Abstract
Ca(2+)-uptake experiments in microsomal fractions from transfected COS-1 cells have revealed a functional difference between the non-muscle SERCA2b Ca2+ pump and its muscle-specific SERCA2a splice variant. Structurally, the two pumps differ only in their C-terminal tail. The last four amino acids of SERCA2a are replaced in SERCA2b by a 49-residue-long peptide chain containing a very hydrophobic stretch which could be an additional transmembrane segment. The functionally important subdomains in the SERCA2b tail were analysed by constructing three SERCA2b deletion mutants lacking 12, 31 or 49 amino acids. The mutants and the parental SERCA2 pumps were expressed in COS-1 cells and analysed for functional difference. SERCA2b had a twofold higher Ca2+ affinity, a twofold lower turnover rate and a 10-fold lower vanadate-sensitivity than SERCA2a and the mutants. Since each of the three truncated versions of SERCA2b acquire the characteristic properties of SERCA2a, it is concluded that the stretch of the last 12 residues of SERCA2b is of critical importance.
Full text
PDF

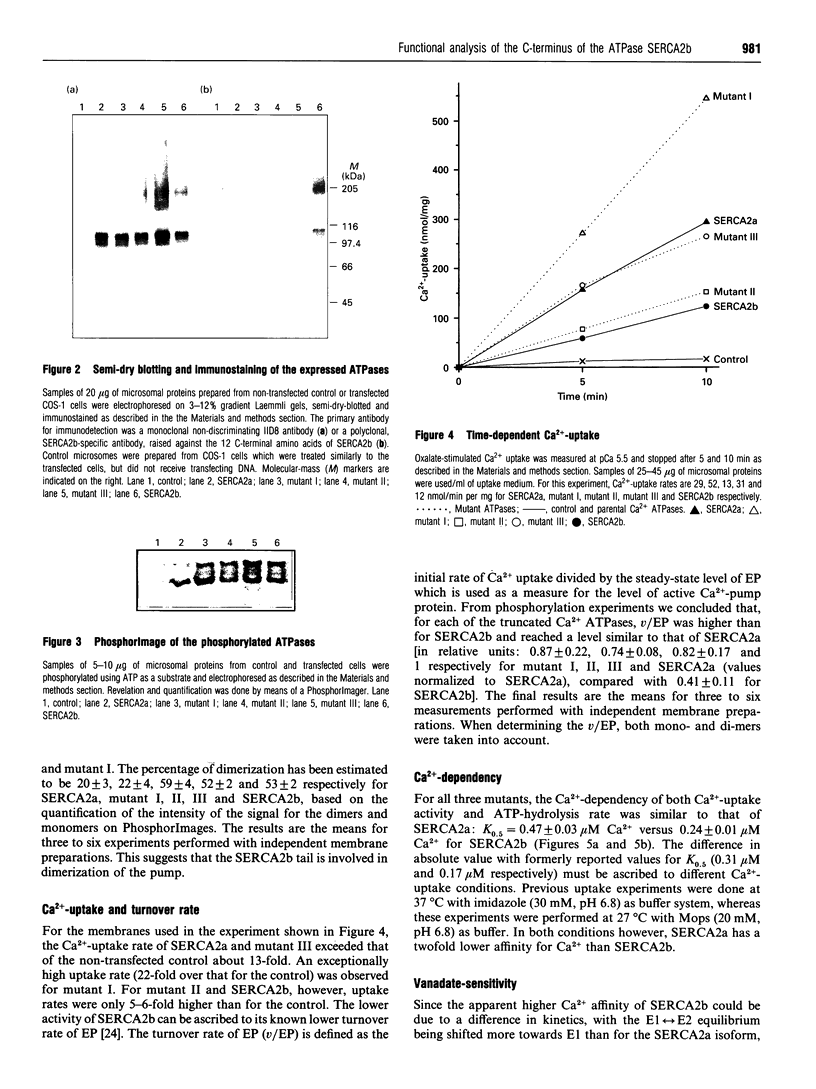
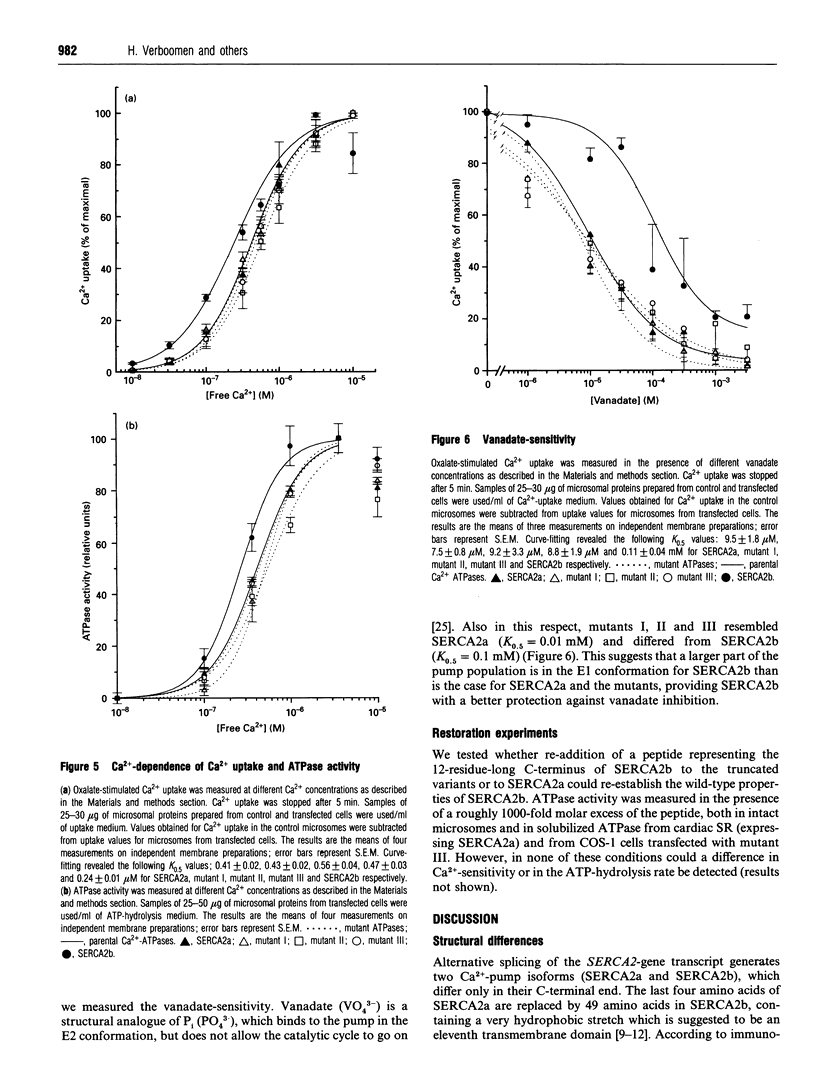


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandl C. J., deLeon S., Martin D. R., MacLennan D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987 Mar 15;262(8):3768–3774. [PubMed] [Google Scholar]
- Burk S. E., Lytton J., MacLennan D. H., Shull G. E. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989 Nov 5;264(31):18561–18568. [PubMed] [Google Scholar]
- Campbell A. M., Kessler P. D., Fambrough D. M. The alternative carboxyl termini of avian cardiac and brain sarcoplasmic reticulum/endoplasmic reticulum Ca(2+)-ATPases are on opposite sides of the membrane. J Biol Chem. 1992 May 5;267(13):9321–9325. [PubMed] [Google Scholar]
- Campbell A. M., Kessler P. D., Sagara Y., Inesi G., Fambrough D. M. Nucleotide sequences of avian cardiac and brain SR/ER Ca(2+)-ATPases and functional comparisons with fast twitch Ca(2+)-ATPase. Calcium affinities and inhibitor effects. J Biol Chem. 1991 Aug 25;266(24):16050–16055. [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Inesi G., MacLennan D. H. Location of high affinity Ca2+-binding sites within the predicted transmembrane domain of the sarcoplasmic reticulum Ca2+-ATPase. Nature. 1989 Jun 8;339(6224):476–478. doi: 10.1038/339476a0. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., MacLennan D. H. Functional consequences of alterations to polar amino acids located in the transmembrane domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990 Apr 15;265(11):6262–6267. [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., De Jaegere S., Nelles L., Casteels R. Evidence for two isoforms of the endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1989 Jun 15;260(3):757–761. doi: 10.1042/bj2600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., Verbist J., Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990 Nov 1;271(3):649–653. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Filoteo A. G., Gardos G., Penniston J. T. Calmodulin-binding domains from isozymes of the plasma membrane Ca2+ pump have different regulatory properties. J Biol Chem. 1991 May 15;266(14):8952–8956. [PubMed] [Google Scholar]
- Enyedi A., Verma A. K., Heim R., Adamo H. P., Filoteo A. G., Strehler E. E., Penniston J. T. The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem. 1994 Jan 7;269(1):41–43. [PubMed] [Google Scholar]
- Escalante R., Sastre L. Similar alternative splicing events generate two sarcoplasmic or endoplasmic reticulum Ca-ATPase isoforms in the crustacean Artemia franciscana and in vertebrates. J Biol Chem. 1993 Jul 5;268(19):14090–14095. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Huylebroeck D., Maertens G., Verhoeyen M., Lopez C., Raeymakers A., Jou W. M., Fiers W. High-level transient expression of influenza virus proteins from a series of SV40 late and early replacement vectors. Gene. 1988 Jun 30;66(2):163–181. doi: 10.1016/0378-1119(88)90354-x. [DOI] [PubMed] [Google Scholar]
- Inesi G., Cantilina T., Yu X., Nikic D., Sagara Y., Kirtley M. E. Long-range intramolecular linked functions in activation and inhibition of SERCA ATPases. Ann N Y Acad Sci. 1992 Nov 30;671:32–48. doi: 10.1111/j.1749-6632.1992.tb43782.x. [DOI] [PubMed] [Google Scholar]
- Korczak B., Zarain-Herzberg A., Brandl C. J., Ingles C. J., Green N. M., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. J Biol Chem. 1988 Apr 5;263(10):4813–4819. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., Westlin M., Burk S. E., Shull G. E., MacLennan D. H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992 Jul 15;267(20):14483–14489. [PubMed] [Google Scholar]
- MacLennan D. H., Clarke D. M., Loo T. W., Skerjanc I. S. Site-directed mutagenesis of the Ca2+ ATPase of sarcoplasmic reticulum. Acta Physiol Scand Suppl. 1992;607:141–150. [PubMed] [Google Scholar]
- Sasaki T., Inui M., Kimura Y., Kuzuya T., Tada M. Molecular mechanism of regulation of Ca2+ pump ATPase by phospholamban in cardiac sarcoplasmic reticulum. Effects of synthetic phospholamban peptides on Ca2+ pump ATPase. J Biol Chem. 1992 Jan 25;267(3):1674–1679. [PubMed] [Google Scholar]
- Tavernier J., Gheysen D., Duerinck F., Van der Heyden J., Fiers W. Deletion mapping of the inducible promoter of human IFN-beta gene. Nature. 1983 Feb 17;301(5901):634–636. doi: 10.1038/301634a0. [DOI] [PubMed] [Google Scholar]
- Van den Bosch L., Eggermont J., De Smedt H., Mertens L., Wuytack F., Casteels R. Regulation of splicing is responsible for the expression of the muscle-specific 2a isoform of the sarco/endoplasmic-reticulum Ca(2+)-ATPase. Biochem J. 1994 Sep 1;302(Pt 2):559–566. doi: 10.1042/bj3020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboomen H., Wuytack F., De Smedt H., Himpens B., Casteels R. Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J. 1992 Sep 1;286(Pt 2):591–595. doi: 10.1042/bj2860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wuytack F., Casteels R. Demonstration of a (Ca2+ + Mg2+)-ATPase activity probably related to Ca2+ transport in the microsomal fraction of porcine coronary artery smooth muscle. Biochim Biophys Acta. 1980 Jan 25;595(2):257–263. doi: 10.1016/0005-2736(80)90088-7. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Eggermont J. A., Raeymaekers L., Plessers L., Casteels R. Antibodies against the non-muscle isoform of the endoplasmic reticulum Ca2(+)-transport ATPase. Biochem J. 1989 Dec 15;264(3):765–769. doi: 10.1042/bj2640765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuytack F., Papp B., Verboomen H., Raeymaekers L., Dode L., Bobe R., Enouf J., Bokkala S., Authi K. S., Casteels R. A sarco/endoplasmic reticulum Ca(2+)-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. J Biol Chem. 1994 Jan 14;269(2):1410–1416. [PubMed] [Google Scholar]
- Yamasaki K., Yamamoto T. Existence of high- and low-affinity vanadate-binding sites on Ca(2+)-ATPase of the sarcoplasmic reticulum. J Biochem. 1991 Dec;110(6):915–921. doi: 10.1093/oxfordjournals.jbchem.a123689. [DOI] [PubMed] [Google Scholar]
- Zarain-Herzberg A., MacLennan D. H., Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2(+)-ATPase gene. J Biol Chem. 1990 Mar 15;265(8):4670–4677. [PubMed] [Google Scholar]