Abstract
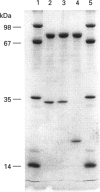
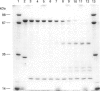
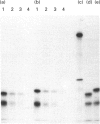
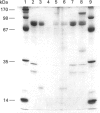
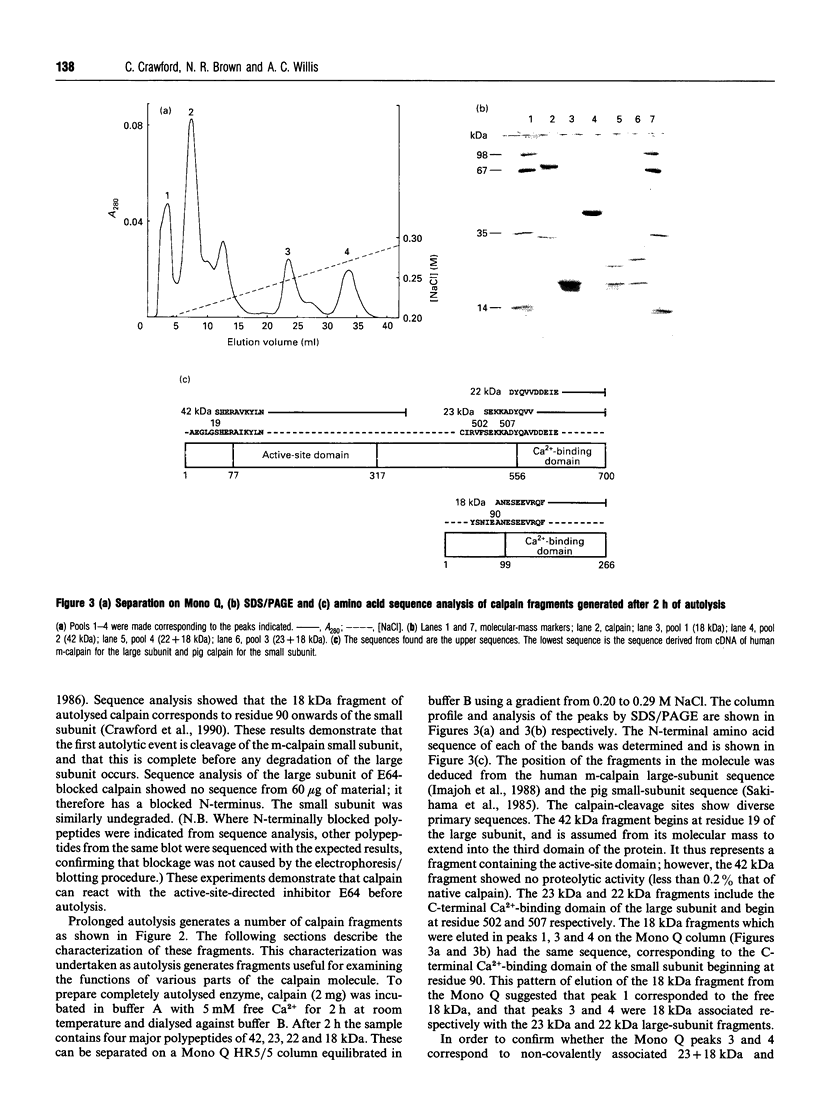
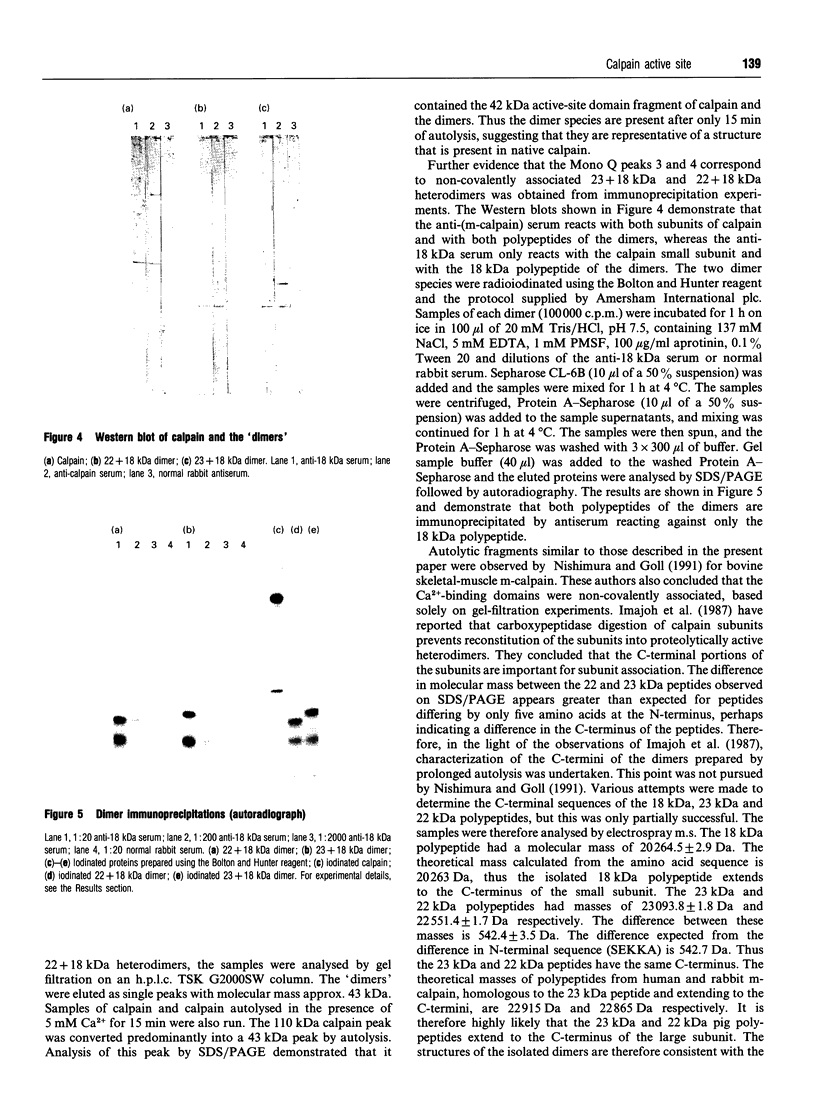
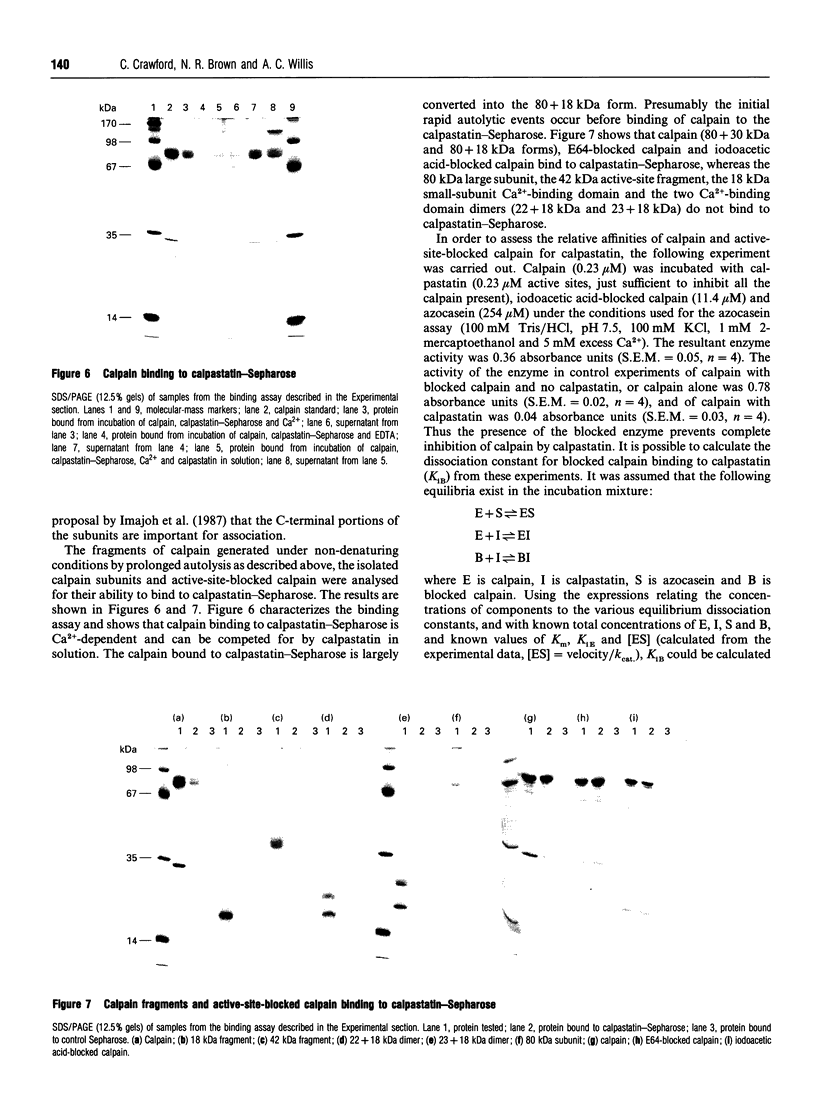
Calpain autolyses in the presence of Ca2+. In the case of m-calpain (80 + 30 kDa) the first product is an 80 + 18 kDa species which has an intact large subunit and the C-terminal Ca(2+)-binding domain of the small subunit. It was possible to bind E64 into the active site of calpain in the presence of Ca2+ before cleavage of either calpain subunit. This suggests that the active site is functional before any autolysis has occurred and that calpain is not a proenzyme. Prolonged autolysis generates several fragments including a 42 kDa active-site domain fragment that showed no proteolytic activity and Ca(2+)-binding domain fragments. Some of the Ca(2+)-binding domain fragments were found to exist as heterodimers (23 + 18 kDa and 22 + 18 kDa), with the Ca(2+)-binding domain of the large subunit interacting with the Ca(2+)-binding domain of the small subunit. These species were true heterodimers, as they showed co-elution of the two Ca(2+)-binding domains on ion-exchange and gel-filtration chromatography, and immunoprecipitation of both polypeptides with an antiserum specific for the small-subunit Ca(2+)-binding domain. The generation of the dimer species after only 15 min autolysis suggests that the interaction between the Ca(2+)-binding domains is present in the native calpain structure. The interaction of calpain with calpastatin was investigated using an assay based on binding to calpastatin-Sepharose and a competitive binding assay. Calpain, active-site-blocked calpain and calpain fragments generated by autolysis were studied. Calpain bound to calpastatin in the presence of Ca2+; however, the isolated active-site-containing 80 kDa large subunit (proteolytically inactive), a 42 kDa active-site-containing fragment (proteolytically inactive) and Ca(2+)-binding domain fragments of calpain did not. Active-site-blocked calpain bound to calpastatin, but with an affinity reduced by approximately two orders of magnitude when compared with native calpain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cong J., Goll D. E., Peterson A. M., Kapprell H. P. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J Biol Chem. 1989 Jun 15;264(17):10096–10103. [PubMed] [Google Scholar]
- Coolican S. A., Haiech J., Hathaway D. R. The role of subunit autolysis in activation of smooth muscle Ca2+-dependent proteases. J Biol Chem. 1986 Mar 25;261(9):4170–4176. [PubMed] [Google Scholar]
- Cottin P., Vidalenc P. L., Merdaci N., Ducastaing A. Evidence for non-competitive inhibition between two calcium-dependent activated neutral proteinases and their specific inhibitor. Biochim Biophys Acta. 1983 Mar 16;743(2):299–302. doi: 10.1016/0167-4838(83)90227-3. [DOI] [PubMed] [Google Scholar]
- Crawford C., Brown N. R., Willis A. C. Investigation of the structural basis of the interaction of calpain II with phospholipid and with carbohydrate. Biochem J. 1990 Jan 15;265(2):575–579. doi: 10.1042/bj2650575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C., Willis A. C., Gagnon J. The effects of autolysis on the structure of chicken calpain II. Biochem J. 1987 Dec 1;248(2):579–588. doi: 10.1042/bj2480579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Huff C. A., Croall D. E. Autoproteolysis of the small subunit of calcium-dependent protease II activates and regulates protease activity. J Biol Chem. 1986 Sep 15;261(26):12047–12052. [PubMed] [Google Scholar]
- Imajoh S., Aoki K., Ohno S., Emori Y., Kawasaki H., Sugihara H., Suzuki K. Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease. Biochemistry. 1988 Oct 18;27(21):8122–8128. doi: 10.1021/bi00421a022. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Kawasaki H., Suzuki K. Limited autolysis of calcium-activated neutral protease (CANP): reduction of the Ca2+-requirement is due to the NH2-terminal processing of the large subunit. J Biochem. 1986 Sep;100(3):633–642. doi: 10.1093/oxfordjournals.jbchem.a121755. [DOI] [PubMed] [Google Scholar]
- Inomata M., Imahori K., Kawashima S. Autolytic activation of calcium-activated neutral protease. Biochem Biophys Res Commun. 1986 Jul 31;138(2):638–643. doi: 10.1016/s0006-291x(86)80544-7. [DOI] [PubMed] [Google Scholar]
- Kapprell H. P., Goll D. E. Effect of Ca2+ on binding of the calpains to calpastatin. J Biol Chem. 1989 Oct 25;264(30):17888–17896. [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Imajoh-Ohmi S., Minami Y., Suzuki K. Identification and characterization of inhibitory sequences in four repeating domains of the endogenous inhibitor for calcium-dependent protease. J Biochem. 1989 Aug;106(2):274–281. doi: 10.1093/oxfordjournals.jbchem.a122844. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maki M., Takano E., Mori H., Sato A., Murachi T., Hatanaka M. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987 Oct 19;223(1):174–180. doi: 10.1016/0014-5793(87)80531-8. [DOI] [PubMed] [Google Scholar]
- Maki M., Takano E., Osawa T., Ooi T., Murachi T., Hatanaka M. Analysis of structure-function relationship of pig calpastatin by expression of mutated cDNAs in Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10254–10261. [PubMed] [Google Scholar]
- Mellgren R. L., Nettey M. S., Mericle M. T., Renno W., Lane R. D. An improved purification procedure for calpastatin, the inhibitor protein specific for the intracellular calcium-dependent proteinases, calpains. Prep Biochem. 1988;18(2):183–197. doi: 10.1080/00327488808062520. [DOI] [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Inomata M., Hayashi M., Imahori K., Kawashima S. Purification and characterization of an inhibitor of calcium-activated neutral protease from rabbit skeletal muscle: purification of 50,000-dalton inhibitor. J Biochem. 1984 Nov;96(5):1399–1407. doi: 10.1093/oxfordjournals.jbchem.a134968. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Goll D. E. Binding of calpain fragments to calpastatin. J Biol Chem. 1991 Jun 25;266(18):11842–11850. [PubMed] [Google Scholar]
- Parkes C., Kembhavi A. A., Barrett A. J. Calpain inhibition by peptide epoxides. Biochem J. 1985 Sep 1;230(2):509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Schoolnik G. K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984 Jul 1;160(1):208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakihama T., Kakidani H., Zenita K., Yumoto N., Kikuchi T., Sasaki T., Kannagi R., Nakanishi S., Ohmori M., Takio K. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6075–6079. doi: 10.1073/pnas.82.18.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S., Kimura Y., Kubota S., Imahori K. Autolysis of calcium-activated neutral protease of chicken skeletal muscle. J Biochem. 1981 Dec;90(6):1787–1793. doi: 10.1093/oxfordjournals.jbchem.a133656. [DOI] [PubMed] [Google Scholar]
- Takano E., Maki M., Mori H., Hatanaka M., Marti T., Titani K., Kannagi R., Ooi T., Murachi T. Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry. 1988 Mar 22;27(6):1964–1972. doi: 10.1021/bi00406a024. [DOI] [PubMed] [Google Scholar]