Abstract
Background
Primary liver cancer is the third leading cause of cancer deaths worldwide and has one of the worst 5-year survival rates. This study examines US primary liver cancer incidence and incidence-based mortality trends over four decades.
Research design and methods
The SEER-9 registry was used to study primary liver cancer cases from 1978 to 2018. The incidence and mortality rates were calculated based on gender, age, race, and stage of diagnosis. Joinpoint regression software was used to calculate the annual percent change.
Results
The overall incidence rate of primary liver cancer from 1978 to 2018 increased by 2.71%/year (p<0.001). Rates in patients <50 years old began to fall in 2002 at a rate of -3.62%/year (p<0.001). Similarly, the incidence-based mortality rates for primary liver cancer increased by 2.15%/year (p<0.001). Whereas Whites incidence-based mortality rates began to plateau in 2012 (0.18%/year; p = 0.84), Blacks rates have declined since 2010 (-2.93%/year; p = 0.03), and Asian rates have declined since 1999 (-1.30%/year; p<0.001).
Conclusion
While the overall primary liver cancer incidence and incidence-based mortality have been increasing over the last four decades, there was an observed decline in incidence and incidence-based mortality in recent years, especially among at-risk subgroups.
Introduction
Primary liver cancer (PLC) is the seventh most diagnosed cancer and the third leading cause of cancer-related death worldwide, causing an estimated 830,180 deaths in 2020 [1]. Worldwide, the PLC incidence rates continue to rise, particularly in the United States (US). Additionally, the 5-year survival rates remain one of the worst among all cancers, with rates below twenty percent [2, 3].
The two main subtypes of PLC are hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), with HCC comprising around eighty-five percent of all liver cancer cases [4]. HCC primarily complicates longstanding cirrhosis, exacerbated by factors such as chronic viral hepatitis, alcoholic and non-alcoholic fatty liver disease, as well as aflatoxin exposure [5]. While risk factors for ICC are less specific, they also include cirrhosis, viral hepatitis, primary sclerosing cholangitis, and liver fluke infections (in Asia) [6]. Regional and age-related variations exist for different exposures and are reflected in the changing incidence trends observed within these subgroups [7]. Implementation of early screening protocols, large public health initiatives, and advanced management protocols for the underlying liver disease has significantly impacted the incidence and survival rates of HCC [8]. This has translated into early detection and improved survival of HCC patients in the last two decades [9]. Reports on the decreasing rates seen among certain at-risk subgroups of HCC suggest that there could be a decline in the incidence of PLC in the near future [10].
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute has been an essential resource for cancer epidemiology for decades [11]. This continuous monitoring of disease patterns and population characteristics has allowed for an increased understanding of the burden of PLC, better assessment of the quality of healthcare delivery, and identifying gaps in preventive measures. Quantitative analysis of incidence rates and etiology-based survival in HCC patients has been reported [5, 12]. PLC incidence-based rates (IBR) and incidence-based mortality rates (IBMR) were examined over the last four decades using the SEER database. Henceforth, this article aims to add an updated understanding of the changing trends of PLC in the US.
Material and methods
The incidence and mortality data for primary liver cancer (PLC) were obtained from SEER-9, a population-based cancer registry that covers approximately 9.4% of the US population from 1978 to 2018 [11]. Cases were identified using specific International Classification of Diseases for Oncology, 3rd edition, codes (site: C22.0 and C22.1; histology: 8160, 8162, 8170–8175, and 8180). Only cases of malignant PLC were included in the analysis, while cases without active follow-up or those diagnosed solely based on autopsy or death certificates were excluded.
Staging information was consolidated using a merged variable. From 1973 to 2015, the ’SEER historic stage A’ was utilized, while from 2016 to 2018, the ’Combined summary stage (+2004)’ was employed.
Statistical analysis
Both incidence-based mortality rates (IBMR) and incidence-based rates (IBR) were age-standardized to the 2000 US population. The calculation of IBMR and IBR took into account variables such as age, sex, ethnicity/race, and stage at the time of diagnosis. IBMR was determined by dividing the number of PLC deaths among cases by the person-time at risk within the SEER sites [11]. Population adjustments for the Katrina and Rita Hurricanes were not applied.
To examine trends over time, annual percent changes (APCs) were estimated using Joinpoint regression. This statistical analysis identifies the calendar years with significant annual percentage changes by selecting the optimal log-linear regression model with the minimum number of joinpoints required to accurately fit the data [13]. The model with the fewest number of Joinpoints, yet providing the best fit, was selected for analysis [14].
Ethics approval and consent to participate
This study is based on the use of de-identified public data from the SEER database. It does not involve interaction with human subjects or the use of personal identifying information. The study did not require informed consent from the SEER-registered cases, and the authors obtained Limited-Use Data Agreements from SEER. No trial registration was necessary.
Results
PLC incidence-based rates
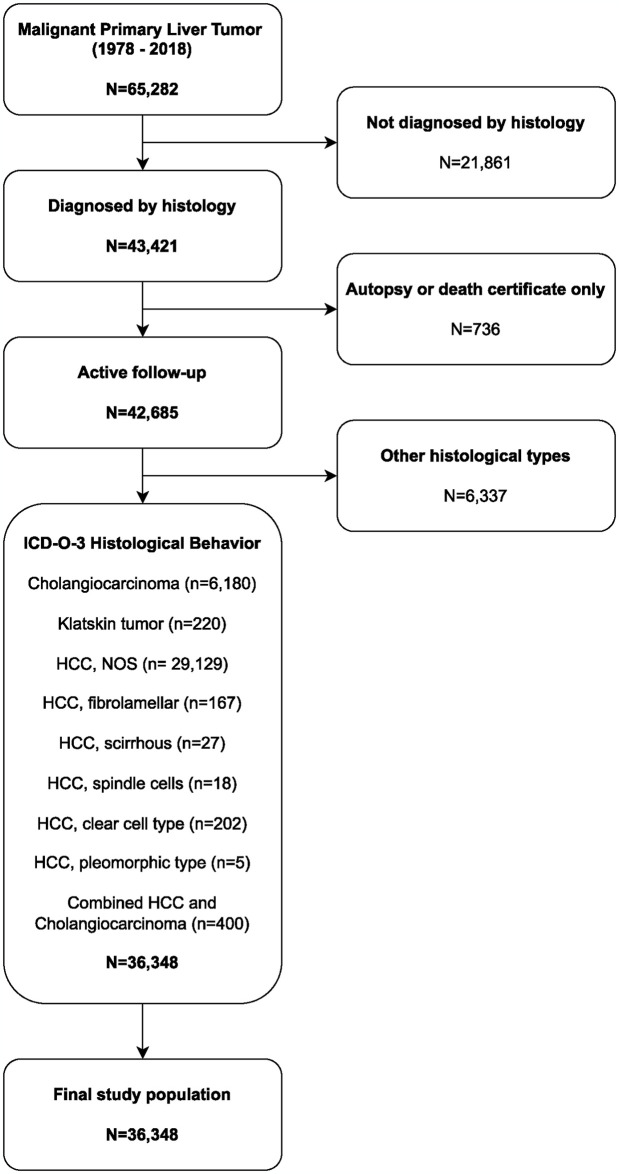
Malignant PLC was diagnosed in 65,282 patients between 1978 and 2018. Our study included 36,348 of them (Fig 1). The majority of the cases (71.39% [n = 25,949]) were men, 66.44% [n = 24,148] were White, 89.61% [n = 32,573] were over 50, and 34.22% [n = 12,439] had localized cancer. The liver (85.25% [n = 30,985]) was the most common subsite for PLC, followed by the intrahepatic biliary duct (14.75% [n = 5,363]). Table 1 summarizes the patient demographics and age-standardized IBR.
Fig 1. Study flowchart design and patient selection.
Table 1. Age-standardized incidence rates and annual percent changes in primary liver cancer rates, 1978–2018.
| Incidence-based Rates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment 1 | Segment 2 | Segment 3 | Segment 4 | Segment 5 | Incidence rates Per 100,000 |
||||||||
| AAPC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | 2017 | 2018 | |
| Overall | 2.71 (2.40, 3.20) | 1978–2007 | 3.86 (3.54, 4.18) | 2007–2018 | -0.26 (-1.07, 0.56) | 4.60 | 4.01** | ||||||
| Sex | |||||||||||||
| Men | 2.52 (2.16, 2.89) | 1978–2007 | 3.78 (3.41, 4.15) | 2007–2018 | -0.72 (-1.64, 0.21) | 6.96 | 6.11** | ||||||
| Women | 2.73 (2.32, 3.13) | 1978–2008 | 3.43 (3.06, 3.81) | 2008–2018 | 0.64 (-0.57, 1.86) | 2.56 | 2.21** | ||||||
| Age, years | |||||||||||||
| <50 | 1.13 (0.48, 1.78) | 1978–2002 | 4.41 (3.58, 5.25) | 2002–2018 | -3.62(-4.71, -2.51) | 0.44 | 0.46 | ||||||
| ≥50 | 2.91 (2.58, 3.25) | 1978–2008 | 3.91 (3.59, 4.24) | 2008–2018 | -0.03(-1.00, 0.95) | 15.48 | 13.33** | ||||||
| Race/ethnicity | |||||||||||||
| White | 2.80 (1.31, 4.31) | 1978–1986 | 1.52 (-1.44, 4.58) | 1986–1998 | 5.75 (4.29, 7.22) | 1998–2001 | -1.50 (-16.55, 16.27) | 2001–2008 | 4.77 (2.13, 7.49) | 2008–2018 | 0.33 (-0.67, 1.34) | 4.09 | 3.55* |
| Black | 2.14 (1.55, 2.73) | 1978–2008 | 3.47 (2.89, 4.04) | 2008–2018 | -1.75 (-3.42, -0.06) | 5.94 | 5.23 | ||||||
| Other races | 0.28 (-0.12, 0.68) | 1978–2005 | 1.80 (1.34, 2.26) | 2005–2018 | -2.80 (-3.61, -1.98) | 6.42 | 5.37** | ||||||
| Stage | |||||||||||||
| Localized | 3.95 (2.97, 4.94) | 1978–1988 | 2.59 (-0.33, 5.60) | 1988–2006 | 8.40 (7.56, 9.24) | 2006–2015 | 0.35 (-1.07, 1.79) | 2015–2018 | -6.13 (-11.96, 0.09) | 1.95 | 1.76 | ||
| Regional | 2.91 (2.41, 3.41) | 1978–2005 | 4.59 (4.01, 5.17) | 2005–2018 | -0.49 (-1.50, 0.53) | 1.24 | 1.00** | ||||||
| Distal *** | 1.98 (1.76, 2.21) | 1.16 | 1.01 | ||||||||||
* Indicates statistical significance (p<0.05)
** Indicates significant declines from 2017 to 2018, p<0.05;
*** Model has no joinpoints; it only has a continuous trend.
AAPC = average annual percent changes, APC = annual percent changes, CI = confidence interval;
Rates were standardized to the 2000 US population in 5-year age categories.
The Joinpoint regression model requires a minimum of two observations between a joinpoint and the first or last data point and between two joinpoints.
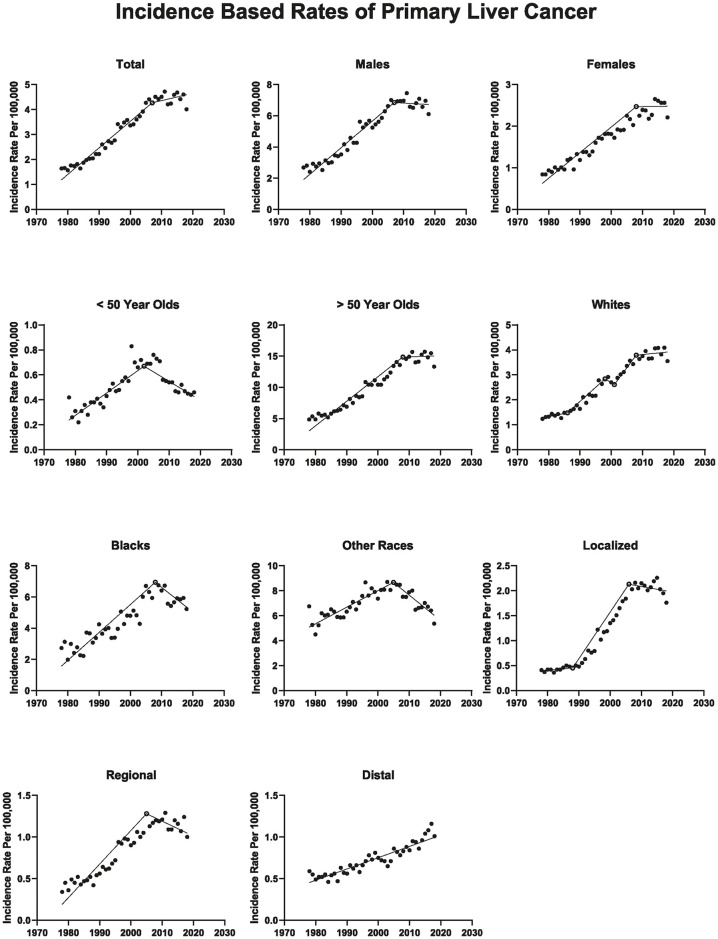
Overall, PLC rates increased by 3.86%/year (p < 0.001) during 1978–2007, then plateaued starting in 2007 (APC, -0.26%/year; p = 0.524). The average APC from 1978 to 2018 was 2.711% yearly (p < 0.001). Males’ and females’ rates increased up to 2007 and 2008, respectively, then plateaued (APC, -0.72%/year; p = 0.126; APC, 0.64%/year; p = 0.291, respectively). For ≥ 50-year-olds, rates increased during 1978–2008 (APC, 3.91%/year; p < 0.001), then plateaued from 2008 to 2018 (APC, -0.03%/year; p = 0.953). In contrast, for < 50-year-olds, rates increased during 1978–2002 by 3.91%/year (p < 0.001), then decreased from 2002 to 2018 (APC, -3.62%/year; p < 0.001) (Fig 2).
Fig 2. Age-standardized incidence rates of primary liver cancer in the SEER-9 US cancer registry, 1978–2018.
Solid circles represent incidence rates, open circles represent years with a significant change in annual percent changes in rates, and lines represent modeled rates using Joinpoint regression. The y-axis varies between figures.
After years of steadily increasing PLC rates, it plateaued among white individuals beginning in 2008 (APC, 0.33%/year; p = 0.505) and declined among black individuals in 2008 (APC, -1.75%/year; p = 0.043). Starting in 2005, among the other races (American Indian/AK Native and Asian/Pacific Islander), PLC rates decreased by -2.80%/year (p < 0.001).
When looking at the IBR of localized PLC there was a significant increase between 1988 to 2006 (APC, 8.40%/year; p < 0.001), then plateaued (2006–2015) before decreasing from 2015 to 2018 (APC, -6.13%/year; p = 0.053). As for regional PLC IBR increased from 1978 to 2005 (APC, 4.60%/year; p < 0.001), then plateaued till 2018. Finally, distant PLC rates have been increasing throughout the study period (APC, 1.98%/year; p < 0.001).
When comparing the PLC IBR between 2017 to those in 2018, we found a significant decrease (RR, 0.87; 95% CI = 0.809–0.932; p < 0.001). Similarly, between 2017 and 2018, in analyses restricted to SEER-21 (RR, 0.96; 95% CI = 0.925–0.991; p = 0.01), SEER-18 (RR, 0.96; 95% CI = 0.922–1.000; p = 0.05) and SEER-13 (RR, 0.86; 95% CI = 0.818–0.921; p < 0.001) registries and when liver/intrahepatic bile duct rates were estimated using delay adjustment (RR, 0.93; p = 0.005).
PLC incidence-based mortality rates
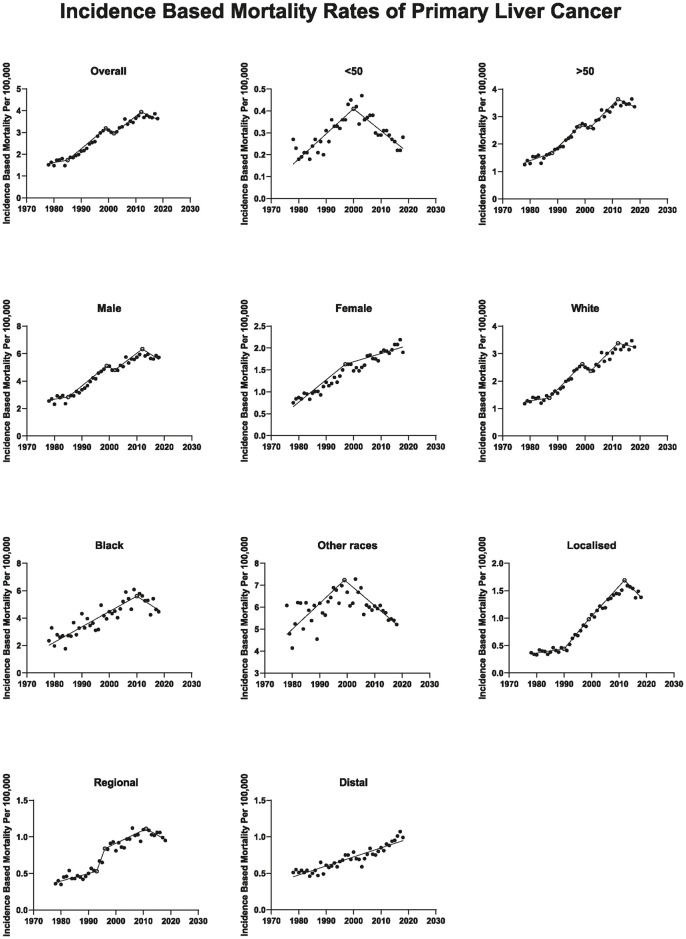
The PLC IBMR increased by 1.11%/year (p = 0.321) during 1978–1985, then increased further to 4.56%/year (p < 0.001) between 1985 to 1999, followed by a slight decrease of -1.62%/year (p = 0.745) between 1999 and 2002, while from 2002 to 2012 the mortality increased to 2.41%/year (p < 0.001) then plateaued starting in 2012 (APC, -0.70%/year; p = 0.299). The average APC from 1978 to 2018 was 2.15%/year (p < 0.001). Males’ IBMR steadily increased up to 2012 and then plateaued from 2012 to 2018 (APC, -1.25%/year; p = 0.092). Whereas females’ rates increased from 1978 to 1997 (APC, 3.624%/year; p < 0.001), then increased at a slower rate to 2018 (APC, 1.651%/year; p < 0.001). For ≥ 50-year-olds, rates steadily increased from 1978 to 2012, then plateaued from 2012 to 2018 (APC, -0.52%/year; p = 0.490). In contrast, < 50-year-old rates increased during 1978–2000 by 3.94%/year (p < 0.001), then decreased from 2000 to 2018 (APC, -3.168%/year; p < 0.001) (Fig 3). Table 2 summarizes the patient demographics and age-standardized IBMR.
Fig 3. Age-standardized mortality rates for primary liver cancer in the SEER-9 US cancer registry, 1978–2018.
Solid circles represent incidence rates, open circles represent years with a significant change in annual percent change in rates, and lines represent modeled rates using Joinpoint regression. The y-axis varies across figures.
Table 2. Age-standardized incidence-based mortality rates, and annual percent changes in primary liver cancer rates, 1978–2018.
| Incidence-based Mortality Rates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment 1 | Segment 2 | Segment 3 | Segment 4 | Segment 5 | Incidence rates Per 100,000 |
||||||||
| AAPC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | Years | APC (95% CI) | 2017 | 2018 | |
| Overall | 2.15* (1.24, 3.06) | 1978–1985 | 1.11 (-1.14, 3.42) | 1985–1999 | 4.56* (3.87, 5.25) | 1999–2002 | -1.62 (-11.15, 8.94) | 2002–2012 | 2.41* (1.56, 3.27) | 2012–2018 | -0.70 (-2.03, 0.66) | 3.86 | 3.64 |
| Sex | |||||||||||||
| Men | 1.94* (0.93, 2.95) | 1978–1985 | 0.69 (-1.84, 3.28) | 1985–1999 | 4.55* (3.78, 5.32) | 1999–2002 | -1.91 (-12.32, 9.74) | 2002–2012 | 2.34* (1.40, 3.28) | 2012–2018 | -1.25 (-2.71, 0.22) | 5.86 | 5.73 |
| Women | 2.58* (2.23, 2.94) | 1978–1997 | 3.62* (2.97, 4.29) | 1997–2018 | 1.65* (1.29, 2.01) | 2.19 | 1.90 | ||||||
| Age, years | |||||||||||||
| <50 | 0.68* (0.02, 1.34) | 1978–2000 | 3.94* (2.99, 4.90) | 2000–2018 | -3.17* (-4.14, -2.19) | 0.22 | 0.28 | ||||||
| ≥50 | 2.41* (1.59, 3.25) | 1978–1988 | 2.23* (0.74, 3.74) | 1988–1998 | 4.97* (3.61, 6.36) | 1998–2002 | -0.43 (-6.28, 5.79) | 2002–2012 | 3.01* (2.02, 4.02) | 2012–2018 | -0.52 (-2.03, 1.01) | 3.64 | 3.36 |
| Race/ethnicity | |||||||||||||
| White | 2.46* (1.26, 3.68) | 1978–1987 | 1.42 (-0.56, 3.44) | 1987–1999 | 5.37* (4.21, 6.54) | 1999–2002 | -3.37 (-15.85, 10.94) | 2002–2012 | 3.17* (1.99, 4.35) | 2012–2018 | 0.18 (-1.62, 2.01) | 3.47 | 3.24 |
| Black | 1.63* (0.95, 2.32) | 1978–2010 | 2.81* (2.25, 3.37) | 2010–2018 | -2.93* (-5.54, -0.23) | 4.63 | 4.46 | ||||||
| Other races | 0.13 (-0.29, 0.55) | 1978–1999 | 1.44* (0.74, 2.13) | 1999–2018 | -1.30* (-1.79, -0.80) | 5.40 | 5.21 | ||||||
| Stage | |||||||||||||
| Localized | 3.50* (2.73, 4.28) | 1978–1990 | 1.92* (0.26, 3.61) | 1990–1999 | 9.44* (7.10, 11.83) | 1999–2012 | 3.84* (3.06, 4.63) | 2012–2018 | -2.53* (-4.25, -0.77) | 1.49 | 1.38 | ||
| Regional | 2.34* (0.55, 4.16) | 1978–1993 | 2.23* (0.94, 3.53) | 1993–1996 | 14.69 (-8.70, 44.08) | 1996–2011 | 1.94* (1.10, 2.79) | 2011–2018 | -1.51 (-3.49, 0.51) | 0.99 | 0.95 | ||
| Distal ** | 1.88* (1.66, 2.10) | 1.07 | 0.99 | ||||||||||
* Indicates statistical significance (p<0.05)
** Model has no joinpoints; it only has a continuous trend.
AAPC = average annual percent changes, APC = annual percent changes, CI = confidence interval;
Rates were standardized to the 2000 US population in 5-year age categories.
The Joinpoint regression model requires a minimum of two observations between a joinpoint and the first or last data point and between two joinpoints.
After years of steadily increasing PLC IBMR, it plateaued among white individuals beginning in 2012 (APC, 0.18%/year; p = 0.843) and declined among Black individuals in 2010 (APC, -2.925%/year; p = 0.034). Starting in 1999, among the other races (American Indian/AK Native and Asian/Pacific Islander), PLC rates decreased by -1.30%/year (p < 0.001).
The analysis of localized PLC IBMR showed an increase from 1978 to 1990, followed by a more rapid increase till 1999 that subsequently slowed till 2012 (APC, 1.92%/year; p = 0.025; APC, 9.44%/year; p < 0.001; APC, 3.84%/year; p < 0.001; respectively), then decreased from 2012 to 2018 (APC, -2.53%/year; p = 0.006). Regional PLC IBMR started to decline from 2011 to 2018 (APC, 1.51%/year; p = 0.136). Finally, distant PLC rates have been increasing throughout the study period (APC, 1.88%/year; p < 0.001).
Subgroup analysis
HCC vs ICC in ≥ 50 years old
The IBR of HCC in patients ≥ 50 years old increased from 1978 to 2009 by 3.81%/year (p < 0.001), followed by a significant decrease between 2009–2018 at a rate of -2.54%/year (p < 0.001). In the same age group, the IBR of ICC increased by 9.98%/year (p < 0.001) from 1978 to 1997, followed by an insignificant decline (APC, -8.94%/year; p = 0.099) between 1997–2003, and then increased by 9.96%/year (p < 0.001) between 2003 and 2018.
Regarding IBMR of HCC in patients ≥ 50 years old increased by 2.98%/year (p < 0.001) from 1978 to 2010, followed by a significant decrease from 2010 to 2018 by -2.05%/year (p < 0.001). In the same age group, the IBMR of ICC rapidly increased by 9.88%/year (p < 0.001) from 1978 to 1997, followed by a significant decrease by -5.49%/year (p = 0.049) from 1997 to 2005, and then a rapid rise by 10.13%/year (p < 0.001) from 2005 to 2018.
Distant group
In the distant subgroup, the PLC IBR for individuals < 50 years old increased by 3.19%/year (p < 0.001) from 1978 to 1998, followed by a significant decline of -1.34%/year (p = 0.034) until 2018. In contrast, those ≥ 50 years old experienced a consistent increase in PLC IBR of 2.19%/year (p < 0.001) from 1978 to 2018.
For males, the distant PLC IBR rose by 1.72%/year (p < 0.001) throughout the study period. On the other hand, females had an initial increase of 1.53%/year (p < 0.001) from 1978 to 2010, followed by a sharper rise of 5.76%/year (p < 0.001) until 2018.
Regarding race, white individuals showed an increasing distant PLC IBR, first by 2.00%/year (p < 0.001) from 1978 to 2010, then accelerating to 4.03%/year (p = 0.001) from 2010 to 2018. Black individuals experienced a steady increase of 1.43%/year (p < 0.001) throughout the study period. In contrast, other races saw a decrease in distant PLC IBR by -0.82%/year (p = 0.001) throughout the study period.
In the distant subgroup, the PLC IBMR for individuals < 50 years old showed a significant decrease of -3.30%/year (p = 0.001) from 1978 to 1997, followed by a decrease of -1.71% per year (p = 0.005) from 1997 to 2018. For those aged ≥ 50, the distant PLC IBMR increased by 2.11%/year (p < 0.001) since 1978.
Regarding distal PLC IBMR in males and females, males had a persistent increase of 1.62%/year (p < 0.001) throughout the study period. Females initially saw an increase of 1.43%/year (p < 0.001) from 1978 until 2011, which then accelerated to 7.03%/year (p = 0.001) until 2018.
In terms of racial differences, the distant PLC IBMR for white individuals was stable between 1978 and 1991 (APC, 0.29%/year; p = 0.708), increased by 7.09% per year (p = 0.016) from 1991 to 1997, declined insignificantly by -2.37%/year (p = 0.338) from 1997 to 2003, and then increased again by 3.63%/year (p < 0.001) from 2003 onwards. For black individuals, there was a persistent increase in distant PLC IBMR by 1.37%/year (p < 0.001) throughout the study period. Other races’ rates declined by -0.85%/year (p = 0.001) over the entire study period.
Discussion
Incidence-based rates
Overall, PLC IBRs have steadily risen over the last four decades; however, since the mid-2000s, rates have plateaued. Since HCC accounts for the majority of PLC cases [4], better control of HCC risk factors likely explains this drop. Hepatitis C virus (HCV) is the most common cause of HCC in the US [15]. Advances in the pre-transfusion screening of blood products, HCV awareness, and treatment have been noted over the last 2 decades [16–18]. Furthermore, HCV direct-acting anti-viral therapy was approved in the US in 2011, which could also be contributing to declining incidence [19]. Although non-alcoholic fatty liver disease (NAFLD) incidences have been on the rise in the US [20, 21], it is unclear how this has affected HCC IBRs. Notably, recent studies suggested that patients with NAFLD, particularly the non-alcoholic steatohepatitis (NASH) subgroup, are more likely to die of cirrhosis, cardiovascular disease, diabetes mellitus, and non-HCC cancers than of HCC [22, 23]. Hence, the decrease in the incidences of PLC might be partially due to the rise of NAFLD. Further studies are needed to explore this correlation and whether PLC IBRs will continue to decline in the future.
PLC rates in individuals younger than fifty have been declining since the early 2000s. This can be partially explained by the introduction of Hepatitis B virus (HBV) vaccination and improved HBV treatment [24]. A randomized control trial showed that treatment of chronic HBV significantly decreases the risk of HCC development [25]. Those two factors might also explain the significant decline in PLC rates among Asians, who suffer from HBV the most [24]. On the other hand, the lack of a significant decline in IBRs in those older than fifty years could be explained by multiple factors. Firstly, with improved cirrhosis management, survival has improved; consequently, the risk of HCC among this group might be increasing. Moreover, a European study showed that a large proportion of patients with chronic HCV are late presenters and often present at an advanced age with complications such as HCC [26]. Finally, ICC is associated with advanced age, and patients with chronic HCV are at an increased risk of ICC [27] all of which might be contributing to the stabilization of the PLC rate among this age group in the setting of the overall decline in the study cohort. This is demonstrated in the subgroup analysis which showed that while HCC rates have started to decline, ICC rates have significantly increased, rising at more than three times the rate of the HCC decline. Although this increase in ICC rates might contribute to the overall rate stabilization in individuals older than 50 years of age, it is unlikely to be the sole factor.
Although it might be premature to conclude that a long-term decline in PLC has begun, the sensitivity analysis based on delay-adjusted rates offers assurance that the decrease in 2018 is not an artifact.
Incidence-based mortality rates
The overall trend of PLC IBM increased till the early 2010s and then plateaued. The initial rise is concurrent with the obesity epidemic, which has spread steadily since the late 1990s [28, 29]. It is noteworthy to mention that obesity is prevalent in around forty percent of the US population above twenty years of age [30] and that obesity in itself is an independent risk factor of HCC mortality [31].
While males continue to have higher IBMRs than females, this study shows that the mortality gap between the two has been shrinking. This difference in IBMR between males and females could be related to the recent narrowing in the trends of alcohol consumption [32, 33]. In recent years, females have been consuming more alcohol [32–35]. Numerous studies have shown that females are more vulnerable to complications of alcohol consumption compared to males [33, 34, 36]. Around 2010, IBMRs in the white population plateaued while those in the black population have been declining. Multiple US studies showed that the prevalence of NAFLD and NASH among Whites was about 1.3 to 1.5 times more than Blacks [37, 38]. These factors could also be contributing to the observed disparity between the IBMRs in both races.
Regarding the subgroup analysis of HCC and ICC IBMR in patients ≥ 50 years old, the initial rise in ICC mortality parallels the increase in incidence and might be related to increased recognition of the disease [39]. The subsequent decrease in ICC mortality could be attributed to earlier detection and increased utilization of surgery, as a study has shown decreased mortality during a similar time frame [39]. However, the IBMR rose again significantly in this age group, which another study has attributed to increased surgical mortality in advanced age [40]. Additionally, another study found that while HCC incidence is decreasing, overall liver cancer mortality is increasing [41]. The former study cited this as evidence that the overall increase in PLC mortality, despite the decrease in HCC mortality, might be explained by the increase in ICC mortality, as our study also speculates [40].
Interestingly, there was an observed significant decline in IBMR amongst those less than fifty years old since 2000. In a similar timeframe, other races/ethnicities, including Asians, also showed a significant decline in IBMR. This could be due to the survival advantages of initiating anti-viral treatment in HCC patients with HBV/HCV; which are leading etiologies among these groups [42–44]. The introduction of screening and surveillance guidelines in the early 2010s also led to improved survival, decreased mortality, and the discovery of HCC at an earlier stage [45, 46]. However, our study also showed that despite an increase in the total PLC IBR, the IBR of localized PLC decreased from 2015–2018. This might seem contradictory, as screening typically leads to an increase in localized cases and a decrease in distal ones. However, the reported decrease in localized IBR was not statistically significant, possibly due to the limited three-year data. As such, unless future data confirms a potential decline, we assume that we are still in the plateau phase.
It is believed that the initiation of screening programs significantly contributed to the initial rise in localized PLC cases, which is supported by a study that showed that screening greatly increased the number of early HCC cases [47]. The subsequent plateau in localized PLC cases between 2015 and 2018 could be due to improved treatment of risk factors, as a recent study in the United Kingdom reported a similar plateau and attributed it to better treatment of hepatitis C [47]. However, as noted in the limitations section, access to data on risk factors was not available to further explain this trend.
Limitations
As with all studies that use SEER as a data source, this study is affected by similar limitations. Firstly, the SEER registries do not provide information about etiologic factors, risk factors, or the presence of comorbidities; hence the proposed explanations for changes in PLC trends are speculative. Secondly, the results are dependent on the histological coding, whereby cases might be missing from the analysis due to variability in the coding. Finally, this paper shares the limitations of any retrospective study. Discussion on the limitations of SEER studies is beyond the scope of this paper; further details have been highlighted in a different report [48].
Conclusion
The incidence and mortality of PLC have been increasing for decades, followed by a plateau in recent years. There seems to be a promising decline in mortality in some age (< 50) and race (Blacks/other races) sub-groups, but more effort is still needed to target modifiable risk factors for PLC that continue to burden a significant portion of the population. This should go hand in hand with the continued development of new diagnostic and therapeutic options.
Abbreviations
- APC
Annual percent change
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- IBMR
Incidence-based mortality rates
- IBR
Incidence-based rates
- ICC
Intrahepatic cholangiocarcinoma
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- PLC
Primary liver cancer
- US
United States
Data Availability
The data used in this study is available free of charge upon registering with the National Cancer Institute SEER database. SEER-9 registry was utilised in this study. Further details regarding access to the minimal data set are available here: https://seer.cancer.gov/data/access.html#researchonly. Furthermore, all of the data generated or analyzed during this study are included in this published article.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries | Enhanced Reader. [DOI] [PubMed]
- 2.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet. 2018;391: 1023–1075. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Frontiers in Oncology. 2020;0: 171. doi: 10.3389/fonc.2020.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macias RIR, Monte MJ, Serrano MA, González-Santiago JM, Martín-Arribas I, Simão AL, et al. Impact of aging on primary liver cancer: epidemiology, pathogenesis and therapeutics. Aging (Albany NY). 2021;13: 23416. doi: 10.18632/aging.203620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Current epidemiology reports. 2019;6: 104. doi: 10.1007/s40471-019-00188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver International. 2019;39: 19–31. doi: 10.1111/liv.14095 [DOI] [PubMed] [Google Scholar]
- 7.Ren J, Tong Y-M, Cui R-X, Wang Z, Li Q-L, Liu W, et al. Comparison of survival between adolescent and young adult vs older patients with hepatocellular carcinoma. World Journal of Gastrointestinal Oncology. 2020;12: 1394. doi: 10.4251/wjgo.v12.i12.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamul Haq M, Drake TM, Lin Goh T, Ahmed A, Forrest E, Barclay S, et al. Clinical Medicine Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study. J Clin Med. 2021;10: 2770. doi: 10.3390/jcm10132770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangkijvanich P, Suwangool P, Mahachai V. Comparison of Clinical Features and Survival of Patients with Hepatitis B-and Hepatitis C-Associated Hepatocellular Carcinoma in Thailand | Tangkijvanich | JOURNAL OF THE MEDICAL ASSOCIATION OF THAILAND. [PubMed]
- 10.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. Journal of Clinical Oncology. 2016;34: 1787. doi: 10.1200/JCO.2015.64.7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SEER*Stat Databases: November 2020 Submission.
- 12.Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, et al. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatology Communications. 2020;4: 1541–1551. doi: 10.1002/hep4.1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che W-Q, Li Y-J, Tsang C-K, Wang Y-J, Chen Z, Wang X-Y, et al. How to use the Surveillance, Epidemiology, and End Results (SEER) data: research design and methodology. Mil Med Res. 2023;10: 50. doi: 10.1186/s40779-023-00488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine. 2000;19: 335–351. doi: [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Parikh ND, Liu H, Wu E, Rao H, Feng B, et al. Incidence and risk factors of hepatocellular carcinoma in patients with hepatitis C in China and the United States. Scientific Reports 2020 10:1. 2020;10: 1–10. doi: 10.1038/s41598-020-77515-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg ES, Rosenthal EM, Hall EW, Barker L, Hofmeister MG, Sullivan PS, et al. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA network open. 2018;1. doi: 10.1001/jamanetworkopen.2018.6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. Journal of hepatology. 2014;60: 691–698. doi: 10.1016/j.jhep.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 18.Drake I, Gullberg B, Sonestedt E, Stocks T, Bjartell A, Wirfält E, et al. Type 2 diabetes, adiposity and cancer morbidity and mortality risk taking into account competing risk of noncancer deaths in a prospective cohort setting. International Journal of Cancer. 2017;141: 1170. doi: 10.1002/ijc.30824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das D, Pandya M. Recent Advancement of Direct-acting Antiviral Agents (DAAs) in Hepatitis C Therapy. Mini reviews in medicinal chemistry. 2018;18: 584–596. doi: 10.2174/1389557517666170913111930 [DOI] [PubMed] [Google Scholar]
- 20.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313: 2263–2273. doi: 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343: 1245. doi: 10.1136/bmj.d6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez CS, Graubard BI, Thistle JE, Petrick JL, McGlynn KA. Attributable Fractions of Nonalcoholic Fatty Liver Disease for Mortality in the United States: Results From the Third National Health and Nutrition Examination Survey With 27 Years of Follow-up. Hepatology. 2020;72: 430–440. doi: 10.1002/hep.31040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality Related to Nonalcoholic Fatty Liver Disease Is Increasing in the United States. Hepatology CommuniCations. 2019;3: 1459. doi: 10.1002/hep4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49. doi: 10.1002/hep.22962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liaw Y-F, Sung JJY, Chow WC, Farrell G, Lee C-Z, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. The New England journal of medicine. 2004;351: 1521–1531. doi: 10.1056/NEJMoa033364 [DOI] [PubMed] [Google Scholar]
- 26.Hansen JF, Hallager S, Øvrehus A, Weis N, Brehm Christensen P, Pedersen C. Late Presentation for Care Among Patients With Chronic Hepatitis C: Prevalence and Risk Factors. Open Forum Infectious Diseases. 2018;5. doi: 10.1093/ofid/ofx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaib YH, El-Serag HB, Davila JA, Morgan R, Mcglynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology. 2005;128: 620–626. doi: 10.1053/j.gastro.2004.12.048 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. International journal of epidemiology. 2020;49: 810–823. doi: 10.1093/ije/dyz273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May AL, Freedman D, Sherry B, Blanck HM. Obesity - United States, 1999–2010. MMWR supplements. 2013;62: 120–128. [PubMed] [Google Scholar]
- 30.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World Journal of Hepatology. 2014;6: 274. doi: 10.4254/wjh.v6.i5.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is Independently Associated with Increased Risk of Hepatocellular Cancer-related Mortality: A Systematic Review and Meta-analysis. American journal of clinical oncology. 2018;41: 874. doi: 10.1097/COC.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keyes KM, Jager J, Mal-Sarkar T, Patrick ME, Rutherford C, Hasin D. Is There a Recent Epidemic of Women’s Drinking? A Critical Review of National Studies. Alcoholism, clinical and experimental research. 2019;43: 1344–1359. doi: 10.1111/acer.14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White AM. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol research: current reviews. 2020;40: 1–13. doi: 10.35946/arcr.v40.2.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCaul ME, Roach D, Hasin DS, Weisner C, Chang G, Sinha R. Alcohol and Women: A Brief Overview. Alcoholism, clinical and experimental research. 2019;43: 774. doi: 10.1111/acer.13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKetta S, Prins SJ, Bates LM, Platt JM, Keyes KM. US trends in binge drinking by gender, occupation, prestige, and work structure among adults in the midlife, 2006–2018. Annals of epidemiology. 2021;62: 22–29. doi: 10.1016/j.annepidem.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong C, Yoon YH, Chen CM, Hsiao-Ye Y. Heavy Alcohol Use and Premature Death from Hepatocellular Carcinoma in the United States, 1999–2006. Journal of Studies on Alcohol and Drugs. 2011;72: 892. doi: 10.15288/jsad.2011.72.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and Ethnic Disparities in Non-alcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. doi: 10.1016/j.cgh.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. doi: 10.3748/wjg.v23.i47.8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology (Baltimore, Md). 2014;60: 1107–1108. doi: 10.1002/hep.26958 [DOI] [PubMed] [Google Scholar]
- 40.Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterology. 2016;16. doi: 10.1186/s12876-016-0527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. The American journal of gastroenterology. 2014;109: 542–553. doi: 10.1038/ajg.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singal AG, Rich NE, Mehta N, Branch AD, Pillai A, Hoteit M, et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology. 2019;157: 1253–1263.e2. doi: 10.1053/j.gastro.2019.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bang CS, Song IH. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: Systematic review and meta-analysis. BMC Gastroenterology. 2017;17: 1–19. doi: 10.1186/S12876-017-0606-9/TABLES/7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singal AG, Rich NE, Mehta N, Branch A, Pillai A, Hoteit M, et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology. 2019;156: 1683–1692.e1. doi: 10.1053/j.gastro.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018;391: 1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 46.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS medicine. 2014;11. doi: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Yang Z, Zeng X, Yu S, Gao L, Jiang Y, et al. Benefits and harms of screening for hepatocellular carcinoma in high-risk populations: systematic review and meta-analysis. Journal of the National Cancer Center. 2023;3: 175–185. doi: 10.1016/j.jncc.2023.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park, NY). 2009;23: 288–295. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study is available free of charge upon registering with the National Cancer Institute SEER database. SEER-9 registry was utilised in this study. Further details regarding access to the minimal data set are available here: https://seer.cancer.gov/data/access.html#researchonly. Furthermore, all of the data generated or analyzed during this study are included in this published article.