Abstract
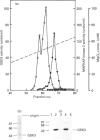
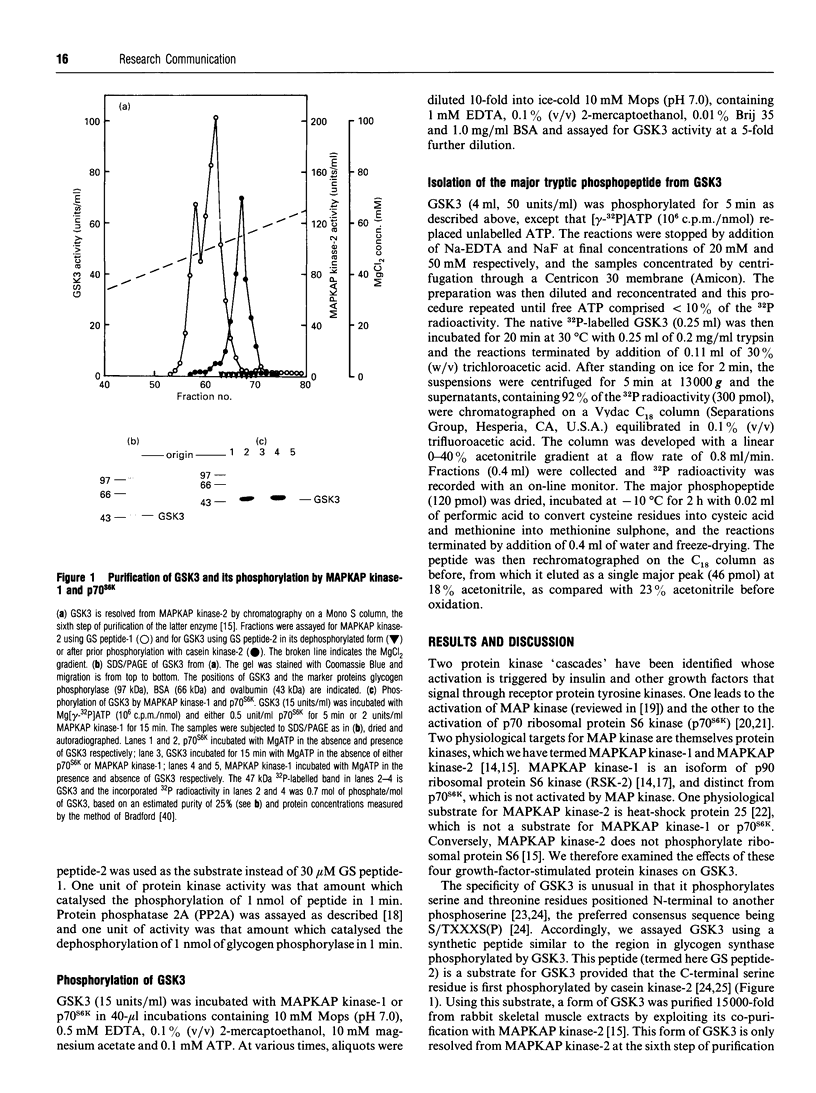
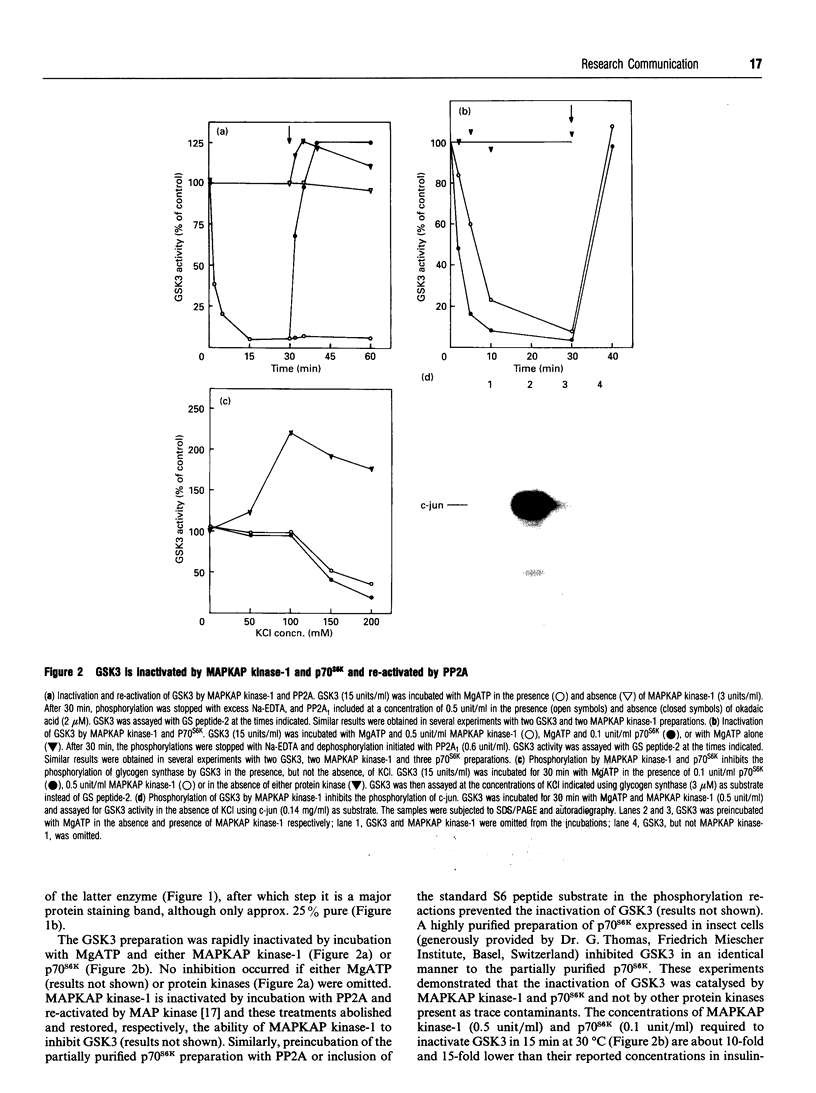
The beta-isoform of glycogen synthase kinase-3 (GSK3 beta) isolated from rabbit skeletal muscle was inactivated 90-95% following incubation with MgATP and either MAP kinase-activated protein kinase-1 (MAPKAP kinase-1, also termed RSK-2) or p70 S6 kinase (p70S6K), and re-activated with protein phosphatase 2A. MAPKAP kinase-1 and p70S6K phosphorylated the same tryptic peptide on GSK3 beta, and the site of phosphorylation was identified as the serine located nine residues from the N-terminus of the protein. The inhibitory effect of Ser-9 phosphorylation on GSK3 beta activity was observed with three substrates, (inhibitor-2, c-jun and a synthetic peptide), and also with glycogen synthase provided that 0.15 M KCl was added to the assays. The results suggest that Ser-9 phosphorylation underlies the reported inhibition of GSK3 beta by insulin and that GSK3 may represent a point of convergence of two major growth-factor-stimulated protein kinase cascades.
Full text
PDF
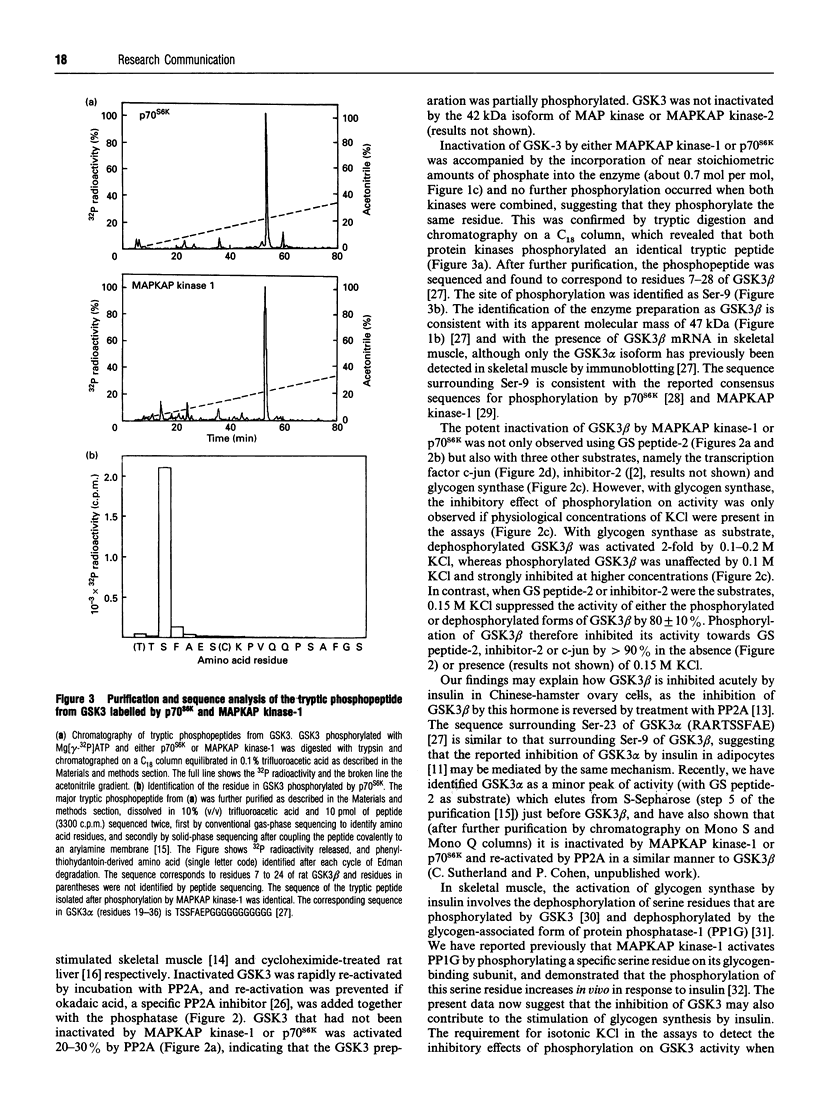

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou L. M., Luther H., Thomas G. MAP2 kinase and 70K S6 kinase lie on distinct signalling pathways. Nature. 1991 Jan 24;349(6307):348–350. doi: 10.1038/349348a0. [DOI] [PubMed] [Google Scholar]
- Blenis J., Chung J., Erikson E., Alcorta D. A., Erikson R. L. Distinct mechanisms for the activation of the RSK kinases/MAP2 kinase/pp90rsk and pp70-S6 kinase signaling systems are indicated by inhibition of protein synthesis. Cell Growth Differ. 1991 Jun;2(6):279–285. [PubMed] [Google Scholar]
- Bourouis M., Moore P., Ruel L., Grau Y., Heitzler P., Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990 Sep;9(9):2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Alemany S., Hemmings B. A., Resink T. J., Strålfors P., Tung H. Y. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- Cohen P. Dissection of the protein phosphorylation cascades involved in insulin and growth factor action. Biochem Soc Trans. 1993 Aug;21(3):555–567. doi: 10.1042/bst0210555. [DOI] [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Donella-Deana A., Lavoinne A., Marin O., Pinna L. A., Cohen P. An analysis of the substrate specificity of insulin-stimulated protein kinase-1, a mammalian homologue of S6 kinase-II. Biochim Biophys Acta. 1993 Aug 18;1178(2):189–193. doi: 10.1016/0167-4889(93)90008-d. [DOI] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [PubMed] [Google Scholar]
- Erikson R. L. Structure, expression, and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991 Apr 5;266(10):6007–6010. [PubMed] [Google Scholar]
- Flotow H., Thomas G. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem. 1992 Feb 15;267(5):3074–3078. [PubMed] [Google Scholar]
- Goode N., Hughes K., Woodgett J. R., Parker P. J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992 Aug 25;267(24):16878–16882. [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Resink T. J., Cohen P. Reconstitution of a Mg-ATP-dependent protein phosphatase and its activation through a phosphorylation mechanism. FEBS Lett. 1982 Dec 27;150(2):319–324. doi: 10.1016/0014-5793(82)80760-6. [DOI] [PubMed] [Google Scholar]
- Hughes K., Ramakrishna S., Benjamin W. B., Woodgett J. R. Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J. 1992 Nov 15;288(Pt 1):309–314. doi: 10.1042/bj2880309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoinne A., Erikson E., Maller J. L., Price D. J., Avruch J., Cohen P. Purification and characterisation of the insulin-stimulated protein kinase from rabbit skeletal muscle; close similarity to S6 kinase II. Eur J Biochem. 1991 Aug 1;199(3):723–728. doi: 10.1111/j.1432-1033.1991.tb16176.x. [DOI] [PubMed] [Google Scholar]
- Matter N., Ritz M. F., Freyermuth S., Rogue P., Malviya A. N. Stimulation of nuclear protein kinase C leads to phosphorylation of nuclear inositol 1,4,5-trisphosphate receptor and accelerated calcium release by inositol 1,4,5-trisphosphate from isolated rat liver nuclei. J Biol Chem. 1993 Jan 5;268(1):732–736. [PubMed] [Google Scholar]
- Nikolakaki E., Coffer P. J., Hemelsoet R., Woodgett J. R., Defize L. H. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993 Apr;8(4):833–840. [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Picton C., Woodgett J., Hemmings B., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982 Dec 13;150(1):191–196. doi: 10.1016/0014-5793(82)81332-x. [DOI] [PubMed] [Google Scholar]
- Plyte S. E., Hughes K., Nikolakaki E., Pulverer B. J., Woodgett J. R. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992 Dec 16;1114(2-3):147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Price D. J., Nemenoff R. A., Avruch J. Purification of a hepatic S6 kinase from cycloheximide-treated Rats. J Biol Chem. 1989 Aug 15;264(23):13825–13833. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Cyclic nucleotide-independent protein kinase from rat liver. Purification and characterization of a multifunctional protein kinase. J Biol Chem. 1985 Oct 5;260(22):12280–12286. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Insulin action rapidly decreases multifunctional protein kinase activity in rat adipose tissue. J Biol Chem. 1988 Sep 5;263(25):12677–12681. [PubMed] [Google Scholar]
- Ruel L., Bourouis M., Heitzler P., Pantesco V., Simpson P. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature. 1993 Apr 8;362(6420):557–560. doi: 10.1038/362557a0. [DOI] [PubMed] [Google Scholar]
- Ruel L., Pantesco V., Lutz Y., Simpson P., Bourouis M. Functional significance of a family of protein kinases encoded at the shaggy locus in Drosophila. EMBO J. 1993 Apr;12(4):1657–1669. doi: 10.1002/j.1460-2075.1993.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E., Chou T. B., Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992 Dec 24;71(7):1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Perkins L. A., Capaci T. M., Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990 Jun 28;345(6278):825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Leevers S. J., Marshall C., Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992 Nov;11(11):3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D., Engel K., Campbell D. G., Cohen P., Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992 Nov 30;313(3):307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Strålfors P., Hiraga A., Cohen P. The protein phosphatases involved in cellular regulation. Purification and characterisation of the glycogen-bound form of protein phosphatase-1 from rabbit skeletal muscle. Eur J Biochem. 1985 Jun 3;149(2):295–303. doi: 10.1111/j.1432-1033.1985.tb08926.x. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Campbell D. G., Cohen P. Identification of insulin-stimulated protein kinase-1 as the rabbit equivalent of rskmo-2. Identification of two threonines phosphorylated during activation by mitogen-activated protein kinase. Eur J Biochem. 1993 Mar 1;212(2):581–588. doi: 10.1111/j.1432-1033.1993.tb17696.x. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993 Sep 15;294(Pt 3):625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R. Use of peptide substrates for affinity purification of protein-serine kinases. Anal Biochem. 1989 Aug 1;180(2):237–241. doi: 10.1016/0003-2697(89)90423-5. [DOI] [PubMed] [Google Scholar]