j.clgc.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 37.ESMO . eUpdate – Renal Cell Carcinoma Treatment Recommendations 2021 [cited 2022 3 October]. Available from: www.esmo.org/guidelines/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations-4
- 38.Powles TB, Oudard S, Grünwald V, et al. 718P A Phase II study of patients with advanced or metastatic renal cell carcinoma (mRCC) receiving pazopanib after previous checkpoint inhibitor treatment. Ann Oncol. 2020;31:S564. doi: 10.1016/j.annonc.2020.08.790 [DOI] [Google Scholar]
- 39.Grande E, Alonso Gordoa T, Reig Torras O, et al. INMUNOSUN-SOGUG trial: a prospective Phase II study to assess the efficacy and safety of sunitinib as second-line (2L) treatment in patients (pts) with metastatic renal cell cancer (RCC) who received immunotherapy-based combination upfront. J Clin Oncol. 2020;38:5060. doi: 10.1200/JCO.2020.38.15_suppl.5060 [DOI] [Google Scholar]
- 40.Ornstein MC, Pal SK, Wood LS, et al. Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, Phase II study. Lancet Oncol. 2019;20:1386–1394. doi: 10.1016/S1470-2045(19)30513-3 [DOI] [PubMed] [Google Scholar]
- 41.Powles T, Motzer RJ, Escudier B, et al. Outcomes based on prior therapy in the Phase III METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;119:663–669. doi: 10.1038/s41416-018-0164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a Phase III, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21:95–104. doi: 10.1016/S1470-2045(19)30735-1 [DOI] [PubMed] [Google Scholar]
- 43.Glen H, Puente J, Heng DYC, et al. A phase 2 trial of lenvatinib 18 mg versus 14 mg once daily (QD) in combination with everolimus (5 mg QD) in renal cell carcinoma (RCC) after 1 prior VEGF-targeted treatment. J Clin Oncol. 2018;36:TPS707–TPS. doi: 10.1200/JCO.2018.36.6_suppl.TPS707 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to applicable laws and regulations pertaining to Swedish administrative and clinical data available for research.


