Abstract
Background
Cerebellar mutism syndrome (CMS) is characterized by deficits of speech, movement, and affect that can occur following tumor removal from the posterior fossa. The role of cerebro-cerebellar tract injuries in the etiology of CMS remains unclear, with recent studies suggesting that cerebro-cerebellar dysfunction may be related to chronic, rather than transient, symptomatology.
Methods
We measured functional connectivity between the cerebellar cortex and functional nodes throughout the brain using fMRI acquired after tumor removal but prior to adjuvant therapy in a cohort of 70 patients diagnosed with medulloblastoma. Surgical lesions were mapped to the infratentorial anatomy, and connectivity with cerebral cortex was tested for statistical dependence on extent of cerebellar outflow pathway injury.
Results
CMS diagnosis was associated with an increase in connectivity between the right cerebellar and left cerebral hemisphere, maximally between cerebellum and ventromedial prefrontal cortex (VM-PFC). Connectivity dependence on cerebellar outflow was significant for some speech nodes but not for VM-PFC, suggesting altered input to the cerebellum. Connectivity between posterior regions of cerebellar cortex and ipsilateral dentate nuclei was abnormal in CMS participants, maximally within the right cerebellar hemisphere.
Conclusions
The functional abnormalities we identified are notably upstream of where causal surgical injury is thought to occur, indicating a secondary phenomenon. The VM-PFC is involved in several functions that may be relevant to the symptomatology of CMS, including emotional control and motor learning. We hypothesize that these abnormalities may reflect maladaptive learning within the cerebellum consequent to disordered motor and limbic function by the periaqueductal gray and other critical midbrain targets.
Keywords: Cerebellar mutism syndrome, cerebellum, functional magnetic resonance imaging, medulloblastoma, posterior fossa syndrome
Key Points.
Input from VM-PFC to the cerebellum is prominent in cerebellar mutism syndrome (CMS).
Cerebellar cortical connectivity with the deep nuclei is altered in CMS.
These changes may affect cerebral function and contribute to cognitive, affective, and motor sequelae in CMS.
Importance of the Study.
Cerebellar mutism syndrome (CMS) is among the most devastating survivorship outcomes for pediatric patients with posterior fossa tumors. Recent evidence has shed light on the postoperative cerebellar and brainstem dysfunction likely responsible for characteristic symptoms like transient mutism, but it remains unclear how cerebello-cerebral circuits are involved in the symptomatology of CMS, or why mutism portends such poor outcome beyond the time of speech recovery. Here, we examine fMRI data collected over a 10-year period to identify patterns of cerebello-cerebral (and intra-cerebellar) dysfunction related to CMS. This is the first case–control study to examine cerebello-cerebral functional connectivity in CMS and uncovers previously obscure phenomena in the disease process affecting brain regions involved in cognitive and affective function.
Cerebellar mutism syndrome (CMS), also known as posterior fossa syndrome, is a disorder of impaired speech, movement, cognitive function, and emotional regulation that can emerge after surgery within the posterior cranial fossa.1 CMS is most commonly seen in pediatric patients following surgical removal of medulloblastoma (MB)—an embryonal tumor of the cerebellum and posterior brainstem, and the most common cancerous brain tumor in children.2 Perhaps the most enigmatic aspect of CMS is that the onset of its defining symptoms may be delayed by several hours or days following surgery, and these tend to resolve spontaneously after days or weeks in less severe cases, or several months to years in more severe cases.1,3 The delayed and transient nature of core symptoms suggests that the acute phase of the syndrome may represent a gain-of-dysfunction in remote areas connected to the site of injury rather than a primary, permanent loss-of-function. Importantly, while the verbal disruption and extreme emotional lability components of the syndrome resolve in most, CMS patients are often burdened by persistent deficits like ataxia, dysmetria, and cerebellar cognitive-affective symptoms that are more severe than patients without CMS diagnosis, negatively impacting the quality of life in this population of survivors.4
The cerebellum is interconnected with various regions of the brainstem, diencephalon, and cerebral cortex, and surgical injury to discrete subsets of these connections may differentially contribute to CMS symptoms that occur in different domains (ie, cognitive vs motor), or on different timescales. Of relevance to CMS, some premotor nuclei of the brainstem have been shown to generate neural patterns for the expression of behaviors such as walking,5 breathing,6 swallowing,7 and eye tracking,8 while others have been shown to gate the expression of conspicuous behaviors like phonation9 and volitional movement.10 Cerebellar nuclei project to the cerebrum via axons that traverse the superior cerebellar peduncles to form synapses in the red nuclei and thalamic nuclei. Through these projections, the cerebellum provides feedback to virtually all regions of the cerebral cortex,11 influencing the timing and coordination of movement sequences and selectively augmenting inter-areal neuronal communication within the cortex for sensorimotor and cognitive function.12
In previous investigations,13,14 we found considerable evidence that a loss in central coordination between phonation and articulation plays a role in the occurrence of mutism, resulting from damage to connections between deep cerebellar nuclei (especially the right fastigial nucleus) and the midbrain periaqueductal gray (PAG),14 an area responsible for the gating of vocalization9 and motor output for speech.15 In CMS participants, we also found evidence of elevated connectivity between cerebral cortical areas involved in the production of speech, which persisted beyond speech recovery.13 In the most affected areas (supplementary motor area and Broca’s area), individuals with the greatest abnormality were those with the least detectable injury to the right SCP—the cerebellar output tract putatively providing those areas with trans-synaptic input. These results highlight the complex role that cerebro-cerebellar loops might play in the manifestation of CMS, showing that surgical injury to cerebello-cerebral connections may be a meaningful source of variance within the group rather than a common driving factor of acute symptoms. Furthermore, if cerebellum-dependent abnormalities in cerebral dynamics impact cognitive and/or motor function, their persistent nature suggests this dysfunction may contribute predominantly to the long-term neurocognitive and motor sequelae, which can negatively impact quality of life for MB survivors years after CMS diagnosis.
A key question that follows is: Why does intact cerebellar output appear to contribute to cerebral dysfunction? Or, more tractably: How is communication between the cerebellum and the rest of the brain altered in patients with CMS? And can specific changes in communication offer clues about the cascade of functional changes that occur during the CMS disease process? To address these questions, we conducted an exploratory study using prospectively gathered fMRI and clinical data from participants enrolled in a clinical trial for the treatment of MB. We estimated functional connectivity between the cerebellum and the rest of the brain using cohort-specific functional nodes derived from independent component analysis of BOLD fMRI data, and compared these connectivity measures to identify functional differences between participants with CMS diagnosis and a group of asymptomatic MB participants of comparable age. We then investigated whether connectivity measures between cerebellum and supratentorial targets of interest exhibited a dependence on the integrity of the superior cerebellar peduncles estimated with T1 structural imaging. Lastly, we interrogated the possibility of intra-cerebellar changes in neuronal signaling by estimating connectivity between regions of the cerebellar cortex and ipsilateral dentate nuclei.
Materials and Methods
Participant Data and Diagnoses
Serial postoperative MR images were acquired in 183 children and adolescents enrolled in a prospective study of patients undergoing treatment for medulloblastoma (SJMB12; NCT 01878617). All patients underwent a standardized postoperative neurologic examination by a neurologist described in detail recently.1 Study participants were assigned to the following diagnostic groups based on the neurologic examination: Asymptomatic (patient experienced no remarkable speech, behavioral, or motor deficits), Complete Mutism (patient experienced an episode of complete mutism, usually with significant motor and behavioral symptoms), Reduced Speech (patient experienced abnormal inhibited speech with inability to complete a 3-word statement, usually with significant motor and behavioral symptoms), Atypical CMS (patient experienced behavioral symptoms and ataxia in the absence of speech deficits), and Ataxia Only (patient experienced ataxia and absence of independent gait without speech deficit or behavioral symptoms).
Image Acquisition and Data Selection
Structural and functional images were acquired during each imaging session using a 3T Siemens Skyra or Siemens Prisma scanner. Structural imaging consisted of a 3D T1 MPRAGE with 1.0mm isotropic resolution. Functional imaging consisted of a 6-minute, 47-second resting state fMRI scan with the following parameters: Single shot T2*-weighted EPI, TR = 2.26 s, TE = 30 ms, FOV = 192 mm, matrix = 64 × 64, voxel size = 3.0 mm isotropic, bandwidth = 2055 Hz/pixel. Imaging sessions occurred at the following timepoints relative to study enrollment: upon arrival at our institution after tumor resection (0 months), after completion of craniospinal radiation therapy (3 months), and at 12-, 18-, and 24 months from enrollment for routine follow up.
Image data from all timepoints in all participants were considered for node discovery analysis (nscans = 411), with exclusions made for structural or functional imaging of insufficient quality. Exploratory analyses were conducted using only 0-month timepoint data, during which participants with CMS diagnosis experienced acute verbal disruption. Three patients were excluded from the study due to recovery of 3-word speech capability more than a week prior to imaging. The asymptomatic MB participants served as the control group for connectivity analyses. Participants with Complete Mutism and Reduced Speech were combined to form the CMS group, as participants in both groups meet the consensus criteria for CMS diagnosis.16 Our investigation was further limited to acquisitions with participants under propofol sedation in accordance with a previous study of this cohort,13 since the use of sedation was necessary for a large majority of participants with CMS diagnosis. Participant diagnoses and process of exclusion are illustrated in Figure 1A, which yielded 32 participants in the CMS group and 38 participants in the asymptomatic MB group for this case–control study. Participant ages in the asymptomatic MB and CMS study groups were comparable (Figure 1B).
Figure 1.
Summary of data used for connectivity analyses. (A) Illustration of diagnostic makeup and exclusion processes for participants enrolled in the study. (B) Ages of participants within the test and control group of the study. After exclusions, there was no age difference between the 2 groups. (C) Functional nodes used in connectivity analyses are projected onto a standard 3D SPM 152 brain. Only projections onto the left cerebral/subcortical hemisphere are shown; node locations on the right side were comparable.
Preprocessing and Parcellation
Data preprocessing for whole-brain functional analyses was performed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), which included slice timing correction, motion correction, spatial normalization, and smoothing. Motion-corrected EPI time-series data were coregistered to the native T1 MPRAGE image within each session, and the T1 image was normalized to an anatomic template. The same transformation was then applied to the EPI data for spatial normalization of the fMRI time series. Intra-cerebellar connectivity analyses were conducted in SUIT space17 for improved inter-subject precision and more accurate alignment with cerebellar lesions that were mapped in SUIT space.
Following preprocessing and quality check, a parcellation was created using independent component analysis. This data-driven approach was chosen for node discovery to ensure an appropriate spatial parcellation scheme for the developmental range and clinical status of the MB cohort. 50 independent components were created using the GIFT toolbox (https://trendscenter.org/software/gift/). Of these 50 components, 27 were deemed likely to be of BOLD origin rather than noise after visual assessment of their spatial projections. Peaks in the spatial projections of these components were considered viable nodes if they contained the global maximum which was at least 5 standard deviations above the mean, or a local maximum that was at least 10 standard deviations above the mean. This resulted in the identification of 40 possible nodes for functional analysis. Thirty-three of these nodes were chosen for analysis after exclusions made for visual cortical nodes, which do not exhibit a functional connection with the cerebellum in healthy subjects.11 Nodes spanning the midline were not divided into left and right halves, with the exception of the cerebellar cortex. The component containing posterior cerebellum was divided into left and right hemispheric nodes by removing vermal tissue from the component mask; the vermis was discarded due to high incidence of vermal surgical damage in the CMS group. The resulting 2 cerebellar node masks overlapped with Crus I and II for each hemisphere, with a partial overlap of the ipsilateral tonsil. The following node ROIs were added from publicly available sources to aid in the study of cerebro-cerebellar interaction: left and right red nuclei,18 which receive input from the contralateral dentate nuclei; left and right dentate nuclei,17 which receive input from the ipsilateral cerebellar hemispheres; and a 100-cluster functional parcellation of the cerebellar cortex,19 which we employed in our final analysis to help reconcile the difference in spatial resolution between lesion mapping information and the fMRI data. Node projections are illustrated in Figure 1C.
Confounding signals from white matter and cerebrospinal fluid were removed from node time course prior to further analysis. For white matter, the signal was averaged from four 6 mm diameter spheroids embedded in the parietal and frontal portions of the corona radiata bilaterally (MNI coordinates: [+/−28, −28, and 29] and [+/−20, 38, and 6], respectively). For cerebrospinal fluid, a conservative mask was created within the lateral ventricles and mean time course was extracted. The time courses of functional nodes were then denoised by taking the residuals after linear regression of the noise signals.
Surgical Lesion Identification
Identification of surgical lesion location in each participant was necessary to help determine whether connectivity patterns were dependent on intact outflow from the cerebellum, and to exclude cerebellar clusters from connectivity analysis when they showed considerable overlap with surgical damage. For lesion identification, we used a customized algorithm and workflow described in detail previously.20 To quantify lesion load to the SCP, we used a probabilistic atlas of cerebellar white matter for anatomic reference21 with a 10% threshold applied. For each participant in the study, the proportion of overlap between the SCP mask and the surgical damage mask within each coronal slice was calculated. The maximum proportional value from all coronal slices from the center of the dentate nuclei (MNI y coordinate = −57) to the SCP decussation in the midbrain tegmentum was taken to represent lesion load for each participant. For connectivity analysis between cerebellar cortical clusters and ipsilateral dentate nuclei, a cluster was excluded for a given participant when its overlap with the surgical lesion exceeded 20%.
Connectivity Analyses
In all cases, connectivity values were calculated as the Pearson correlation coefficient between the time courses of functional node pairs. Connectivity values were first calculated between the cerebellar hemispheres and their potential efferent targets normatively connected by a direct (polysynaptic) neuronal pathway (including contralateral or midline cerebral nodes, ipsilateral dentate nuclei, contralateral red nuclei, and the rostral periaqueductal gray). Cortico-cortical node pairs were not analyzed since cerebral interaction is beyond the scope of the current study, nor were ipsilateral cerebellar and cerebral nodes since these structures are not connected by a direct pathway. Individual connectivity values were z-transformed prior to grouping, and a 2-tailed t-test was conducted for each node pair to quantify group differences in connectivity. For nodes of particular interest, the relationship between connectivity and integrity of the corresponding cerebellar outflow tract was estimated using the Spearman correlation method. All p values shown are uncorrected due to the exploratory nature of this study.
To identify regions of cerebellar cortex with CMS-related changes in connectivity to the ipsilateral dentate nucleus, a Monte Carlo hypothesis test was designed. Diagnoses among the participants were randomized 2000 times, surrogate t values were then calculated using a 2-tailed t-test, and the t values derived from real connectivity values were compared to the distribution of surrogate values for each cluster-dentate pair to estimate probability. Visualization of a smoothed t-value map and boundary of statistical significance (P < .05) was accomplished by smoothing the volumetric t-value map with a 3mm isotropic box kernel, then transforming this volume into a 2D map using the SUIT toolbox for surface-based rendering of cerebellar cortical anatomy.22
Ethical Approval
The protocol was approved by the St. Jude Institutional Review Board and was performed in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients and/or their legal guardians provided written informed consent prior to participation in the study.
Data Availability
The data which support the findings of this study are available from the corresponding authors, MAS and SSM, upon reasonable request.
Results
Increased Measures of Cerebro-Cerebellar Connectivity
We compared connectivity measures from CMS study participants to our asymptomatic MB group to test whether CMS-associated changes in brain function are apparent in measures of cerebro-cerebellar connectivity (Figure 2). We found that cerebro-cerebellar connectivity was elevated between right cerebellum and left cerebrum globally (P = .0145), and that the ventromedial prefrontal cortex was an outlier among these nodes (VM-PFC, t = 3.847, P = .0002). Individual t and p values for each node pair are listed in Supplementary Table 1.
Figure 2.
Cerebellar connectivity. Left: Cerebellar cortical connectivity with functional nodes throughout the brain. Colors indicate the putative resting state network association of the node. Activity of the VM-PFC has the strongest positive correlation with the cerebellar cortex. Right: Mean cerebello-cerebral connectivity in asymptomatic MB and cerebellar mutism syndrome (CMS) participants (not inclusive of Putamen, PAG, RN, and Dentate nodes). Connectivity was significantly increased between the left cerebral cortex and the right cerebellar cortex in participants with CMS.
Connectivity Between Right Cerebellum and VM-PFC
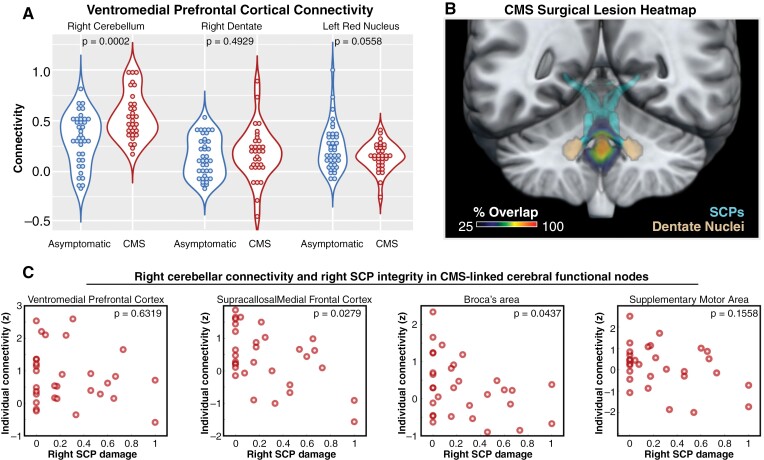
Having identified connectivity between right cerebellum and VM-PFC as uniquely affected in our CMS group, we chose to examine functional connectivity along this cerebello-cerebral pathway in greater detail (Figure 3A). We found that while VM-PFC connectivity was elevated in the cerebellar hemisphere in CMS (P = .0002), it was not significantly changed in the corresponding dentate (P = .4929) or red nucleus (P = .0558) relative to asymptomatic MB participants.
Figure 3.
Evaluation of Cortico-cerebello-cortical loop involving VM-PFC, and dependence of cerebellar outflow on cerebello-cortical functional connectivity. (A) Violin plots showing functional connectivity between VM-PFC and functional nodes along its putative cortico-cerebellar loop. Heightened influence of VM-PFC in the cerebellar cortex is not conveyed to the right dentate nucleus or the left red nucleus. (B): Heat map of surgical resections in study participants with cerebellar mutism syndrome. As in more focused studies of this cohort, complete transection of SCP or dentate nucleus was not the norm. (C) Analysis of the relationship between right SCP integrity (Per T1 imaging) and right cerebellar connectivity with nodes of interest. Damage of 0 indicates no detectable injury while 1 indicates complete transection. Notably, VM-PFC connectivity with the cerebellum does not show a dependence on SCP integrity, which might indicate a directional influence predominantly from the VM-PFC to the right cerebellar hemisphere. Other nodes implicated in speech disruption in a prior study did show varying degrees of dependence on SCP integrity, suggesting a directionality from cerebellum to cerebrum, based on dependence on cerebellar output.
SCP Integrity Influences Cerebellar Connectivity With Certain Cerebral Nodes
Having previously found cerebral functional abnormalities with statistical dependence on SCP integrity,13 we applied this approach to current measures to discern the impact of incidental cerebellar de-efferentation on cerebro-cerebellar connectivity (Figure 3B, C). Thus, we may be able to discriminate influences on connectivity that are predominantly afferent to the cerebellar cortex versus those that are dependent on cerebellar efference. We found that connectivity measures with VM-PFC were not dependent on the right SCP (Spearman’s P = .6319). As a check on the validity of this approach, we also evaluated SCP-connectivity dependence for cerebral nodes involved in speech that were found to be acutely affected in the previous study,13 namely Broca’s area, the supracallosal medial frontal cortex (M-FC), and the supplementary motor area. Cerebellar connectivity with M-FC and Broca’s area did exhibit a dependence on SCP integrity (Spearman’s P = .0279 and P = .0437, respectively), whereas the supplementary motor area did not (Spearman’s P = .1558).
Focal Changes in Cerebellar Cortico-Dentato Connectivity
Lastly, having found that connectivity with VM-PFC was elevated in the cerebellar cortex but not the dentate, and that the elevated connectivity was not dependent on SCP integrity, we investigated whether functional connectivity changed within the cerebellum to create this apparent mismatch between cerebellar input and output. Using a 100-cluster parcellation of the cerebellar cortex,19 we did indeed find regions with significant CMS-associated differences in connectivity to the ipsilateral dentate nuclei (Figure 4). 13 clusters contributed to a significant group difference, and the cluster with the greatest CMS-associated change in connectivity was located in the medial portion of right Crus II (t = −3.4759). Notably, the group difference was not solely due to diminished connectivity measures, but rather increased inverse connectivity in this area which exhibited weak cortico-dentato connectivity in asymptomatic MB participants (Figure 4A). Significant changes in the right hemisphere encompassed most of the posterior cerebellum, spanning the inferior edge of Crus I to the posterior limit of lobule IX (Figure 4B). While abnormalities were maximal in the right posterior cerebellar hemisphere, 2 groups of clusters in the left cerebellar hemisphere and one in the vermis also contributed to the significant group difference. Left hemispheric changes were centered on medial Crus II and lateral lobule VIIIA, whereas vermal changes spanned lobules VIIB, VIIIA, and VIIIB.
Figure 4.
Functional changes within the cerebellum. (A) Connectivity of cerebellar cortical clusters with the ipsilateral dentate nucleus. Areas that exhibit a greater functional association with ipsilateral dentate in asymptomatic patients are largely unaffected, while areas with a lesser connection have a diminished or inverse relationship. Colors indicate the putative resting state network association of the cluster, based on a canonical parcellation.11 (B) Flattened statistical map of the cerebellar cortex showing areas with significant changes in cortico-dentato connectivity.
Discussion
Cerebro-Cerebellar Connectivity in CMS
In this work, we studied how functional relationships between the cerebellum and contralateral cerebrum are altered in CMS as a potential contributor to cerebral dysfunction and long-term sequelae. For methodological context, the fMRI signal within the cerebellum is thought to be dominated by metabolic activity of the granular layer,23 and therefore its fluctuations largely reflect changes in synaptic input to the cerebellum rather than the product of computations that determine cerebellar cortical output.24 The outsized influence of synaptic input in cerebellar fMRI presumably obscures some degree of the efferent influence of the cerebellum in cerebro-cerebellar loops of interest. With this in mind, we leveraged information about individual surgical injuries to the efferent cerebellar pathway to help determine whether CMS-related connectivity changes were more dependent on efferent or afferent influence, with respect to the cerebellum.
Interestingly, we found functional connectivity between the right cerebellum and left cerebrum to be significantly elevated in participants with CMS. This is intriguing not only because these corresponding hemispheres are canonically involved in language function,25,26 but also because the currently prevailing theory of CMS pathogenesis contends that language disruption stems from the detrimental disconnection of these 2 key brain regions, due to surgical injury.27 We did find that SCP injury led to decreasing cerebellar connectivity with language-related areas within the CMS group, but only a minority of CMS participants (7 of the 32) had a lesion load over 50% in the right SCP, and connectivity appeared to be abnormally high in participants with little or no detectable SCP injury (for the 15 CMS participants with < 10% right SCP lesion load compared to asymptomatic MB group: MFC P = .0019, Broca P = .0263). Thus, the elevated connectivity we highlight here appears to be CMS-related, although driven by factors independent of SCP injury. In fact, the exacerbated difference in CMS participants with less apparent harm to the right SCP suggests that cerebellar efference might contribute to acute measures of cortico-PAG desynchronization related to mutism in those participants, as was demonstrated in a previous study of the current cohort.13
Ventromedial PFC-Cerebellar Interaction in Motor Learning
The cortical region found to be most affected in this study was the VM-PFC. In CMS participants, connectivity between VM-PFC and the posterior cerebellum was abnormally high, although this was not reflected in connectivity measures downstream of the cerebellar cortex, or in SCP-dependence of VM-PFC-cerebellar connectivity. Together this suggests that elevated VM-PFC-cerebellar connectivity reflects an increased influence of afferent input to the cerebellum temporally aligned with VM-PFC activity.
Interestingly, the right dentate nucleus did not exhibit any CMS-associated change in connectivity with VM-PFC, which led us to question whether cerebellar cortico-dentato signaling was appreciably altered in CMS patients. Indeed, posterior cerebellar cortex showed focal CMS-associated changes in signaling, marked by an acquired inverse correlation in cerebellar cortical areas where cortico-dentato connectivity was typically weak.
It is not obvious why patients with CMS exhibit these functional changes within the cerebellum, especially given that afferent influence and cortico-dentato signaling occur upstream of the causal surgical injury. We are left to hypothesize that, rather than a primary consequence of surgical injury, these abnormalities reflect a secondary consequence, such as adaptation in the cerebro-cerebellar network meant to compensate during the CMS disease process. Cerebellar learning is manifest as changes in synaptic efficacy between cortical neurons that lead to changes in signaling properties between cortex and deep nuclei,28 thus these abnormalities may reflect an outcome associated with cerebellar learning, which may have led to malfunction when operating in a pathologic context where surgical disruption of cerebello-midbrain circuits is superseding volitional efforts to speak and move.
To substantiate this point, it is important to review some key principles of cerebellar learning in the context of action planning involving the VM-PFC. Conceptually, appropriate motor action requires the processing of large amounts of spatial and contextual information. Motor sequences for appropriate action are precipitated by neocortical-basal-ganglia-cerebellar subnetwork interactions that decrease in abstraction as one (1) gains understanding of a context, (2) orients towards a specific goal, and (3) selects specific actions which align with that goal.29,30 These steps in the action planning process are thought to be predominantly subserved by (1) limbic, (2) associative, and (3) motor subnetworks, respectively.29 The VM-PFC is an instrumental part of the limbic subnetwork and is thought to contribute to cerebellar learning by providing the cerebellum with teaching signals reflecting success or failure in the execution of an action plan.29
The distributed limbic subnetwork comprised of VM-PFC, ventromedial striatum, and posterior cerebellum is particularly active during times of early motor learning,30 or when action choices do not reliably produce expected outcomes. Cerebellar learning relies on afferent signals that convey a chosen plan of action in conjunction with teaching signals, which include information about expectation-outcome mismatch after a selected action is executed,31 and prime the cerebellar cortex to make synaptic changes.28 Learning in the cerebellar system eventually crystallizes as changes in Purkinje cell responses to a given cerebellar afference pattern, which begets predictive responses to the neocortex and midbrain that can facilitate task performance.32
Applying this framework to CMS in the current study, the predominance of VM-PFC activity in the posterior cerebellar cortex may be due to the high rate of failure to carry out motor plans during a prolonged “model-free” state, induced by pathologic inhibition of motor and speech output by the midbrain. Additionally, we should consider that pathologic inhibition per se may be detrimental to cerebellar learning and function given the abundance of expectation-outcome mismatch it is likely to produce. In this case, maladaptive “learning” may result when the brain is over-primed to learn, especially in cerebellar cortical regions involved in volitional motor coordination for speech. Whether the mere fact of midbrain dysfunction leads to maladaptive changes in intact areas of the cerebellum remains to be explored, but it is tempting to consider similarities between cerebellar activation during verb generation25 and the map of cortico-dentato abnormalities found here, and to hypothesize that synaptic efficacy has been meaningfully shifted in the cerebellar modules responsible for speech-related planning. It is also interesting that inferior olivary hypertrophy has a well-established association with CMS,33,34 given that the inferior olive is considered the last common source in the pathway that provides teaching signals to the cerebellar cortex. Specific pathogenic mechanisms for this trans-synaptic injury remain obscure, but we would predict hyper-activation of inferior olivary neurons in the current framing, which could possibly contribute.
Ventromedial PFC in Emotional Control
The VM-PFC is involved in the emotional appraisal of behavioral options during action planning,35,36 and regulation of emotional response is instrumental to this process. Indeed, dysfunction of the VM-PFC has been implicated in range of psychological disorders with prominent affective components.35 The VM-PFC is unique among neocortical areas in its direct projections to subcortical structures which mediate emotional reaction, including the lateral hypothalamus, amygdalae, ventral striatum, and ventrolateral PAG.37 These connections may provide additional anatomical avenues where dysfunction in VM-PFC could mediate deficits in emotional regulation and memory function in CMS patients, and should be examined in future studies.
An Emerging Theory of CMS Pathogenesis
As its delayed onset suggests, CMS likely represents a cascade of functional changes consequent to a causal surgical injury, and untangling the primary effects of the injury from secondary effects that progress throughout the nervous system remains a defining challenge for CMS research. Previously, we identified the inferior vermis and fastigial nuclei as areas where surgical injury was associated with CMS diagnosis,14 and we uncovered evidence suggesting that remote dysfunction in the PAG likely contributes to transient mutism in CMS. We also found that cerebral function was persistently altered in CMS participants, and that the greatest abnormality was diminished by SCP injury, suggesting intact cerebellar outflow contributes to secondary cerebral dysfunction in CMS.
The current study adds a new layer to our understanding of the CMS disease process, by highlighting specific patterns of intrinsic cerebellar dysfunction that are independent of SCP injury. An emerging view of CMS is one in which fastigial-PAG dysfunction could drive core CMS symptoms like mutism and apraxia, while dentato-cerebral dysfunction could drive cerebellar motor and/or cognitive-affective symptoms that are often present but nonspecific to CMS. Here we acknowledge that dysfunction in one pathway could indirectly affect the other, and advance a mechanism by which fastigial-PAG dysfunction could aggravate dentato-cerebral network dysfunction, via maladaptive cerebellar learning. This may help explain why patients with complete mutism and apraxia almost always have more severe dysarthria and ataxia after the resolution of their acute symptoms.1
Theoretical interpretations aside, this study provides further evidence that dentato-cerebral tract injury and cerebello-cerebral diaschisis of speech-related cortices, specifically, are unlikely to serve as the primary drivers of the emblematic symptoms of CMS, as is generally accepted in contemporary scientific literature.38,39 Involvement of more primitive centers for phonation, volitional movement, and oculomotor control—adjacently located in the midbrain—has emerged as a more elegant explanation for the coincidence of these symptoms, and provides an anatomical and functional basis for the distinction between acute symptoms that are CMS-specific and chronic symptoms that are non-CMS-specific.
This study provides further evidence that cerebello-cerebral disconnection and cerebellar dysfunction in the absence of disconnection may have differential impacts on brain function and clinical presentation. Future studies of posterior fossa tumor survivors may be able to link specific patterns of cerebellar hemisphere, dentate, and SCP injury to specific areas of cerebral hypofunction, independent of CMS diagnosis. Surgical models of CMS-associated apraxia may also be tractable in rodents since fastigial-PAG projections are conserved,40 and these models could prove invaluable for the study of secondary plastic changes that may occur during the CMS disease process.
At this nascent stage of fMRI research in CMS, the implications of these findings for clinical practice are limited. Foremost, this study reemphasizes the need for surgical preservation of the brainstem, the superior cerebellar peduncles, the cerebellar commissure, the deep cerebellar nuclei, and the cerebellar vermis whenever possible to avoid the most devastating clinical outcomes for patients with posterior fossa tumors.
Limitations
This study has several important limitations that should be considered. The use of propofol sedation during acquisition is probably not ideal for the study of brain dynamics,41 but is necessary for diagnostic-quality imaging, especially in pediatric patients diagnosed with CMS. As in a previous study of this MB cohort,13 we selected asymptomatic patients with imaging acquired under sedation as our control group. We suspect that sedation may reduce neuronal signal-to-noise ratio in our study, but there is no reason to anticipate differential effects of sedation in CMS and asymptomatic MB patients that would otherwise confound the current findings.
While our question is not focused on neuronal mechanisms of acute mutism, we focused our analyses on data gathered during the acute period of the syndrome, typically weeks removed from the time of surgery (range 11 to 28 days from surgery for CMS participants, median 20 days). This was necessary to avoid potential confounds of cancer treatment, with posterior fossa radiation that varied between patients. We were further motivated to avoid challenges with cerebellar normalization for functional analyses, as signs of cerebellar atrophy accumulated further from surgery and adjuvant therapy.
The estimation of SCP injury based on T1 imaging was chosen as a conservative measure, but does not preclude the possibility that undetected microstructural injury has altered the viability of axons which appear intact. While we have tractographic data at our disposal, our view is that our conservative and binary estimation based on 3D T1 imaging provides the least ambiguity in terms of an individual’s loss of function. We also used a conservative measure for surgical injury within the cerebellum, based on an estimation of the anatomy that was resected.20 Since it remains unclear precisely how postsurgical factors with imaging signatures (eg, focal edema) affect neuronal function, surgically spared cerebellar regions with focal abnormalities were not included in the binary cerebellar injury mask.
We excluded the posterior vermis from whole-brain analysis with the knowledge that vermal injury was common in CMS, but included clusters located in the posterior vermis within our cerebellar cortico-dentate analysis since the parcellation scheme offered full coverage of the cerebellar cortex. As mentioned in the methodology, clusters were excluded from analysis on an individual basis when they overlapped with the surgical lesion by 20% or more, and statistically significant changes were still localized to the vermis after the appropriate exclusions were made. This should be interpreted with caution relative to other areas in the cerebellar analysis, because of previously established differences in the incidence of damage in vermal tissue between these groups.14 Additionally, use of ipsilateral dentate nucleus as the reference node for connectivity analysis is not anatomically proper for the vermis, since vermal cortex projects predominantly to the fastigial and interposed nuclei.
Relatively little is known about the neuronal codes that govern communication between Purkinje and deep nuclei neurons at the individual and population level, and still less is known about how signaling properties between the cerebellar cortex and deep cerebellar nuclei are reflected on the scale we can evaluate using fMRI. Analytic tools for precision analysis of cerebellar function in humans are still relatively new,17,42 and often require extensive customization for use in disease study and/or pediatric populations.20 This history may be to blame for the lack of relevant studies for comparison, and as such we cannot make any specific inference about what synaptic (or other) changes occur in the cerebellar cortex leading to the highlighted cortico-dentato abnormality. However, these tools have proven useful in recent years to highlight functional changes in cortico-dentato connectivity associated with aging,43 autism spectrum disorder,44 and multiple sclerosis,45 suggesting that these measures do reflect meaningful physiological change.
This study was designed to highlight changes in cerebellar and cerebral function that may underlie symptom etiologies that are specific to CMS among participants with MB, and as such there is no comparison to a healthy control group. Due to this study design, there may be meaningful patterns of dysfunction in the CMS group which may have gone undetected if they are also present in the Asymptomatic MB group.
Lastly, CMS is a very complex disorder with many associated symptoms, and methods of diagnosing and categorizing patients with these symptoms can vary between clinics. In our study of the current cohort, we have attempted to reduce the complexity of the issue by focusing primarily on mutism and inhibited speech as the defining feature of the CMS group, and using largely asymptomatic MB participants as our control group. Furthermore, clinical data were collected prospectively irrespective of presumption of CMS by a single pediatric neurologist (RBK) to reduce inter-examiner variability that might influence diagnosis.
Conclusions
We used fMRI and diagnostic data acquired in a prospective study of patients undergoing treatment for medulloblastoma to investigate CMS-associated changes in cerebellar function that may contribute to patterns of cerebral dysfunction identified in a previous study.13 We found that the most striking abnormalities were upstream of the causal surgical injury within the cerebellum, suggesting that these specific CMS-associated abnormalities are secondary to the primary functional consequences of cerebellar surgical injury. We hypothesize that these functional abnormalities are indirectly caused by pathologic inhibition of volitional action by the midbrain. Their apparent secondary nature suggests that they may be modifiable, and reducing the severity of these functional abnormalities may lead to improved long-term outcomes in MB survivors after CMS diagnosis.
Supplementary material
Supplementary material is available online at Neuro-Oncology (https://academic.oup.com/neuro-oncology).
Contributor Information
Samuel S McAfee, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Giles Robinson, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Amar Gajjar, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Nicholas S Phillips, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Silu Zhang, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Ping Zou Stinnett, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Ranganatha Sitaram, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Darcy Raches, Department of Psychology and Biobehavioral Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Heather M Conklin, Department of Psychology and Biobehavioral Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Raja B Khan, Division of Neurology, Department of Pediatrics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Matthew A Scoggins, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Funding
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and the National Cancer Institute grant (P30 CA021765; Cancer Center Support Grant). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest statement
None to declare.
Authorship statement
Study design: S.S.M. and M.A.S. Clinical trial: A.G., G.R. Development of diagnostic criteria: R.K., H.M.C., and D.R. fMRI data preprocessing: S.S.M. and P.Z. Lesion analyses: S.Z., S.S.M., and M.A.S. fMRI data analysis and interpretation: S.S.M., N.P., R.S., and M.A.S. Writing: S.S.M. Editing: S.S.M. and M.A.S.
References
- 1. Khan RB, Patay Z, Klimo P, et al. Clinical features, neurologic recovery, and risk factors of post-operative posterior fossa syndrome and delayed recovery: A prospective study. Neuro Oncol. 2021;23(9):1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cassia GSE, Alves C, Taranath A, et al. Childhood medulloblastoma revisited. Top Magn Reson Imaging. 2018;27(6):479–502. [DOI] [PubMed] [Google Scholar]
- 3. Catsman-Berrevoets CE. Cerebellar mutism syndrome: Cause and rehabilitation. Curr Opin Neurol. 2017;30(2):133–139. [DOI] [PubMed] [Google Scholar]
- 4. Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caggiano V, Leiras R, Goni-Erro H, et al. Midbrain circuits that set locomotor speed and gait selection. Nature. 2018;553(7689):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krohn F, Novello M, van der Giessen RS, et al. The integrated brain network that controls respiration. Elife. 2023;12:e83654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuhuber W, Bieger D.. Brainstem control of deglutition: brainstem neural circuits and mediators regulating swallowing. In: Shaker R, Belafsky PC, Postma GN, Easterling C, eds. Principles of Deglutition: A Multidisciplinary Text for Swallowing and its Disorders. New York, NY: Springer New York; 2013:89–113. [Google Scholar]
- 8. Fukushima K. The interstitial nucleus of Cajal and its role in the control of movements of head and eyes. Prog Neurobiol. 1987;29(2):107–192. [DOI] [PubMed] [Google Scholar]
- 9. Michael V, Goffinet J, Pearson J, et al. Circuit and synaptic organization of forebrain-to-midbrain pathways that promote and suppress vocalization. Elife. 2020;9:e63493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tovote P, Esposito MS, Botta P, et al. Midbrain circuits for defensive behaviour. Nature. 2016;534(7606):206–212. [DOI] [PubMed] [Google Scholar]
- 11. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT.. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McAfee SS, Liu Y, Sillitoe RV, Heck DH.. Cerebellar coordination of neuronal communication in cerebral cortex. Front Syst Neurosci. 2021;15:781527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAfee SS, Robinson G, Gajjar A, et al. Cerebellar mutism is linked to midbrain volatility and desynchronization from speech cortices. Brain. 2023;146(11):4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McAfee SS, Zhang S, Zou P, et al. Fastigial nuclei surgical damage and focal midbrain disruption implicate PAG survival circuits in cerebellar mutism syndrome. Neuro Oncol. 2023;25(2):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holstege G, Subramanian HH.. Two different motor systems are needed to generate human speech. J Comp Neurol. 2016;524(8):1558–1577. [DOI] [PubMed] [Google Scholar]
- 16. Gudrunardottir T, Morgan AT, Lux AL, et al. ; Iceland Delphi Group. Consensus paper on post-operative pediatric cerebellar mutism syndrome: The Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 17. Diedrichsen J, Maderwald S, Kuper M, et al. Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage. 2011;54(3):1786–1794. [DOI] [PubMed] [Google Scholar]
- 18. Keuken MC, Forstmann BU.. A probabilistic atlas of the basal ganglia using 7 T MRI. Data Brief. 2015;4:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ren Y, Guo L, Guo CC.. A connectivity-based parcellation improved functional representation of the human cerebellum. Sci Rep. 2019;9(1):9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang S, McAfee S, Patay Z, Pinto S, Scoggins MA.. Automatic detection and segmentation of postoperative cerebellar damage based on normalization. Neurooncol. Adv. 2023;5(1):vdad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Baarsen KM, Kleinnijenhuis M, Jbabdi S, Sotiropoulos SN, Grotenhuis JA, van Cappellen van Walsum AM.. A probabilistic atlas of the cerebellar white matter. Neuroimage. 2016;124(Pt A):724–732. [DOI] [PubMed] [Google Scholar]
- 22. Diedrichsen J, Zotow E.. Surface-based display of volume-averaged cerebellar imaging Data. PLoS One. 2015;10(7):e0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howarth C, Peppiatt-Wildman CM, Attwell D.. The energy use associated with neural computation in the cerebellum. J Cereb Blood Flow Metab. 2010;30(2):403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Léna C, Popa D.. Chapter 6 - cerebrocerebellar loops in the rodent brain. In: Heck DH, ed. The Neuronal Codes of the Cerebellum. San Diego: Academic Press; 2016:135–153. [Google Scholar]
- 25. King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J.. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22(8):1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marien P, Verhoeven J, Engelborghs S, et al. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin Neurol Neurosurg. 2006;108(5):518–522. [DOI] [PubMed] [Google Scholar]
- 27. Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31(2):288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carey MR. Synaptic mechanisms of sensorimotor learning in the cerebellum. Curr Opin Neurobiol. 2011;21(4):609–615. [DOI] [PubMed] [Google Scholar]
- 29. Bostan AC, Strick PL.. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat Rev Neurosci. 2018;19(6):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fermin AS, Yoshida T, Yoshimoto J, et al. Model-based action planning involves cortico-cerebellar and basal ganglia networks. Sci Rep. 2016;6:31378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito M. Error detection and representation in the olivo-cerebellar system. Front Neural Circuits. 2013;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Welniarz Q, Worbe Y, Gallea C.. The forward model: A unifying theory for the role of the cerebellum in motor control and sense of agency. Front Syst Neurosci. 2021;15:644059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avula S, Spiteri M, Kumar R, et al. Post-operative pediatric cerebellar mutism syndrome and its association with hypertrophic olivary degeneration. Quant Imaging Med Surg. 2016;6(5):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patay Z, Enterkin J, Harreld JH, et al. MR imaging evaluation of inferior olivary nuclei: Comparison of postoperative subjects with and without posterior fossa syndrome. AJNR Am J Neuroradiol. 2014;35(4):797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hiser J, Koenigs M.. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83(8):638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy M, Shohamy D, Wager TD.. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. [DOI] [PubMed] [Google Scholar]
- 38. Ashida R, Nazar N, Edwards R, Teo M.. Cerebellar mutism syndrome: An overview of the pathophysiology in relation to the cerebrocerebellar anatomy, risk factors, potential treatments, and outcomes. World Neurosurg. 2021;153:63–74. [DOI] [PubMed] [Google Scholar]
- 39. Catsman-Berrevoets C, Patay Z.. Cerebellar mutism syndrome. Handb Clin Neurol. 2018;155:273–288. [DOI] [PubMed] [Google Scholar]
- 40. Vaaga CE, Brown ST, Raman IM.. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. Elife. 2020;9:e54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu X, Lauer KK, Douglas Ward B, et al. Propofol attenuates low-frequency fluctuations of resting-state fMRI BOLD signal in the anterior frontal cortex upon loss of consciousness. Neuroimage. 2017;147:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N.. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. [DOI] [PubMed] [Google Scholar]
- 43. Bernard JA, Ballard HK, Jackson TB.. Cerebellar dentate connectivity across adulthood: A large-scale resting state functional connectivity investigation. Cereb Cortex Commun. 2021;2(3):tgab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anteraper SA, Guell X, Taylor HP, et al. Intrinsic functional connectivity of dentate nuclei in autism spectrum disorder. Brain Connect. 2019;9(9):692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tona F, De Giglio L, Petsas N, et al. Role of cerebellar dentate functional connectivity in balance deficits in patients with multiple sclerosis. Radiology. 2018;287(1):267–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data which support the findings of this study are available from the corresponding authors, MAS and SSM, upon reasonable request.