Abstract
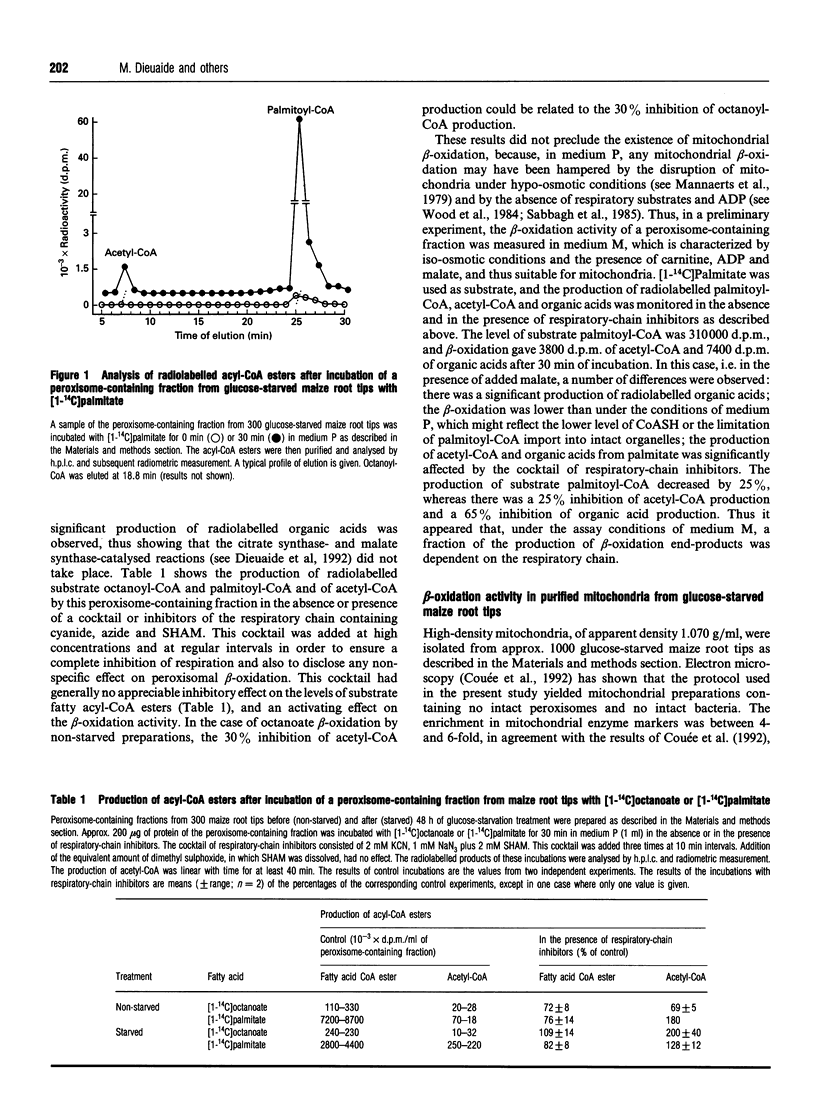
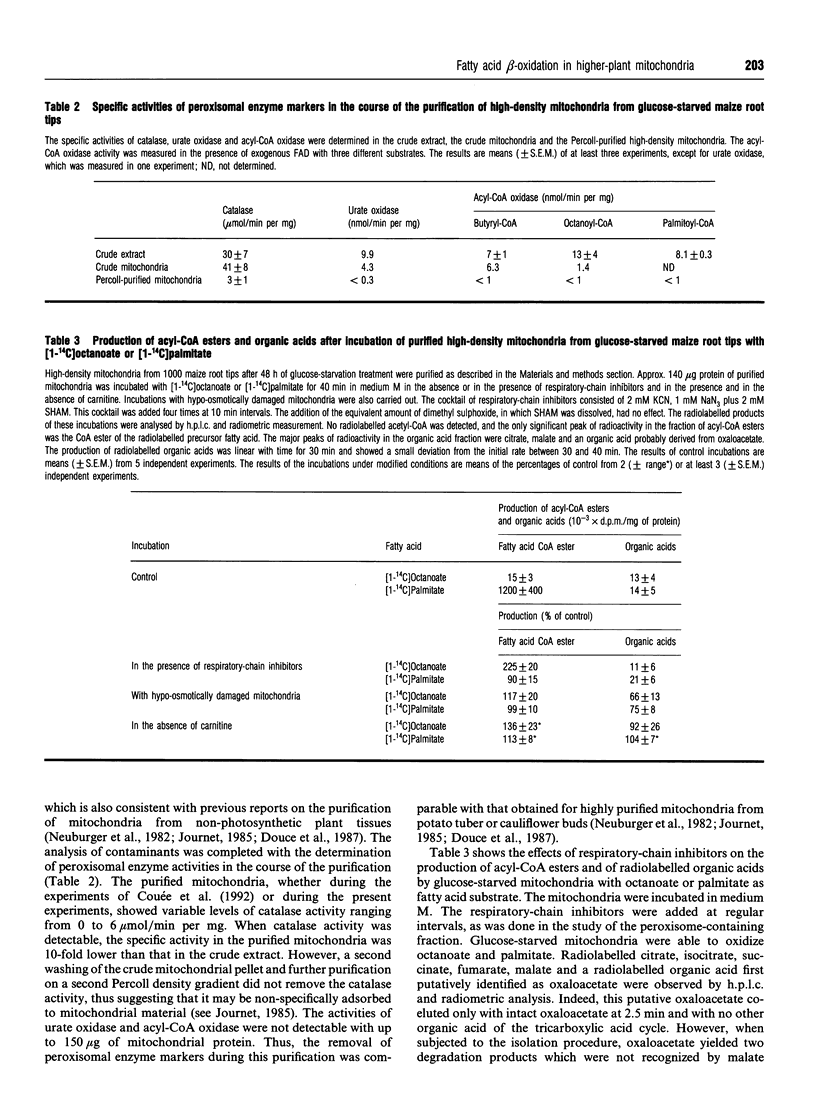
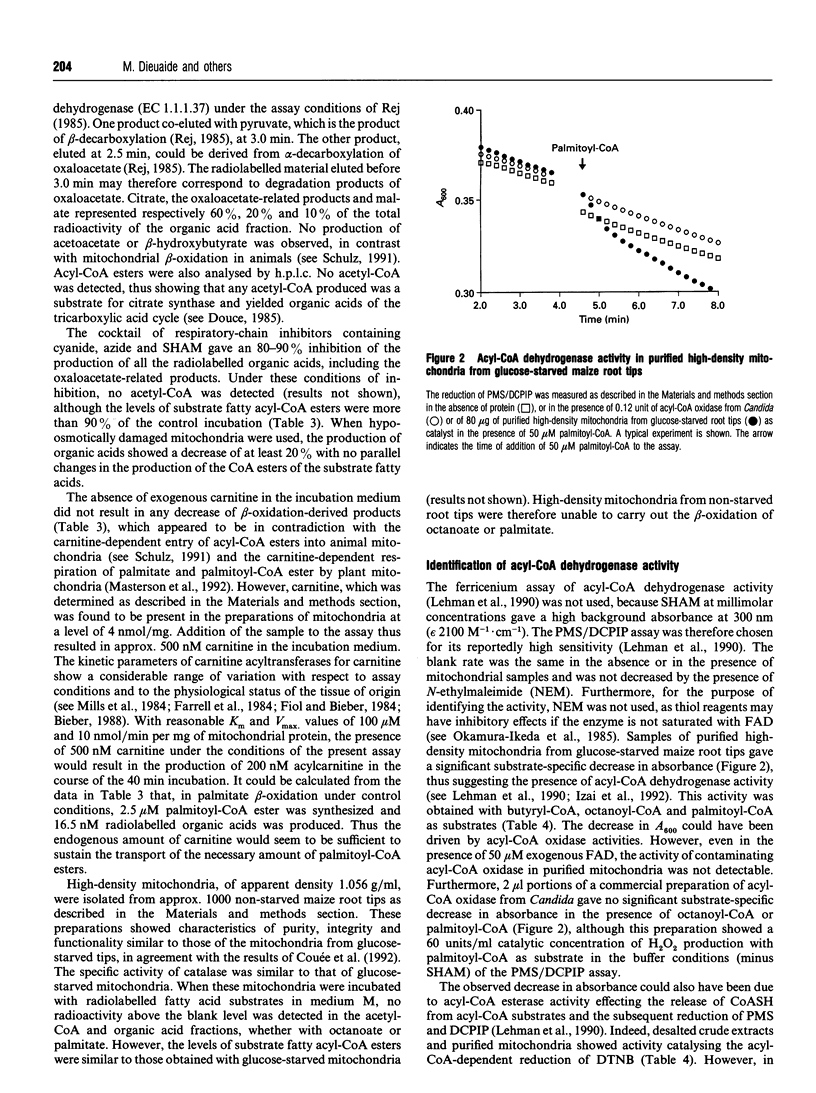
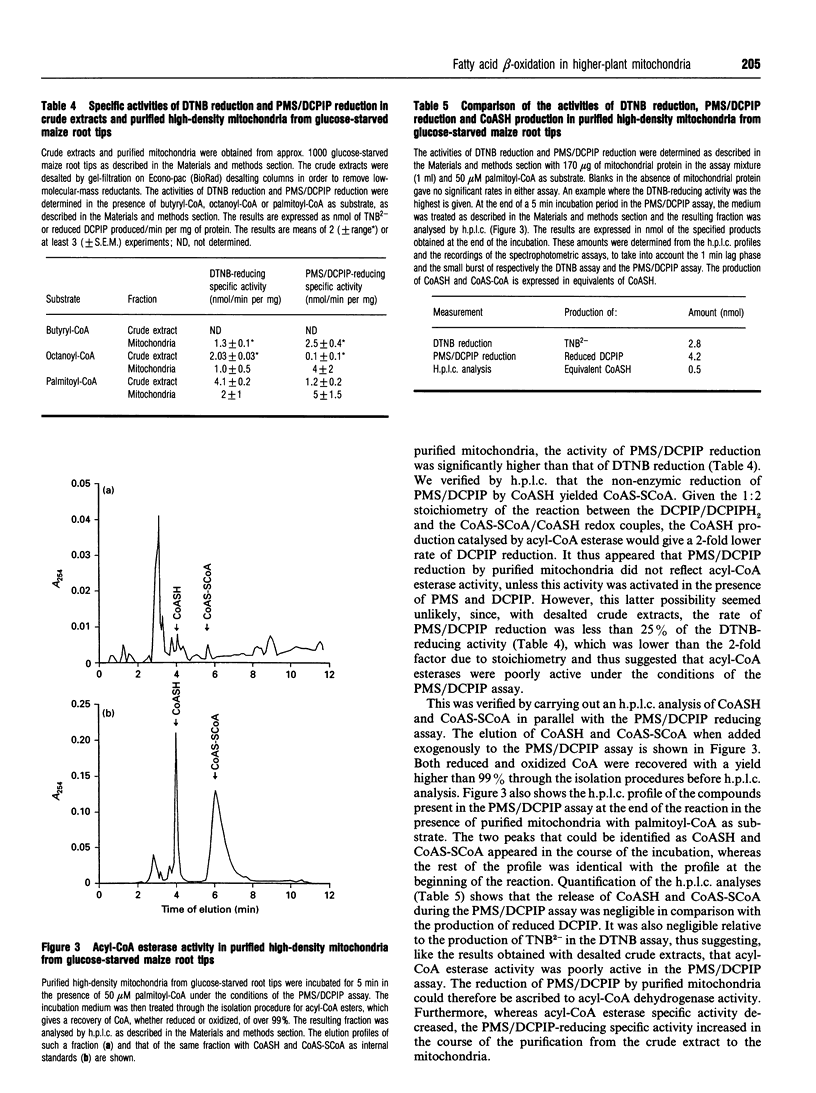
Fatty acid beta-oxidation was studied in organellar fractions from maize root tips by h.p.l.c. and radiometric analysis of the products of incubations with [1-14C]octanoate and [1-14C]palmitate. In crude organellar fractions containing both mitochondria and peroxisomes, octanoate and palmitate beta-oxidation, as determined by the production of acetyl-CoA, was functional and, for palmitate, was activated 4-12-fold after subjecting the root tips to 48 h of glucose starvation. The sensitivity to a 'cocktail' of respiratory-chain inhibitors containing cyanide, azide and salicylhydroxamate depended on the conditions of incubation, with no inhibition in a medium facilitating peroxisomal beta-oxidation and a significant inhibition in a medium potentially facilitating mitochondrial beta-oxidation. Indeed, preparations of highly purified mitochondria from glucose-starved root tips were able to oxidize octanoate and palmitate to give organic acids of the tricarboxylic acid cycle. This activity was inhibited 5-10-fold by the above cocktail of respiratory-chain inhibitors, with no parallel accumulation of acetyl-CoA, thus showing that the inhibition affected beta-oxidation rather than the pathway from acetyl-CoA to the organic acids. This provides the first evidence that the complete beta-oxidation pathway from fatty acids to citrate was functional in mitochondria from a higher plant. Moreover, an acyl-CoA dehydrogenase activity was shown to be present in the purified mitochondria. In contrast with the peroxisomal activity, mitochondrial beta-oxidation showed the same efficiency with octanoate and palmitate and was strictly dependent on glucose starvation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker F. C., Schooley D. A. Analysis and purification of acyl coenzyme A thioesters by reversed-phase ion-pair liquid chromatography. Anal Biochem. 1979 Apr 15;94(2):417–424. doi: 10.1016/0003-2697(79)90384-1. [DOI] [PubMed] [Google Scholar]
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brouquisse R., James F., Raymond P., Pradet A. Study of glucose starvation in excised maize root tips. Plant Physiol. 1991 Jun;96(2):619–626. doi: 10.1104/pp.96.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couée I., Defontaine S., Carde J. P., Pradet A. Effects of anoxia on mitochondrial biogenesis in rice shoots: modification of in organello translation characteristics. Plant Physiol. 1992 Feb;98(2):411–421. doi: 10.1104/pp.98.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Neuburger M., Douce R. Activation of NAD-linked malic enzyme in intact plant mitochondria by exogenous coenzyme A. Arch Biochem Biophys. 1984 May 15;231(1):233–242. doi: 10.1016/0003-9861(84)90383-7. [DOI] [PubMed] [Google Scholar]
- Dieuaide M., Brouquisse R., Pradet A., Raymond P. Increased Fatty Acid beta-Oxidation after Glucose Starvation in Maize Root Tips. Plant Physiol. 1992 Jun;99(2):595–600. doi: 10.1104/pp.99.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell S. O., Fiol C. J., Reddy J. K., Bieber L. L. Properties of purified carnitine acyltransferases of mouse liver peroxisomes. J Biol Chem. 1984 Nov 10;259(21):13089–13095. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Hosokawa Y., Shimomura Y., Harris R. A., Ozawa T. Determination of short-chain acyl-coenzyme A esters by high-performance liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):45–49. doi: 10.1016/0003-2697(86)90058-8. [DOI] [PubMed] [Google Scholar]
- Izai K., Uchida Y., Orii T., Yamamoto S., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J Biol Chem. 1992 Jan 15;267(2):1027–1033. [PubMed] [Google Scholar]
- Lehman T. C., Hale D. E., Bhala A., Thorpe C. An acyl-coenzyme A dehydrogenase assay utilizing the ferricenium ion. Anal Biochem. 1990 May 1;186(2):280–284. doi: 10.1016/0003-2697(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- Miernyk J. A., Thomas D. R., Wood C. Partial purification and characterization of the mitochondrial and peroxisomal isozymes of enoyl-coenzyme a hydratase from germinating pea seedlings. Plant Physiol. 1991 Feb;95(2):564–569. doi: 10.1104/pp.95.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Okamura-Ikeda K., Ikeda Y., Tanaka K. An essential cysteine residue located in the vicinity of the FAD-binding site in short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. J Biol Chem. 1985 Jan 25;260(2):1338–1345. [PubMed] [Google Scholar]
- Osmundsen H., Bremer J., Pedersen J. I. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta. 1991 Sep 11;1085(2):141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- Panter R. A., Mudd J. B. Some aspects of carnitine metabolism in avocado (Persea americana) (Short Communication). Biochem J. 1973 Jun;134(2):655–658. doi: 10.1042/bj1340655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Estimation of peroxisomal and mitochondrial fatty acid oxidation in rat hepatocytes using tritiated substrates. Biochem J. 1991 Oct 1;279(Pt 1):147–150. doi: 10.1042/bj2790147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh E., Cuebas D., Schulz H. 3-Mercaptopropionic acid, a potent inhibitor of fatty acid oxidation in rat heart mitochondria. J Biol Chem. 1985 Jun 25;260(12):7337–7342. [PubMed] [Google Scholar]
- Saglio P. H., Pradet A. Soluble Sugars, Respiration, and Energy Charge during Aging of Excised Maize Root Tips. Plant Physiol. 1980 Sep;66(3):516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Rancillac M., Bruzan F., Pradet A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984 Sep;76(1):151–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salon C., Raymond P., Pradet A. Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J Biol Chem. 1988 Sep 5;263(25):12278–12287. [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991 Jan 28;1081(2):109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Sumegi B., Porpaczy Z., Alkonyi I. Kinetic advantage of the interaction between the fatty acid beta-oxidation enzymes and the complexes of the respiratory chain. Biochim Biophys Acta. 1991 Jan 28;1081(2):121–128. doi: 10.1016/0005-2760(91)90016-b. [DOI] [PubMed] [Google Scholar]


